Lentil Waste Extracts for Inflammatory Bowel Disease (IBD) Symptoms Control: Anti-Inflammatory and Spasmolytic Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Lentil Materials
2.2. Lentil Hull Extraction Procedures
2.2.1. Conventional Extraction
2.2.2. Microwave-Assisted Extraction (MAE)
2.3. Determination of Total Phenolic Content (TPC)
2.4. Anti-Inflammatory Activity
2.4.1. Cell Cultures and Treatments
2.4.2. Cell Viability Assay
2.4.3. Electrophoresis and Western Blotting
2.4.4. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) and Quantitative Real-Time PCR Analyses
2.4.5. Data Presentation and Statistical Analysis
2.5. Spasmolytic Activity
Spontaneous Contractility
2.6. Lentil Hull Metabolite Profiling
2.6.1. Sample Preparation
2.6.2. Untargeted High-Resolution Mass Spectrometry Analysis
2.6.3. Metabolite Identification
3. Results
3.1. Lentil Hull Bioactive Compounds Extraction and Determination of Total Phenolic Content (TPC)
3.2. Anti-Inflammatory Activity
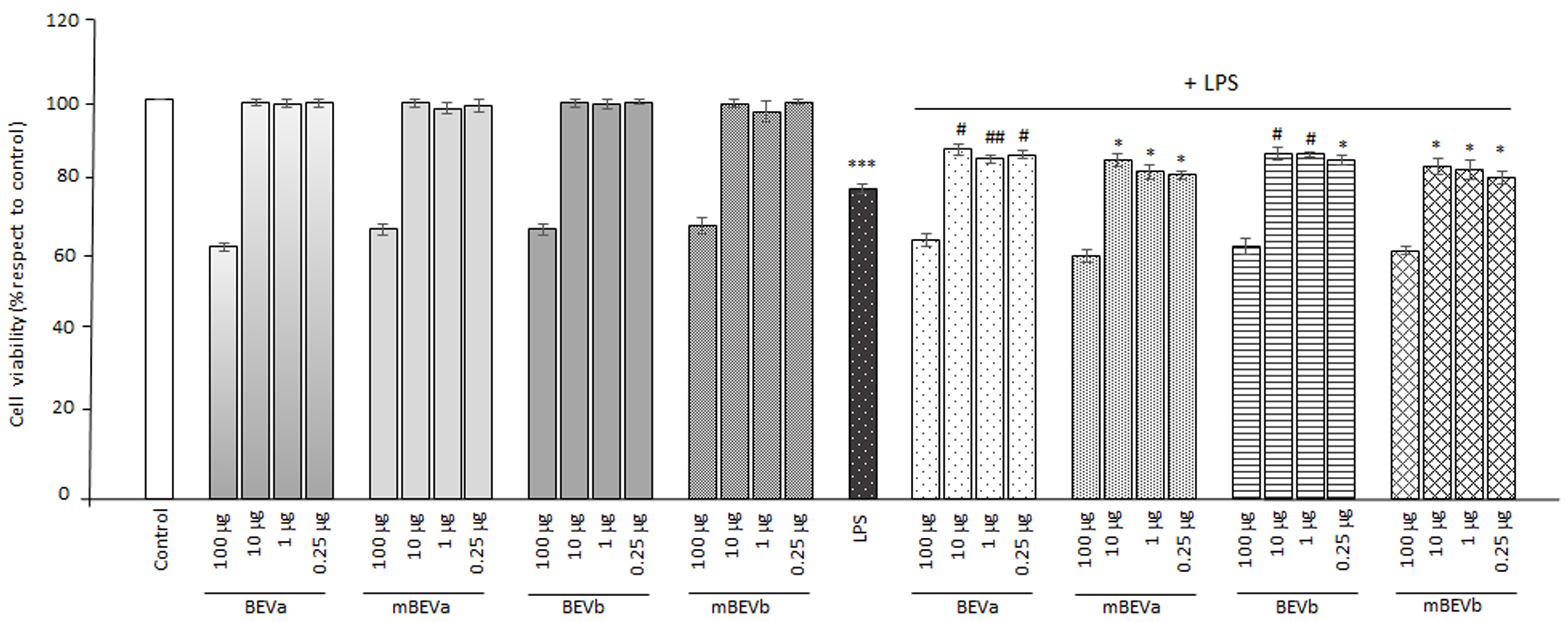
3.2.1. Viability Assay on the Intestinal Cells
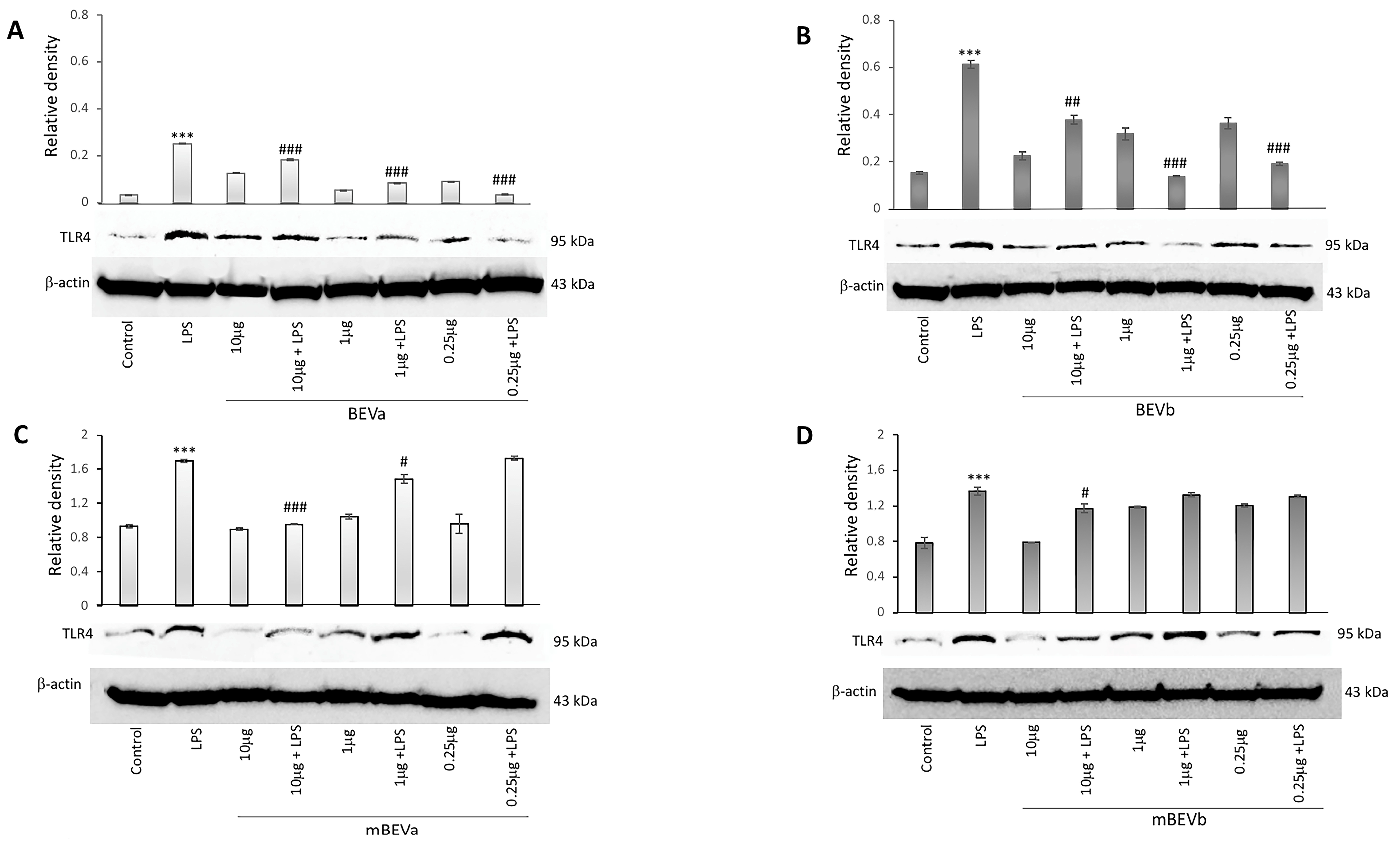
3.2.2. Expression of Toll-like Receptors 4 on the Intestinal Cells
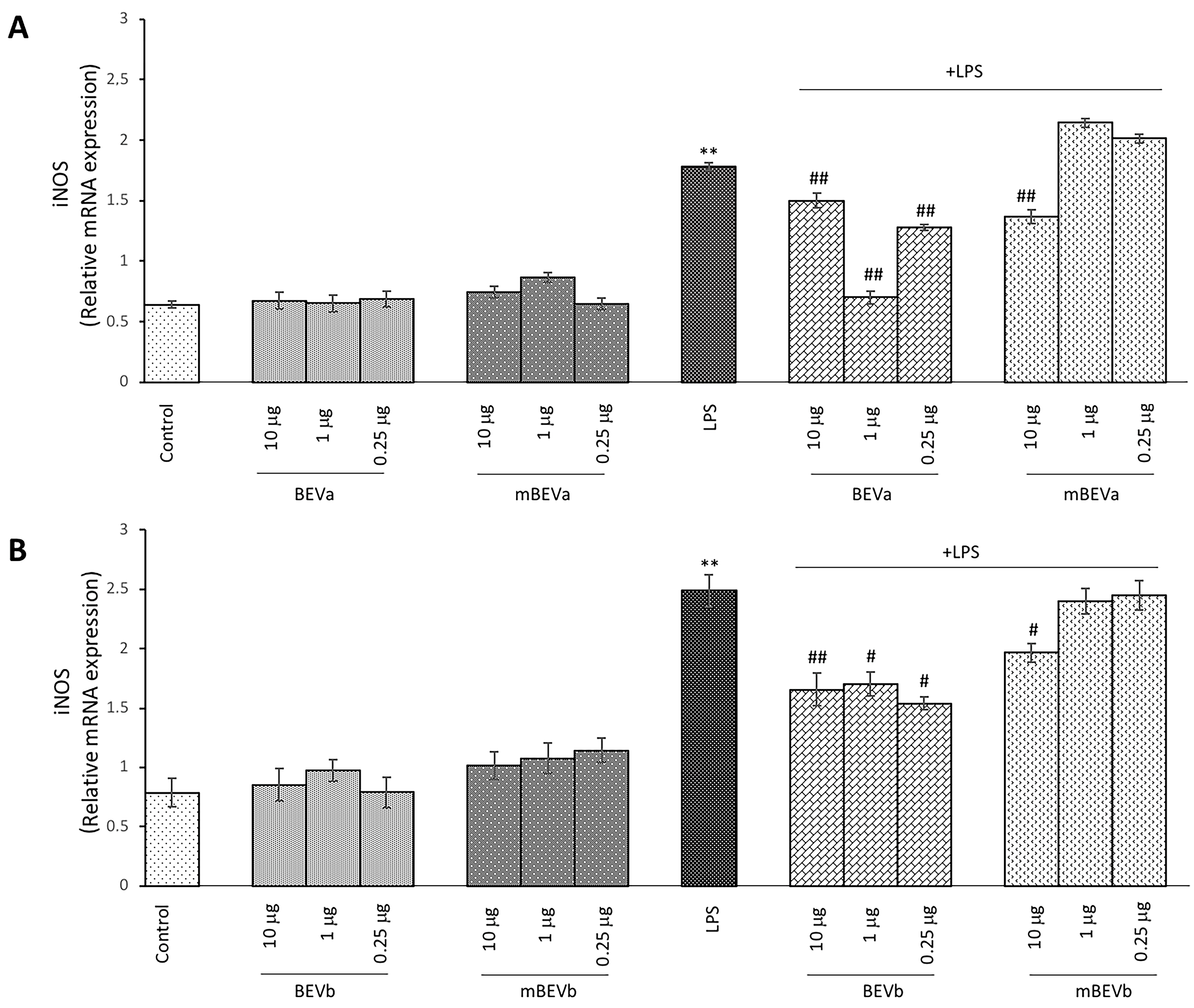
3.2.3. Effect of Lentil Hull Extracts on Inducible Nitric Oxide Synthase (iNOS) Expression in Intestinal Cells
3.2.4. Effect of Lentil Hull Extracts on Pro-Inflammatory IL-1 Cytokine Expression in Intestinal Cells
3.2.5. Effect of Lentil Hull Extracts on Anti-Inflammatory IL-10 Cytokine Expression in Intestinal Cells
3.3. Spasmolytic Activity
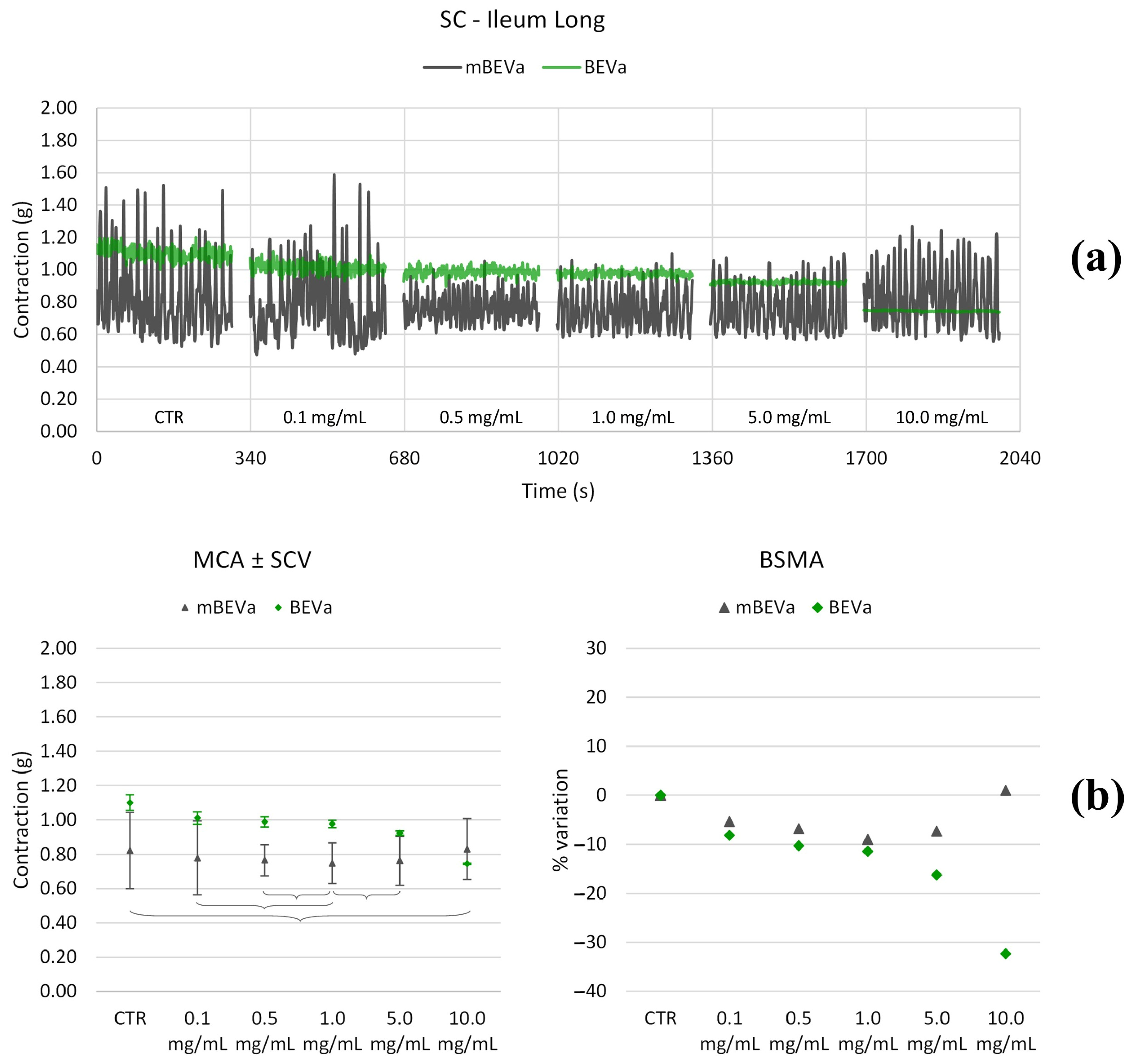
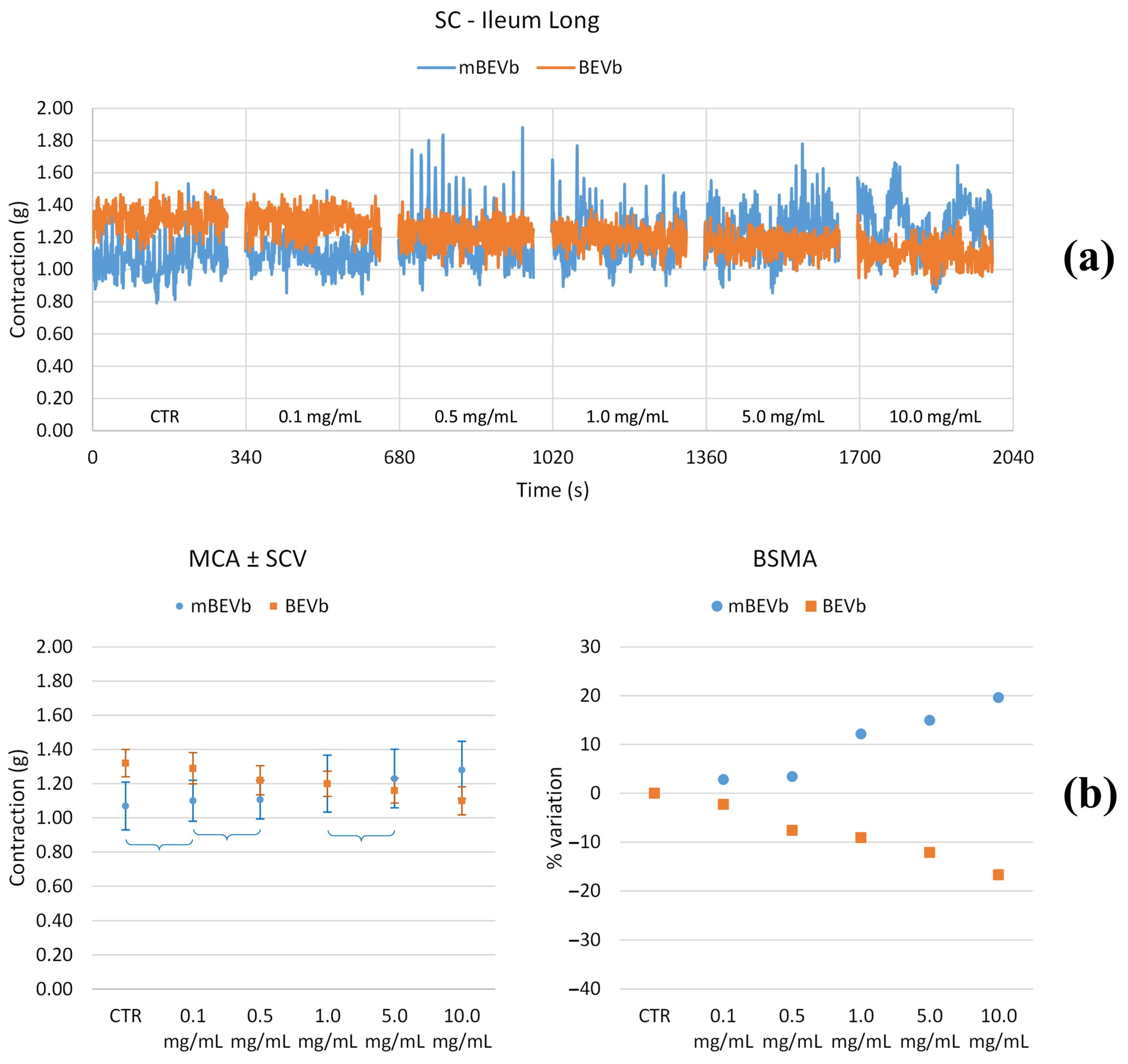
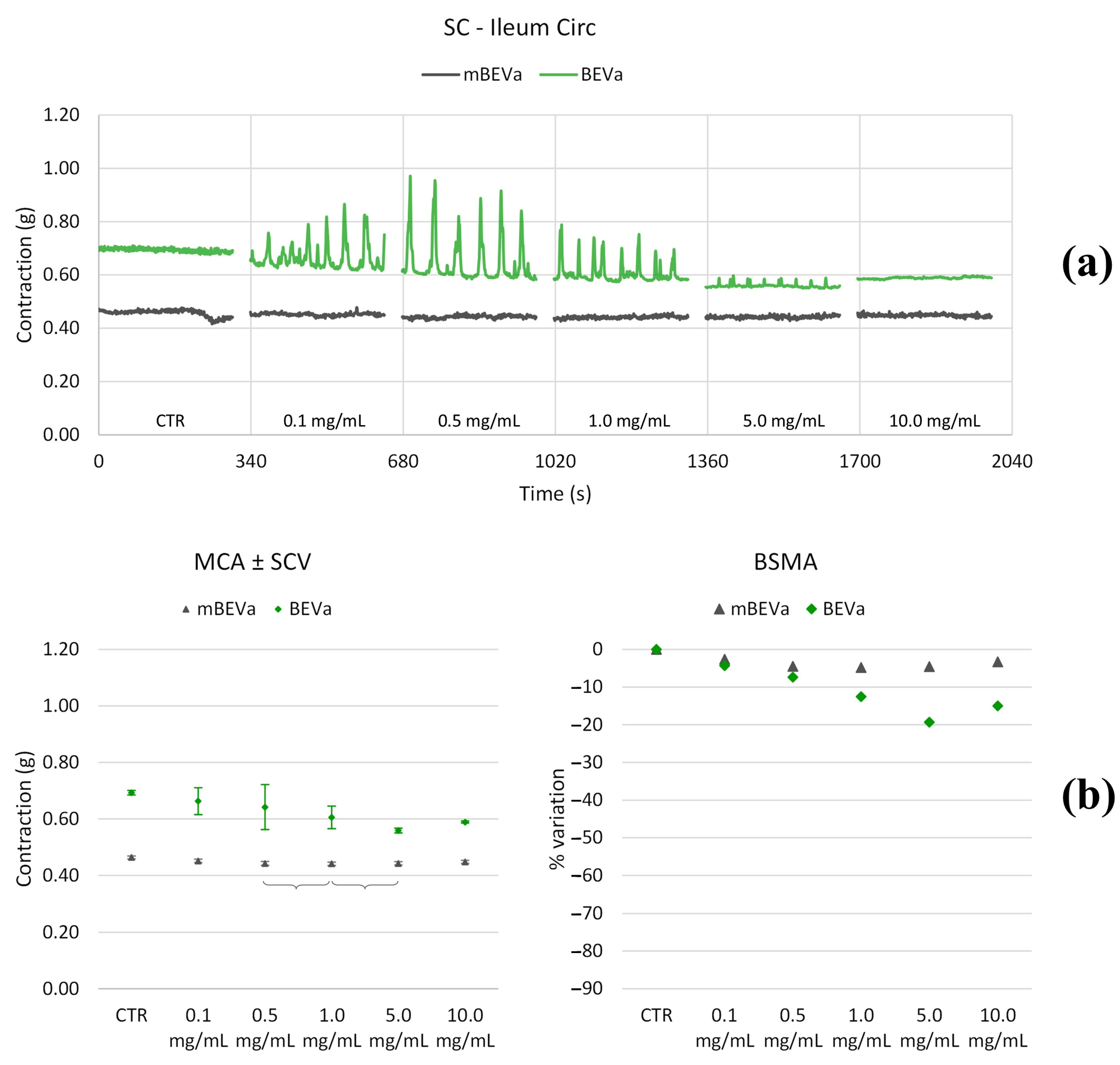
3.3.1. Effects of Lentil Hull Extracts on Ileum Muscles
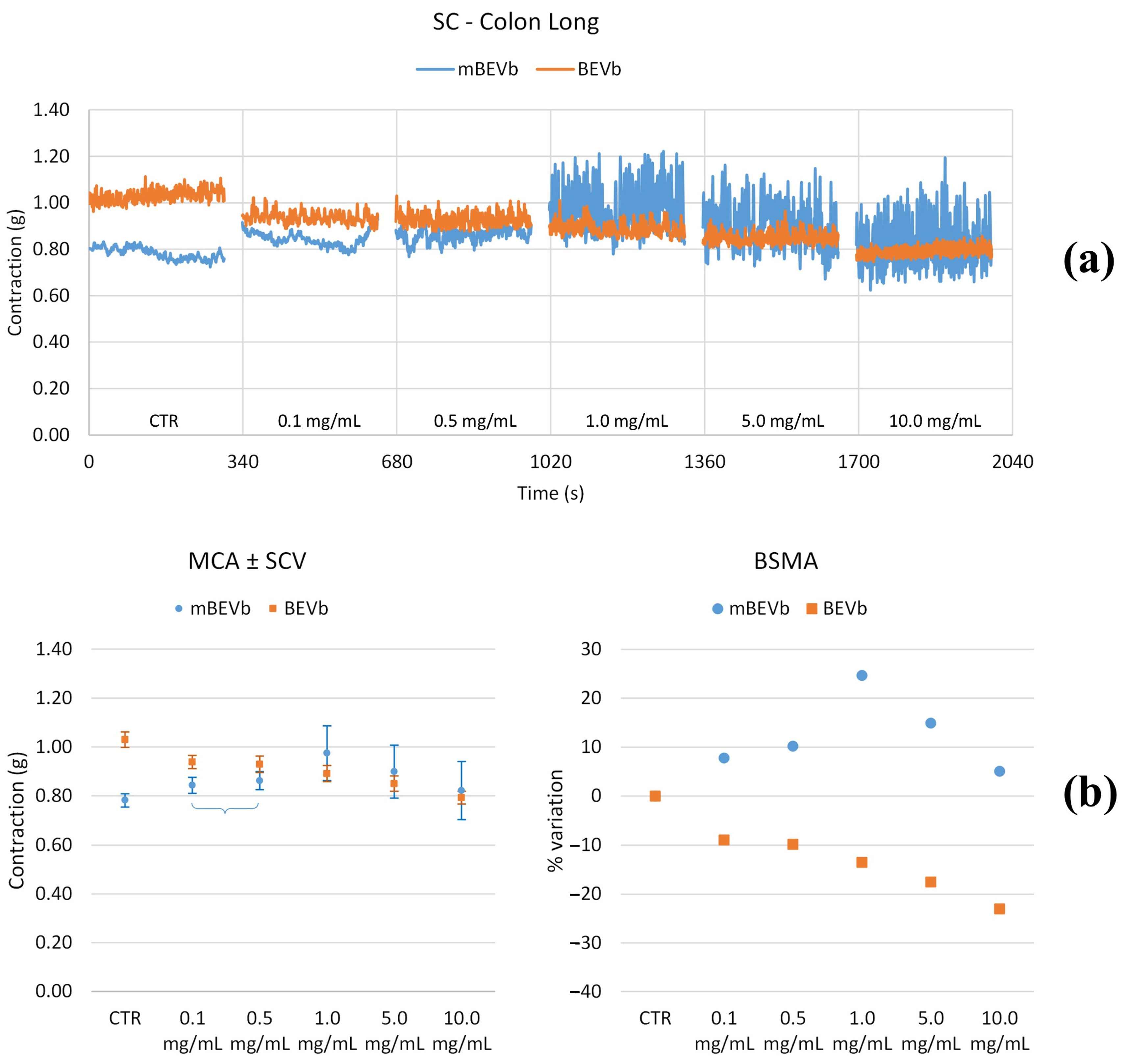
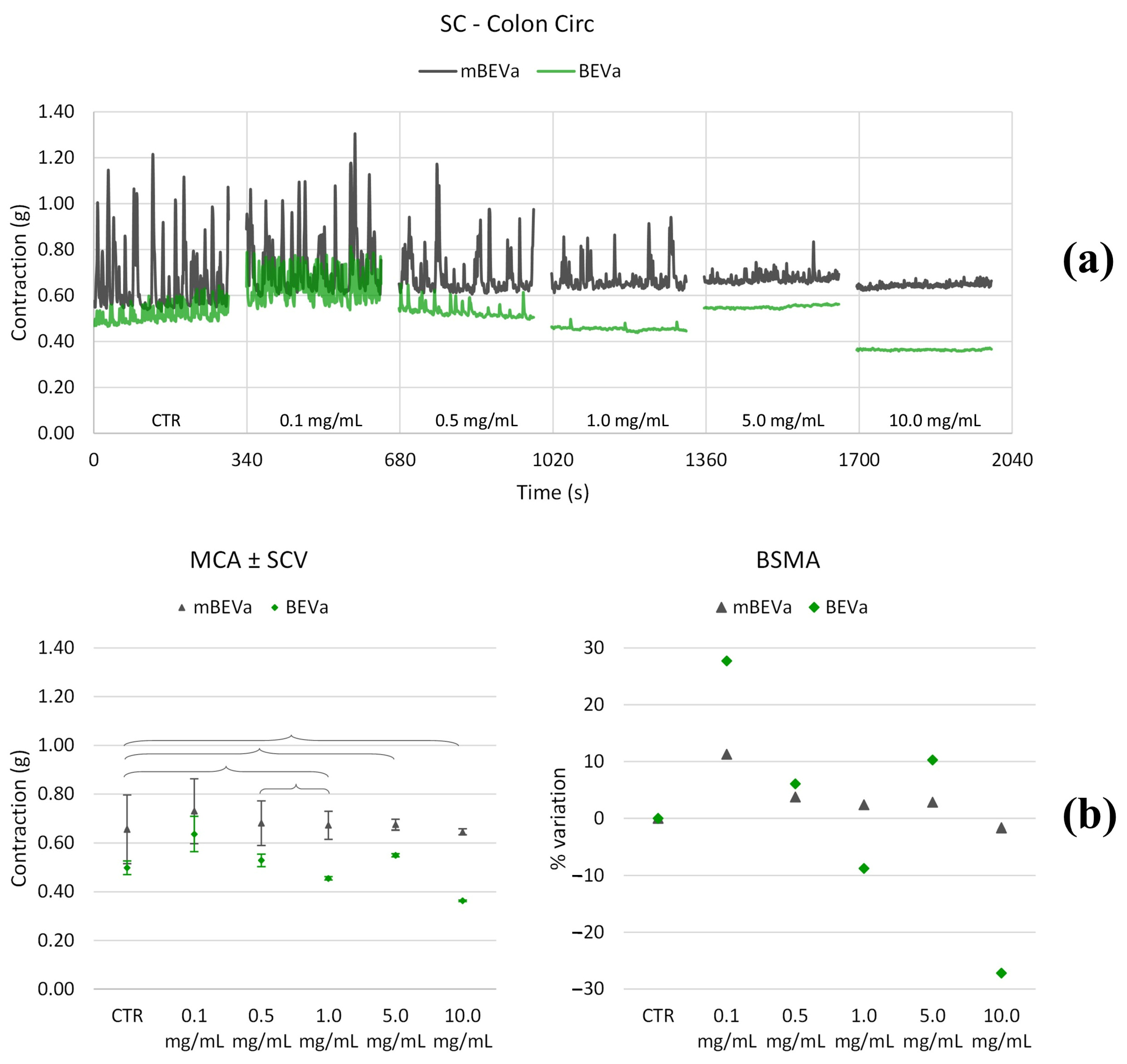
3.3.2. Effects of Lentil Hull Extracts on Colon Muscles
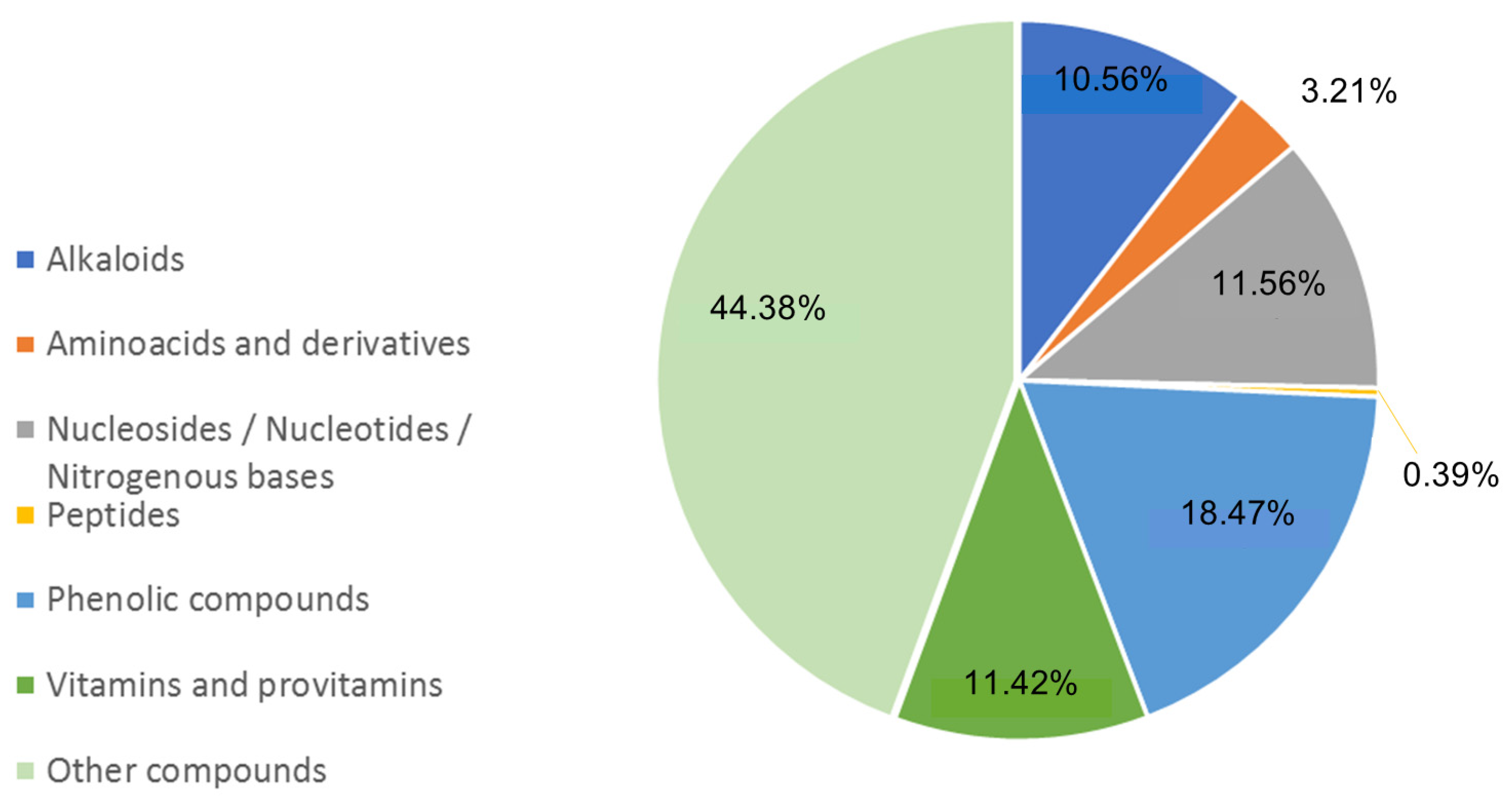
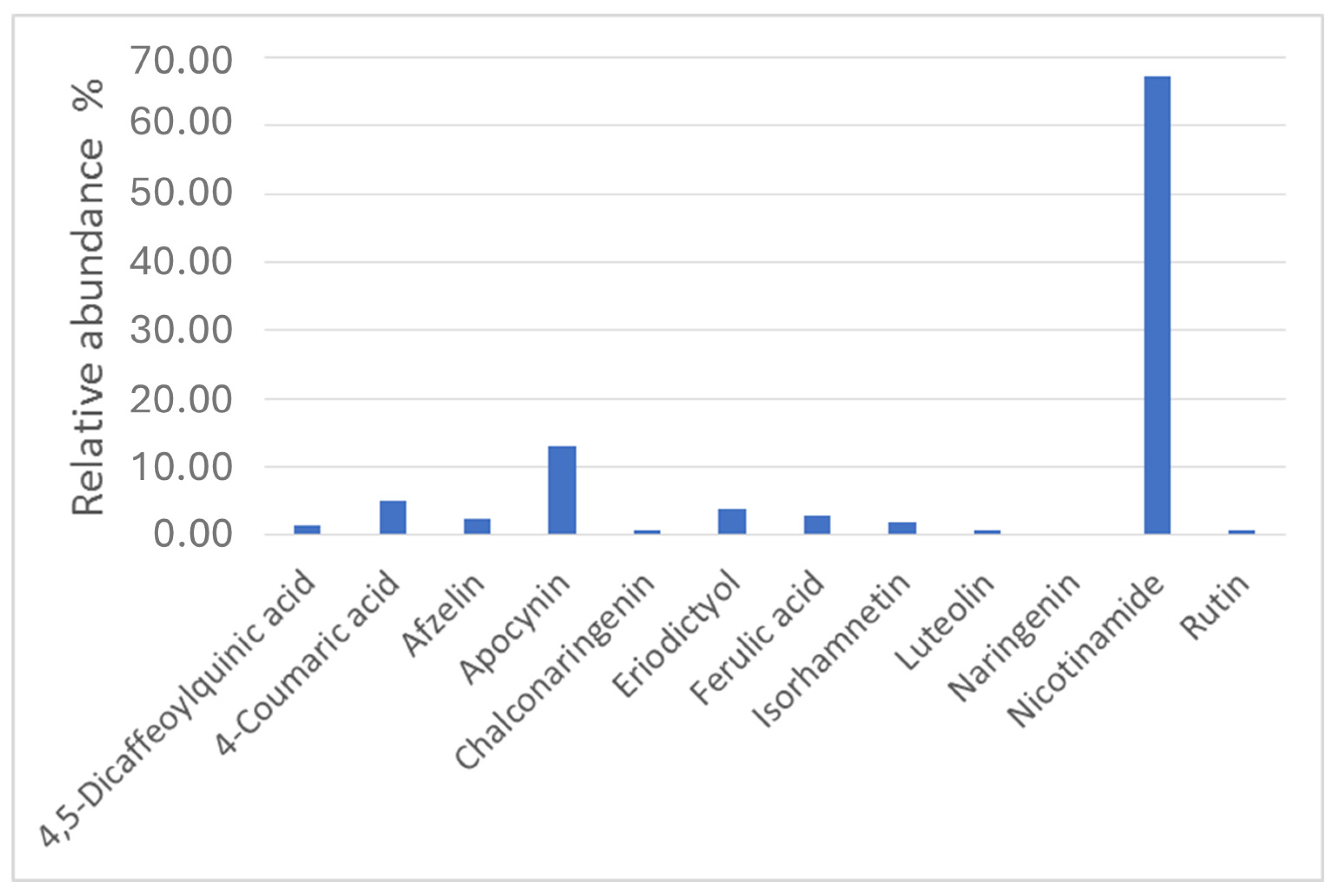
3.4. Metabolites Profiling of Lentil Hulls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garber, A.; Regueiro, M. Extraintestinal manifestations of inflammatory bowel disease: Epidemiology, etiopathogenesis, and management. Curr. Gastroenterol. Rep. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Stocker, F.; Ministro, P.; Gonçalves, R.; Carvalho, D.; Portela, F.; Correia, L.; Lago, P.; Trindade, E.; Dias, C.C.; et al. Incidence trends of inflammatory bowel disease in a southern European country: A mirror of the western world? Clin. Transl. Gastroenterol. 2022, 13, e00481. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Garrone, A.; Bertolino, C.; Vanni, R.; Bretto, E.; Poshnjari, A.; Tribocco, E.; Frara, S.; Armandi, A.; Astegiano, M.; et al. Epidemiology of inflammatory bowel diseases: A population study in a healthcare district of north-west Italy. J. Clin. Med. 2023, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, A.N.; Priyamvada, S.; Alrefai, W.A.; Dudeja, P.K. Pathophysiology of IBD associated diarrhea. Tissue Barriers 2018, 6, e1463897. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of inflammatory bowel disease: A comprehensive review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- de Castro, M.M.; Pascoal, L.B.; Steigleder, K.M.; Siqueira, B.P.; Corona, L.P.; Ayrizono, M.L.S.; Milanski, M.; Leal, R.F. Role of diet and nutrition in inflammatory bowel disease. World J. Exp. Med. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Reznikov, E.A.; Suskind, D.L. Current nutritional therapies in inflammatory bowel disease: Improving clinical remission rates and sustainability of long-term dietary therapies. Nutrients 2023, 15, 668. [Google Scholar] [CrossRef]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Polyphenol content of Green Pea (Pisum sativum L.) hull under in vitro digestion and effects of digestive products on anti-inflammatory activity and intestinal barrier in the Caco-2/Raw264.7 Coculture Model. J. Agric. Food Chem. 2022, 70, 3477–3488. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, H.; Huang, H.; Xiao, Y.; Wu, X.; Li, M.; Shen, J.; Xiao, Z.; Zhao, Y.; Du, F.; et al. The dietary supplement Rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food Funct. 2021, 12, 3142–3158. [Google Scholar] [CrossRef]
