Omega-3 Supplementation in Coronary Artery Bypass Graft Patients: Impact on ICU Stay and Hospital Stay—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.1.1. Eligibility Criteria
2.1.2. Selection of the Studies
2.2. Data Extraction
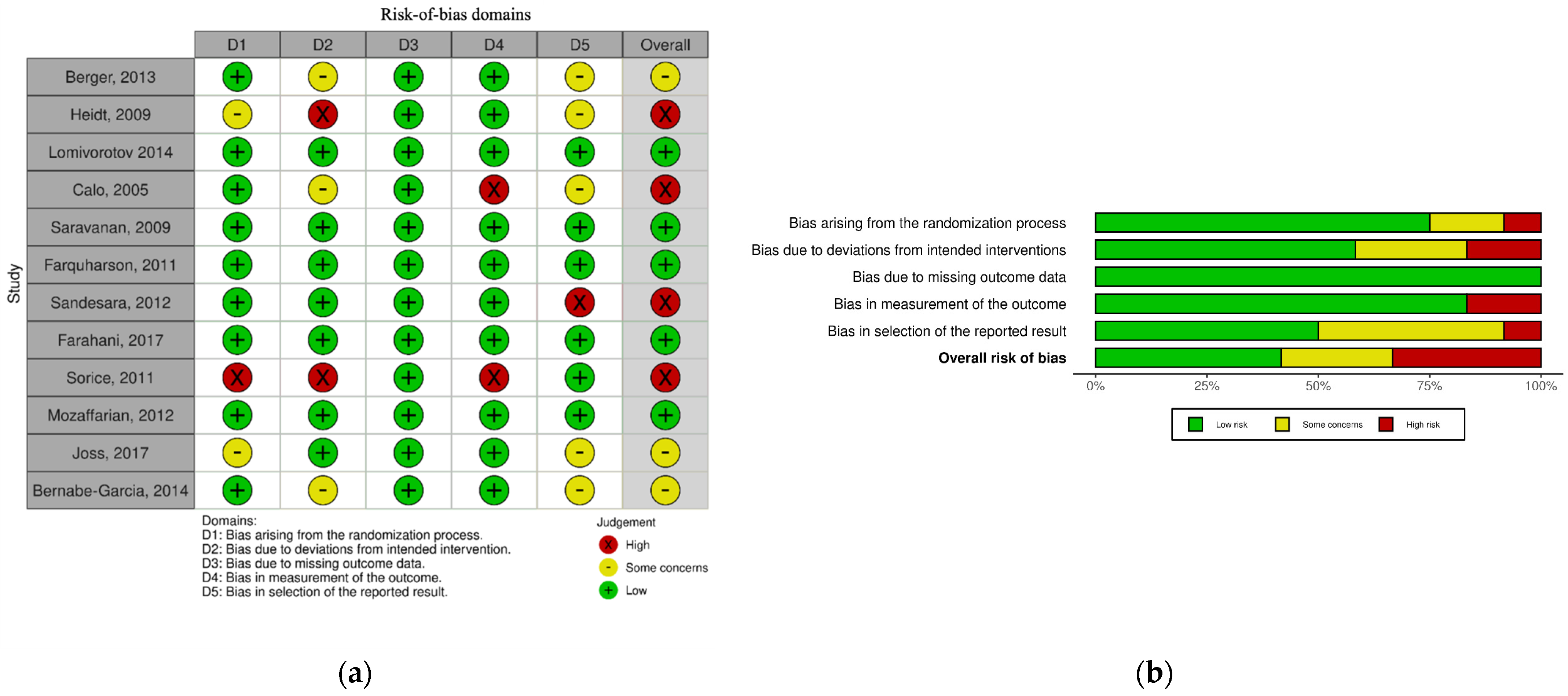
2.3. Assessment of Articles Quality
2.4. Statistical Analysis
3. Results
3.1. Search Outcome
3.2. Characteristics of Included Studies
| Study | Country | Design | Surgery | n (I/C) | Age (Mean ± SD) | Men (%) | Dose | EPA/DHA Ratio | Duration of Supplementation | Length of Hospital Stay Length of ICU stay |
|---|---|---|---|---|---|---|---|---|---|---|
| Berger, M.M., 2013 [29] | Germany | RCT, SB | CABG ± valve surgery (CPB) | I: 14 | 64.7 ± 10.5 | 14 (100%) | 400 mg FO/kg over 2 infusions of 200 mg FO/kg at 12 h + 2 h Pre-OD 200 mg FO/kg Post-OD | Not specified | Pre-OD 1 to Post-OD 1 | (=) ICU stay (p = 0.118) |
| C: 14 | 66.3 ± 9.5 | 11 (78.5%) | Saline | |||||||
| Heidt, M.C., 2009 [42] | Germany | RCT, DB | CABG | I: 52 | M: 61.2 ± 14.1 F: 74.4 ± 9.2 | 38 (73%) | 100 mg FO/kg/day | Not specified | 12 h Pre-OD to ICU discharge | Comparison between pts with AF to without AF (variance analysis) (=) ICU stays (p > 0.05) * (=) Hospital stays (p > 0.05) * |
| C: 50 | M: 66.6 ±10.7 F: 70.7 ± 8.2 | 32 (64%) | FFA 100 mg Soya oil/kg/day containing 10 g Soya oil | |||||||
| Lomivorotov, V.V., 2014 [43] | Russia | RCT, DB | CABG (CPB) | I: 18 | 61 | 17 (94.4%) | Pre-OD 1: 200 mg/kg/day Post-OD 2–7: 100 mg/kg/day | Not specified | Pre-OD 1 to 2–7 Post-OD | (=) ICU stay (p = 0.97) (=) Hospital Stay (p = 0.56) |
| C: 21 | 58 | 20 (95%) | Pre-OD 1: 2 mL/kg/day Post-OD 2–7: 1 mL/kg/day Intralipid | |||||||