- Moon, H.-J.; Cha, Y.-S.; Kim, K.-A. Blackcurrant alleviates dextran sulfate sodium (DSS)-induced colitis in mice. Foods 2023, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Song, M.; Gu, M.; Ren, D.; Zhu, X.; Cao, X.; Li, F.; Wang, W.; Cai, X.; Yuan, B.; et al. Dietary intake of whole strawberry inhibited colonic inflammation in dextran-sulfate-sodium-treated mice via restoring immune homeostasis and alleviating gut microbiota dysbiosis. J. Agric. Food Chem. 2019, 67, 9168–9177. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, T.; Zeng, L.; Shi, L.; Li, Y.; Xia, Z.; Xia, X.; Lin, Q.; Luo, F. Crude extract of Fuzhuan brick tea ameliorates DSS-induced colitis in mice. Int. J. Food Sci. Technol. 2016, 51, 2574–2582. [Google Scholar] [CrossRef]
- Lu, K.; Liu, L.; Lin, P.; Dong, X.; Ni, L.; Che, H.; Xie, W. Saccharina japonica ethanol extract ameliorates dextran sulfate sodium-induced colitis via reshaping intestinal microenvironment and alleviating inflammatory response. Foods 2023, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, G.; Malekshahi, H.; Miraghaee, S.; Madani, H.; Babaei, A.; Mohammadi, B.; Hatami, R. Protective and therapeutic effects of aloe vera gel on ulcerative colitis induced by acetic acid in rats. Clin. Nutr. Res. 2020, 9, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.; Yan, W. Treatment effects of natural products on inflammatory bowel disease in vivo and their mechanisms: Based on animal experiments. Nutrients 2023, 15, 1031. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Landa, P.; Kutil, Z.; Qayum, M.; Ahmad, S. Evaluation of anti-inflammatory activity of selected legumes from Pakistan: In vitro inhibition of cyclooxygenase-2. Pak. J. Pharm. Sci. 2013, 26, 185–187. [Google Scholar]
- Mazewski, C.; Luna-Vital, D.; Berhow, M.; Gonzalez de Mejia, E. Reduction of colitis-associated colon carcinogenesis by a black lentil water extract through inhibition of inflammatory and immunomodulatory cytokines. Carcinogenesis 2020, 41, 790–803. [Google Scholar] [CrossRef]
- Peng, L.; Guo, F.; Pei, M.; Tsao, R.; Wang, X.; Jiang, L.; Sun, Y.; Xiong, H. Anti-inflammatory effect of lentil hull (Lens culinaris) extract via MAPK/NF-κB signaling pathways and effects of digestive products on intestinal barrier and inflammation in Caco-2 and Raw264.7 co-culture. J. Funct. Foods 2022, 92, 105044. [Google Scholar] [CrossRef]
- Guo, F.; Peng, L.; Xiong, H.; Wang, J.; Tsao, R.; Peng, X.; Jiang, L.; Sun, Y. Free and bound phenolics of laird lentil (Lens culinaris) hulls and the anti-inflammatory activity of their digestive products via crosstalk between NF-κB and Keap1-Nrf2 signaling pathways in HT-29 cells. J. Agric. Food Chem. 2022, 70, 13251–13263. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.W.; Bairagi, S.; Bhattacharyya, D. Valorization of agricultural wastes: An approach to impart environmental friendliness. In Handbook of Biomass Valorization for Industrial Applications; Shahid-ul-Islam, A.H.S., Khan, S.A., Eds.; Scrivener Publishing LCC: Beverly, MA, USA, 2022; pp. 369–393. [Google Scholar]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel approaches in the valorization of agricultural wastes and their applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A review on the challenges and choices for food waste valorization: Environmental and economic impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Cobo, S.; Dominguez-Ramos, A.; Irabien, A. Minimization of resource consumption and carbon footprint of a circular organic waste valorization system. ACS Sustain. Chem. Eng. 2018, 6, 3493–3501. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Cavalluzzi, M.M.; Lamonaca, A.; Rotondo, N.P.; Miniero, D.V.; Muraglia, M.; Gabriele, P.; Corbo, F.; De Palma, A.; Budriesi, R.; De Angelis, E.; et al. Microwave-assisted extraction of bioactive compounds from lentil wastes: Antioxidant activity evaluation and metabolomic characterization. Molecules 2022, 27, 7471. [Google Scholar] [CrossRef]
- Panaro, M.A.; Cavallo, P.; Acquafredda, A.; Cianciulli, A.; Calvello, R.; Mitolo, V. Expression of UDP-glucuronosyltransferase 1A6 isoform in Caco-2 cells stimulated with lipopolysaccharide. Innate Immun. 2010, 16, 302–309. [Google Scholar] [CrossRef]
- Tafurt-Cardona, Y.; Suares-Rocha, P.; Fernandes, T.C.; Marin-Morales, M.A. Cytotoxic and genotoxic effects of two hair dyes used in the formulation of black color. Food Chem. Toxicol. 2015, 86, 9–15. [Google Scholar] [CrossRef]
- Mattioli, L.B.; Frosini, M.; Amoroso, R.; Maccallini, C.; Chiano, E.; Aldini, R.; Urso, F.; Corazza, I.; Micucci, M.; Budriesi, R. Olea europea L. leaves and Hibiscus sabdariffa L. petals extracts: Herbal mix from cardiovascular network target to gut motility dysfunction application. Nutrients 2022, 14, 463. [Google Scholar] [CrossRef]
- Budriesi, R.; Vivarelli, F.; Canistro, D.; Aldini, R.; Babot Marquillas, C.; Corazza, I.; Fato, R.; Cirillo, S.; Bergamini, C.; D’Errico, A.; et al. Liver and intestinal protective effects of Castanea sativa Mill. bark extract in high-fat diet rats. PLoS ONE 2018, 13, e0201540. [Google Scholar] [CrossRef]
- Sumner, L.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of agro-waste into value-added bioproducts and bioactive compounds: Micro/nano formulations and application in the agri-food-pharma sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorization as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Lovece, A.; Bruno, C.; Cavalluzzi, M.M.; Laghezza, A.; Mercurio, A.; Lentini, G.; Corbo, F.; la Forgia, F.; Fontana, S.; et al. Antioxidant activity of Uva di Troia Canosina: Comparison of two extraction methods. Clin. Immunol. Endocr. Metab. Drugs 2015, 2, 8–12. [Google Scholar] [CrossRef]
- Caputo, L.; Quintieri, L.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. Antimicrobial and antibiofilm activities of citrus water-extracts obtained by microwave-assisted and conventional methods. Biomedicines 2018, 6, 70. [Google Scholar] [CrossRef]
- Milani, G.; Curci, F.; Cavalluzzi, M.M.; Crupi, P.; Pisano, I.; Lentini, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Optimization of microwave-assisted extraction of antioxidants from bamboo shoots of Phyllostachys pubescens. Molecules 2020, 25, 215. [Google Scholar] [CrossRef]
- Admane, N.; Cavallo, G.; Hadjila, C.; Cavalluzzi, M.M.; Rotondo, N.P.; Salerno, A.; Cannillo, J.; Difonzo, G.; Caponio, F.; Ippolito, A.; et al. Biostimulant formulations and Moringa oleifera extracts to improve yield, quality, and storability of hydroponic lettuce. Molecules 2023, 28, 373. [Google Scholar] [CrossRef]
- Gargano, M.L.; Balenzano, G.; Venturella, G.; Cavalluzzi, M.M.; Rotondo, N.P.; Lentini, G.; Cirlincione, F.; Mirabile, G.; Zapora, E.; Wołkowycki, M.; et al. Nutritional contents and antimicrobial activity of the culinary-medicinal mushroom Leccinum scabrum. Mycology 2024, 1–11. [Google Scholar] [CrossRef]
- Ferrero, D.; Moscato, E.; Spina, F.; Cavalluzzi, M.M.; Rotondo, N.; Oddon, S.B.; Gargano, M.L.; Venturella, G.; Lentini, G.; Bertea, C.M.; et al. The fungal alternative: Insights on medicinal mushrooms-based protein-rich biomasses by submerged fermentation of agro-industrial by-products. Innov. Food Sci. Emerg. Technol. 2024, 95, 103721. [Google Scholar] [CrossRef]
- Chemat, F.; Cravotto, G. Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Springer Science & BusinessMedia: New York, NY, USA, 2012. [Google Scholar]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Caricilli, A.M.; Castoldi, A.; Câmara, N.O. Intestinal barrier: A gentlemen’s agreement between microbiota and immunity. World J. Gastrointest. Pathophysiol. 2014, 5, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Microbial recognition by Toll-like receptors. J. Dermatol. Sci. 2004, 34, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Simiantonaki, N.; Kurzik-Dumke, U.; Karyofylli, G.; Jayasinghe, C.; Michel-Schmidt, R.; Kirkpatrick, C.J. Reduced expression of TLR4 is associated with the metastatic status of human colorectal cancer. Int. J. Mol. Med. 2007, 20, 21–29. [Google Scholar] [CrossRef]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef]
- Baumann, A.; Rajcic, D.; Brandt, A.; Sánchez, V.; Jung, F.; Staltner, R.; Nier, A.; Trauner, M.; Staufer, K.; Bergheim, I. Alterations of nitric oxide homeostasis as trigger of intestinal barrier dysfunction in non-alcoholic fatty liver disease. J. Cell. Mol. Med. 2022, 26, 1206–1218. [Google Scholar] [CrossRef]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Piątek-Guziewicz, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Targosz, A.; Ptak-Belowska, A.; Szczyrk, U.; et al. The importance of nutritional aspects in the assessment of inflammation and intestinal barrier in patients with inflammatory bowel disease. Nutrients 2022, 14, 4622. [Google Scholar] [CrossRef]
- Oh, H.; Park, S.H.; Kang, M.K.; Kim, Y.H.; Lee, E.J.; Kim, D.Y.; Kim, S.I.; Oh, S.; Lim, S.S.; Kang, Y.H. Asaronic acid attenuates macrophage activation toward M1 phenotype through inhibition of NF-kappaB pathway and JAK-STAT signaling in glucose-loaded murine macrophages. J. Agric. Food Chem. 2019, 67, 10069–10078. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Sun, A.; Zhang, E.; Ding, L.; Mukherjee, S.; Wei, X.; Chou, G.; Wang, Z.; Mani, S. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013, 110, 599–608. [Google Scholar] [CrossRef]
- Zong, X.; Hu, W.; Song, D.; Li, Z.; Du, H.; Lu, Z.; Wang, Y. Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response. Biochem. Pharmacol. 2016, 104, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Turner, J. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, H.; Sun, X.; Zhu, M.J. Metformin improves ileal epithelial barrier function in interleukin-10 deficient mice. PLoS ONE 2016, 11, e0168670. [Google Scholar] [CrossRef] [PubMed]
- Correa, I.; Veny, M.; Esteller, M.; Pique, J.M.; Yague, J.; Panes, J.; Salas, A. Defective IL-10 production in severe phenotypes of Crohn’s disease. J. Leukoc. Biol. 2009, 85, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Curci, F.; Corbo, F.; Clodoveo, M.L.; Salvagno, L.; Rosato, A.; Corazza, I.; Budriesi, R.; Micucci, M.; Mattioli, L.B. Polyphenols from olive-mill wastewater and biological activity: Focus on irritable bowel syndrome. Nutrients 2022, 14, 1264. [Google Scholar] [CrossRef]
- Yates, K.; Pohl, F.; Busch, M.; Mozer, A.; Watters, L.; Shiryaev, A.; Lin, P.K.T. Determination of sinapine in rapeseed pomace extract: Its antioxidant and acetylcholinesterase inhibition properties. Food Chem. 2019, 276, 768–775. [Google Scholar] [CrossRef]
- Marques, C.; Hadjab, F.; Porcello, A.; Lourenço, K.; Scaletta, C.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Mechanistic insights into the multiple functions of niacinamide: Therapeutic implications and cosmeceutical applications in functional skincare products. Antioxidants 2024, 13, 425. [Google Scholar] [CrossRef]
- Ungerstedt, J.S.; Blömback, M.; Söderström, T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin. Exp. Immunol. 2003, 131, 48–52. [Google Scholar] [CrossRef]
- Gerner, R.R.; Klepsch, V.; Macheiner, S.; Arnhard, K.; Adolph, T.E.; Grander, C.; Wieser, V.; Pfister, A.; Moser, P.; Hermann-Kleiter, N.; et al. NAD metabolism fuels human and mouse intestinal inflammation. Gut 2018, 67, 1813–1823. [Google Scholar] [CrossRef]
- Xue, X.; Miao, Y.; Wei, Z. Nicotinamide adenine dinucleotide metabolism: Driving or counterbalancing inflammatory bowel disease? FEBS Lett. 2023, 597, 1179–1192. [Google Scholar] [CrossRef]
- Kang, K.S. Phytochemical constituents of medicinal plants for the treatment of chronic inflammation. Biomolecules 2021, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Ruddock, M.W.; Burns, D.M.; Murphy, L.E.; O’Rourke, M.; Hirst, D.G. The effect of nicotinamide on spontaneous and induced activity in smooth and skeletal muscle. Radiother. Oncol. 2000, 56, 253–257. [Google Scholar] [CrossRef] [PubMed]
- ‘t Hart, B.A.; Copray, S.; Philippens, I. Apocynin, a low molecular oral treatment for neurodegenerative disease. BioMed Res. Int. 2014, 2014, 298020. [Google Scholar] [CrossRef] [PubMed]
- Boshtam, M.; Kouhpayeh, S.; Amini, F.; Azizi, Y.; Najaflu, M.; Shariati, L.; Khanahmad, H. Anti-inflammatory effects of apocynin: A narrative review of the evidence. All Life 2021, 14, 997–1010. [Google Scholar] [CrossRef]
- Stefanska, J.; Pawliczak, R. Apocynin: Molecular aptitudes. Mediat. Inflamm. 2008, 2008, 106507. [Google Scholar] [CrossRef]
- Li, Y.R.; Li, Y.; Jin, Y.; Xu, M.; Fan, H.W.; Zhang, Q.; Tan, G.H.; Chen, J.; Li, Y.Q. Involvement of nitrergic neurons in colonic motility in a rat model of ulcerative colitis. World J. Gastroenterol. 2022, 28, 3854–3868. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Venkatesan, A.; Samy, J.V.R.A.; Balakrishnan, K.; Natesan, V.; Kim, S.J. In Vitro antioxidant, anti-inflammatory, antimicrobial, and antidiabetic activities of synthesized chitosan-loaded p-coumaric acid nanoparticles. Curr. Pharm. Biotechnol. 2023, 24, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Luceri, C.; Guglielmi, F.; Lodovici, M.; Giannini, L.; Messerini, L.; Dolara, P. Plant phenolic 4-coumaric acid protects against intestinal inflammation in rats. Scand. J. Gastroenterol. 2004, 39, 1128–1133. [Google Scholar] [PubMed]
- Wahid, M.; Saqib, F.; Akhtar, S.; Ali, A.; Wilairatana, P.; Mubarak, M.S. Possible mechanisms underlying the antispasmodic, bronchodilator, and antidiarrheal activities of polarity-based extracts of Cucumis sativus L. seeds in in silico, in vitro, and in vivo studies. Pharmaceuticals 2022, 15, 641. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Helkar, P.B.; Sahoo, A.K.; Patil, N.J. Review: Food industry by-products used as a functional food ingredients. Int. J. Waste Resour 2016, 6, 1–6. [Google Scholar]
- Sharma, S.K.; Bansal, S.; Mangal, M.; Dixit, A.K.; Gupta, R.K.; Mangal, A.K. Utilization of food processing by-products as dietary, functional, and novel fiber: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1647–1661. [Google Scholar] [CrossRef]
- Gemechu, F.G. Embracing Nutritional Qualities, Biological Activities and Technological Properties of Coffee Byproducts in Functional Food Formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]





| Entry | Method | Solvent | Extract Abbreviation | Yield (%) a | TPC b |
|---|---|---|---|---|---|
| 1 | MAE | abs EtOH | BEVb | 4.5 | 2.14 ± 0.02 |
| 2 | MAE | EtOAc | BEVa | 4.1 | 0.22 ± 0.05 |
| 3 | maceration | abs EtOH | mBEVb | 4.6 | 1.30 ± 0.08 |
| 4 | maceration | EtOAc | mBEVa | 4.3 | 0.35 ± 0.07 |
| Concentration (mg/mL) | BEVa | mBEVa | BEVb | mBEVb | ||
|---|---|---|---|---|---|---|
| Ileum long | LF | 0.1 | * | * | * | −70% |
| 0.5 | * | −90% | * | −90% | ||
| 1.0 | −70% | −70% | * | * | ||
| 5.0 | −90% | −60% | * | * | ||
| 10.0 | −100% | * | * | * | ||
| MF | 0.1 | * | * | * | * | |
| 0.5 | −60% | −70% | * | * | ||
| 1.0 | −80% | −70% | * | * | ||
| 5.0 | −100% | −70% | * | +100% | ||
| 10.0 | −100% | −60% | * | * | ||
| HF | 0.1 | * | * | * | +100% | |
| 0.5 | −50% | * | * | * | ||
| 1.0 | −50% | * | * | +70% | ||
| 5.0 | −70% | * | * | * | ||
| 10.0 | −100% | * | * | −60% | ||
| Ileum circ | LF | 0.1 | >+500% | * | * | −100% |
| 0.5 | >+500% | +100% | * | −100% | ||
| 1.0 | >+500% | * | * | −100% | ||
| 5.0 | >+500% | +50% | * | −100% | ||
| 10.0 | * | * | >+500% | −100% | ||
| MF | 0.1 | +60% | * | −100% | * | |
| 0.5 | +290% | * | * | +80% | ||
| 1.0 | +130% | * | * | * | ||
| 5.0 | −60% | * | * | +70% | ||
| 10.0 | −100% | * | +70% | +290% | ||
| HF | 0.1 | +100% | * | * | * | |
| 0.5 | +150% | * | * | * | ||
| 1.0 | +140% | * | * | * | ||
| 5.0 | −60% | * | * | * | ||
| 10.0 | −90% | * | +60% | +100% |
| ILEUM | Transit Speed Variation % | Pain % | Mixing % | Fragmentation % | Longitudinal Contraction Variation % | Circular Contraction Variation % | |
|---|---|---|---|---|---|---|---|
| BEVa | 0.1 mg/mL | = | = | +++ | + | - | - |
| 0.5 mg/mL | = | - | +++ | + | - | - | |
| 1.0 mg/mL | - | - | +++ | + | - | - | |
| 5.0 mg/mL | - | - | +++ | - | - | - | |
| 10.0 mg/mL | - | - | = | - | -- | - | |
| mBEVa | 0.1 mg/mL | = | = | = | = | - | - |
| 0.5 mg/mL | - | - | + | = | - | - | |
| 1.0 mg/mL | - | - | = | = | - | - | |
| 5.0 mg/mL | - | - | + | = | - | - | |
| 10.0 mg/mL | = | - | = | = | = | - | |
| BEVb | 0.1 mg/mL | = | = | = | - | - | - |
| 0.5 mg/mL | = | = | = | = | - | -- | |
| 1.0 mg/mL | = | = | = | = | - | -- | |
| 5.0 mg/mL | = | = | = | = | - | -- | |
| 10.0 mg/mL | = | = | +++ | + | - | -- | |
| mBEVb | 0.1 mg/mL | - | + | - | = | = | -- |
| 0.5 mg/mL | - | = | - | + | + | --- | |
| 1.0 mg/mL | = | + | - | = | + | --- | |
| 5.0 mg/mL | = | + | - | + | + | ---- | |
| 10.0 mg/mL | = | - | - | + | ++ | ---- | |
| Concentration (mg/mL) | Concentration (mg/mL) | BEVa | mBEVa | BEVb | mBEVb | ||
|---|---|---|---|---|---|---|---|
| Colon long | LF | 0.1 | * | * | +100% | * | +100% |
| 0.5 | +250% | +250% | −60% | >+500% | * | ||
| 1.0 | >+500% | >+500% | −80% | >+500% | +120% | ||
| 5.0 | >+500% | >+500% | −90% | >+500% | * | ||
| 10.0 | >+500% | >+500% | −90% | >+500% | −50% | ||
| MF | 0.1 | * | * | * | * | −50% | |
| 0.5 | >+500% | >+500% | −80% | +420% | * | ||
| 1.0 | >+500% | >+500% | −100% | >+500% | * | ||
| 5.0 | >+500% | >+500% | −100% | >+500% | * | ||
| 10.0 | >+500% | >+500% | −100% | >+500% | * | ||
| HF | 0.1 | * | * | * | * | * | |
| 0.5 | +165% | +165% | −80% | * | * | ||
| 1.0 | +350% | +350% | −90% | +190% | * | ||
| 5.0 | +480% | +480% | −100% | +400% | * | ||
| 10.0 | +400% | +400% | −90% | >+500% | * | ||
| Colon circ | LF | 0.1 | 0.1 | >+500% | * | +150% | * |
| 0.5 | 0.5 | * | −70% | +100% | +80% | ||
| 1.0 | 1.0 | −90% | −90% | * | +120% | ||
| 5.0 | 5.0 | −100% | −100% | * | * | ||
| 10.0 | 10.0 | −100% | −100% | −90% | +60% | ||
| MF | 0.1 | 0.1 | >+500% | * | +250% | +210% | |
| 0.5 | 0.5 | −70% | * | +230% | +210% | ||
| 1.0 | 1.0 | −100% | −50% | +90% | +70% | ||
| 5.0 | 5.0 | −100% | −90% | * | +230% | ||
| 10.0 | 10.0 | −100% | −100% | −70% | +200% | ||
| HF | 0.1 | 0.1 | +500% | * | >+500% | +70% | |
| 0.5 | 0.5 | −60% | −60% | +240% | +80% | ||
| 1.0 | 1.0 | −90% | −90% | +180% | +60% | ||
| 5.0 | 5.0 | −90% | −100% | +130% | +70% | ||
| 10.0 | 10.0 | −90% | −100% | * | +80% |
| COLON | Transit Speed Variation % | Pain % | Mixing % | Fragmentation % | Longitudinal Contraction Variation % | Circular Contraction Variation % | |
|---|---|---|---|---|---|---|---|
| BEVa | 0.1 mg/mL | + | = | +++ | +++ | - | ++ |
| 0.5 mg/mL | - | - | = | - | -- | + | |
| 1.0 mg/mL | - | - | - | - | -- | - | |
| 5.0 mg/mL | - | - | - | - | -- | + | |
| 10.0 mg/mL | - | - | - | - | -- | -- | |
| mBEVa | 0.1 mg/mL | = | = | = | = | + | + |
| 0.5 mg/mL | +++ | ++ | - | - | -- | + | |
| 1.0 mg/mL | +++ | +++ | - | - | - | = | |
| 5.0 mg/mL | +++ | +++ | - | - | - | = | |
| 10.0 mg/mL | +++ | +++ | - | - | - | = | |
| BEVb | 0.1 mg/mL | + | - | + | +++ | - | + |
| 0.5 mg/mL | = | = | + | + | - | + | |
| 1.0 mg/mL | + | = | = | + | - | = | |
| 5.0 mg/mL | = | = | = | + | - | = | |
| 10.0 mg/mL | - | = | - | - | -- | -- | |
| mBEVb | 0.1 mg/mL | = | = | = | + | + | = |
| 0.5 mg/mL | + | +++ | + | + | + | + | |
| 1.0 mg/mL | +++ | +++ | + | + | ++ | + | |
| 5.0 mg/mL | +++ | +++ | = | + | + | + | |
| 10.0 mg/mL | +++ | +++ | + | + | + | - | |
| Class | Name | Chemical Formula | Adduct | m/z | RT (min) | Identification Level |
|---|---|---|---|---|---|---|
| Alkaloids | Sinapine | C16H23NO5 | [M + H]+ | 310.16486 | 32.376 | ** |
| Tetramethylpyrazine | C8H12N2 | [M + H]+ | 137.1073 | 46.486 | ** | |
| Aminoacids and derivatives | Gamma-hydroxyhomoarginine | C7H16N4O3 | [M + H]+ | 205.12943 | 1.871 | * |
| Histidinate | C6H8N3O2 | [M − H]− | 153.05456 | 14.859 | * | |
| l-Tyrosine methyl ester | C10H13NO3 | [M − H]− | 194.08155 | 19.552 | ** | |
| N-Acetyl-l-phenylalanine | C11H13NO3 | [M − H]− | 206.08167 | 45.068 | ** | |
| Nucleosides/Nucleotides/Nitrogenous bases | Adenine | C5H5N5 | [M + H]+ | 136.06175 | 7.539 | ** |
| Adenosine | C10H13N5O4 | [M + H]+ | 268.1039 | 7.543 | ** | |
| Thymidine | C10H14N2O5 | [M − H]− | 241.08281 | 12.52 | * | |
| Uracil | C4 H4N2O2 | [M + H]+ | 113.03482 | 5.256 | ** | |
| Uric acid | C5H4N4O3 | [M + H]+ | 169.03555 | 4.157 | ** | |
| Uridine | C9H12N2O6 | [M − H]− | 243.06219 | 5.248 | ** | |
| Peptides | Beta-Ala-Lys-N(epsilon)-AMCA | C21H28N4O6 | [M − H]− | 431.19264 | 46.18 | * |
| Trp-asp-glu | C20H24N4O8 | [M − H]− | 447.15133 | 40.191 | * | |
| Phenolic compounds | 2-[({[4-({[(2-Hydroxyphenyl)methylene]amino}methyl)cyclohexyl]methyl}imino)methyl]phenol | C22H26N2O2 | [M + H]+ | 351.20609 | 49.224 | ** |
| 3,4-Dihydroxybenzaldehyde | C7H6O3 | [M + H]+ | 139.03893 | 20.307 | ** | |
| 3,5-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl hexopyranoside | C21H22O11 | [M − H]− | 449.10943 | 34.313 | ** | |
| 4-(Isothiocyanatomethyl)phenol | C8H7NOS | [M + H]+ | 166.0321 | 47.176 | * | |
| 4-(Phenylethynyl)phenol | C14H10O | [M + H]+ | 195.08044 | 53.625 | * | |
| 4,5-Dicaffeoylquinic acid | C25H24O12 | [M − H]− | 515.11992 | 51.061 | ** | |
| 4-Coumaric acid | C9H8O3 | [M + H]+ | 165.05458 | 30.554 | ** | |
| 6-O-[(2E)-3-(4-Hydroxyphenyl)-2-propenoyl]-1-O-(3,4,5-trihydroxybenzoyl)hexopyranose | C22H22O12 | [M − H]− | 477.10409 | 53.366 | ** | |
| Afzelin | C21H20O10 | [M − H]− | 431.09862 | 53.643 | ** | |
| Apigenin 7-(3”-acetyl-6”-E-p-coumaroylglucoside) | C32H28O13 | [M + H]+ | 621.15947 | 45.897 | * | |
| Apocynin | C9H10O3 | [M + H]+ | 167.07021 | 44.149 | ** | |
| Astragalin | C21H20O11 | [M − H]− | 447.09416 | 53.331 | ** | |
| Catechin | C15H14O6 | [M − H]− | 289.07211 | 27.963 | ** | |
| Chalconaringenin | C15H12O5 | [M + H]+ | 273.0754 | 49.069 | * | |
| Eriodictyol | C15H12O6 | [M + H]+ | 289.07028 | 53.188 | ** | |
| Ferulic acid | C10H10O4 | [M − H]− | 193.04989 | 47.919 | ** | |
| Hyperoside | C21H20O12 | [M + H]+ | 465.10236 | 52.356 | ** | |
| Isorhamnetin | C16H12O7 | [M + H]+ | 317.06503 | 53.379 | ** | |
| Kaempferol | C15H10O6 | [M + H]+ | 287.0547 | 53.638 | ** | |
| Luteolin | C15H10O6 | [M − H]− | 285.04052 | 53.883 | ** | |
| Mitoxantrone | C22H28N4O6 | [M − H]− | 443.19273 | 29.803 | * | |
| Myricetin 3-O-beta-d-galactopyranoside | C21H20O13 | [M − H]− | 479.08365 | 49.938 | ** | |
| Myricitrin | C21H20O12 | [M − H]− | 463.08897 | 51.464 | ** | |
| Naringenin | C15H12O5 | [M − H]− | 271.06119 | 53.81 | ** | |
| Naringeninchalcone | C15H12O5 | [M + H]+ | 273.07531 | 51.595 | ** | |
| N-Feruloyloctopamine | C18H19NO5 | [M − H]− | 328.11941 | 52.055 | * | |
| Phaseolic acid | C12H22O6 | [M − H]− | 261.13446 | 51.523 | * | |
| Phloretin | C15H14O5 | [M + H]+ | 275.09114 | 53.461 | * | |
| Quercetin | C15H10O7 | [M + H]+ | 303.04963 | 53.338 | ** | |
| Quercetin-3β-d-glucoside | C21H20O12 | [M − H]− | 463.08923 | 52.362 | ** | |
| Resveratrol | C14H12O3 | [M + H]+ | 229.08556 | 48.286 | ** | |
| Rutin | C27H30O16 | [M − H]− | 609.14705 | 50.539 | ** | |
| Syringaldehyde | C9H10O4 | [M + H]+ | 183.06521 | 41.581 | * | |
| Taxifolin | C15H12O7 | [M + H]+ | 305.06522 | 51.303 | ** | |
| Vitamins and provitamins | Choline | C5H13NO | [M + H]+ | 104.10739 | 1.877 | ** |
| Nicotinamide | C6H6N2O | [M + H]+ | 123.05548 | 3.465 | ** | |
| Pantothenic acid | C9H17NO5 | [M − H]− | 218.10293 | 14.431 | ** | |
| Other compounds | (±)-Abscisic acid | C15H20O4 | [M − H]− | 263.12911 | 53.545 | ** |
| 1-(4-Methylphenyl)pyrrolidine-2,5-dione | C11H11NO2 | [M + H]+ | 190.08625 | 53.563 | ** | |
| 1-(Carboxymethyl)cyclohexanecarboxylic acid | C9H14O4 | [M + H]+ | 187.09649 | 51.399 | ** | |
| 1H-Pyrazole-4-carbonitrile | C4H3N3 | [M − H]− | 92.0252 | 49.38 | * | |
| 1-Methyl-4-(1-methyl-2-propenyl)-benzene | C13H18 | [M + H]+ | 175.14814 | 53.606 | * | |
| 2-(1H-Indol-3-yl)acetic acid | C10H9NO2 | [M + H]+ | 176.07055 | 52.517 | ** | |
| 2,4-Heptadienal | C7H10O | [M + H]+ | 111.08073 | 52.938 | * | |
| 2,5,8,11,14,17-Hexaoxaoctadecane | C12H26O6 | [M + Na]+ | 289.16181 | 44.217 | * | |
| 2,6-Dimethylpyrazine | C6H8N2 | [M + H]+ | 109.07632 | 2.042 | ** | |
| 2-Hydroxydecanedioic acid | C10H18O5 | [M − H]− | 217.10763 | 52.081 | * | |
| 3-(2-Hydroxyethyl)indole | C10H11NO | [M + H]+ | 162.09119 | 49.171 | ** | |
| 4-Dimethylaminocinnamaldehyde | C11H13NO | [M + H]+1 | 176.1069 | 52.277 | ** | |
| 4-Indolecarbaldehyde | C9H7NO | [M + H]+ | 146.05999 | 47.077 | ** | |
| 4-Methoxycinnamaldehyde | C10H10O2 | [M + H]+ | 163.07535 | 49.332 | ** | |
| 4-Oxo-5-phenylpentanoic acid | C11H12O3 | [M + H]+ | 193.08605 | 46.049 | ** | |
| 4-Oxododecanedioic acid | C12H20O5 | [M + H]+ | 245.13827 | 52.666 | ** | |
| 5-Oxo-7-octenoic acid | C8H12O3 | [M + H]+ | 157.08599 | 53.706 | * | |
| 8-Hydroxyquinoline | C9H7NO | [M + H]+ | 146.05998 | 23.334 | ** | |
| 9-(Methylsulfinyl) nonanoic acid | C10H20O3S | [M − H]− | 219.10549 | 53.471 | * | |
| Asperitaconic acid C | C11H16O5 | [M − H]− | 227.09209 | 50.274 | * | |
| Cyclo(phenylalanyl-prolyl) | C14H16N2O2 | [M + H]+ | 245.12826 | 48.365 | ** | |
| d-(–)-Quinic acid | C7H12O6 | [M − H − H2O]− | 173.04454 | 2.096 | ** | |
| Dibenzylamine | C14H15N | [M + H]+ | 198.12771 | 46.296 | ** | |
| d-iditol | C6H14O6 | [M + H]+ | 183.08638 | 1.945 | * | |
| Dulcitol | C6H14O6 | [M − H]− | 181.07088 | 2.001 | ** | |
| Ethyl sorbate | C8H12O2 | [M + H]+ | 141.09091 | 51.34 | ** | |
| Gabapentin | C9H17NO2 | [M + H]+ | 172.1331 | 45.282 | ** | |
| Gluconic acid | C6H12O7 | [M − H]− | 195.05033 | 1.993 | ** | |
| Hmmtic | C6H10N6O2 | [M + H]+ | 199.094 | 52.156 | * | |
| Indole-3-lactic acid | C11H11NO3 | [M − H]− | 204.06601 | 46.717 | ** | |
| Lariciresinol 4-O-glucoside | C26H34O11 | [M − H]− | 521.2034 | 48.819 | ** | |
| Methyl 2E,4E,6Z-decatrienoate | C11H16O2 | [M + H]+ | 181.12232 | 53.863 | * | |
| Mexiletine | C11H17NO | [M + H]+ | 180.13823 | 48.866 | ** | |
| N-acetyldopamine | C10H13NO3 | [M + H]+ | 196.09681 | 19.569 | ** | |
| N-acetyltyramine | C10H13NO2 | [M + H]+ | 180.10183 | 27.328 | ** | |
| Nootkatone | C15H22O | [M + H]+ | 219.17417 | 53.659 | ** | |
| Oxepanone | C6H10O2 | [M + H]+ | 115.0756 | 19.494 | ** | |
| Pimelic dialdehyde | C7H12O2 | [M + H]+ | 129.09116 | 53.704 | * | |
| Repirinast | C20H21NO5 | [M − H]− | 354.13494 | 1.078 | * | |
| Sedanolide | C12H18O2 | [M + H]+ | 195.13799 | 52.389 | ** | |
| Sjg 136 | C31H32N4O6 | [M − H]− | 555.22424 | 52.552 | * | |
| Sucrose | C12H22O11 | [M − H]− | 341.10925 | 2.072 | ** | |
| Tetraethylene glycol dimethyl ether | C10H22O5 | [M+Na]+ | 245.13581 | 36.844 | * | |
| Trans-Cinnamaldehyde | C9H8O | [M + H]+ | 133.06482 | 51.824 | ** | |
| Δ-Gluconic acid δ-lactone | C6H10O6 | [M − H]− | 177.03951 | 2.038 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panaro, M.A.; Budriesi, R.; Calvello, R.; Cianciulli, A.; Mattioli, L.B.; Corazza, I.; Rotondo, N.P.; Porro, C.; Lamonaca, A.; Ferraro, V.; et al. Lentil Waste Extracts for Inflammatory Bowel Disease (IBD) Symptoms Control: Anti-Inflammatory and Spasmolytic Effects. Nutrients 2024, 16, 3327. https://doi.org/10.3390/nu16193327
Panaro MA, Budriesi R, Calvello R, Cianciulli A, Mattioli LB, Corazza I, Rotondo NP, Porro C, Lamonaca A, Ferraro V, et al. Lentil Waste Extracts for Inflammatory Bowel Disease (IBD) Symptoms Control: Anti-Inflammatory and Spasmolytic Effects. Nutrients. 2024; 16(19):3327. https://doi.org/10.3390/nu16193327
Chicago/Turabian StylePanaro, Maria Antonietta, Roberta Budriesi, Rosa Calvello, Antonia Cianciulli, Laura Beatrice Mattioli, Ivan Corazza, Natalie Paola Rotondo, Chiara Porro, Antonella Lamonaca, Valeria Ferraro, and et al. 2024. "Lentil Waste Extracts for Inflammatory Bowel Disease (IBD) Symptoms Control: Anti-Inflammatory and Spasmolytic Effects" Nutrients 16, no. 19: 3327. https://doi.org/10.3390/nu16193327
APA StylePanaro, M. A., Budriesi, R., Calvello, R., Cianciulli, A., Mattioli, L. B., Corazza, I., Rotondo, N. P., Porro, C., Lamonaca, A., Ferraro, V., Muraglia, M., Corbo, F., Clodoveo, M. L., Monaci, L., Cavalluzzi, M. M., & Lentini, G. (2024). Lentil Waste Extracts for Inflammatory Bowel Disease (IBD) Symptoms Control: Anti-Inflammatory and Spasmolytic Effects. Nutrients, 16(19), 3327. https://doi.org/10.3390/nu16193327