| Calò, 2005 [52] | Italy | OL, RCT, Parallel groups | CABG | I: 81 | 64.9 ± 9.1 | 68 (84%) | 1.7 g/day 850 mg EPA, 882 mg DHA | 1:2 | Pre-OD 5 to discharge | (-) length of hospital stays after surgery (p = 0.017) |
| C: 79 | 66.2 ± 8.0 | 68 (84%) | Usual care | |||||||
| Saravanan, P., 2009 [44] | UK | SC, RCT, DB | CABG (CPB) | I: 52 | 64 ± 11 | 40 (77%) | 2 g/day | 1.2:1 | 17 days (median) Pre-OD 12 to 21 until discharge | (=) Hospital stay (p = 0.49) (=) ICU stay (p > 0.05) * |
| C: 51 | 64 ± 10 | 42 (82%) | 2 g/day Olive oil | 16.5 (median) (13–21 days) | ||||||
| Farquharson, A.L., 2011 [46] | Australia | RCT, DB | CABG or valve repair/replacement | I: 97 | 64 ± 11 | 80 (82%) | 4.6 g/day as Liquid oil 15 mL/day EPA: 2.7, DHA: 1.9 g/day) | Not specified | 3 week Pre-OP to Post-OD 6 or discharge 22 days (median) | (-) Length of time in cardiothoracic ICU (p = 0.006) (=) Total hospital length of stay (p = 0.24) |
| C: 97 | 64 ± 10 | 62 (64%) | 15 mL/day High monounsaturated sunflower oil | 21 days (median) | ||||||
| Sandesara, 2012 [48] (The FISH trial) | US | MC, DB, RCT | CABG with or without valve surgery | I: 120 | 63.4 ± 9.5 | 94 (78%) | Pre-operative: ≥4 g/day with minimum loading dose of 6 g over 2 days Post-operative: 2 g/day 465 mg EPA 375 mg DHA | 1.24:1 | Pre-OD 2 until occurrence of AF or 14 days | (=) hospital stay (p = 0.27) (=) ICU rehospitalization (p = 0.64) |
| C: 123 | 62 ± 11.4 | 102 (83%) | Corn oil 2 g/day | |||||||
| Farahani, 2017 [50] | Iran | SC, RCT, DB | CABG | I: 202 | 60.62 ± 8.95 | 132 (65%) | 2 g/day fish oil 300 mg EPA 200 DHA | 1.5:1 | Pre-OD 5 to discharge | (-) ICU stay (p = 0.003) (-) Hospital stay (p = 0.04) |
| C: 199 | 61.28 ± 10 | 127 (64%) | Olive oil soft gelatin capsules | |||||||
| Sorice, M., 2011 [49] | Italy | RCT, DB | CABG “on-pump” and “off-pump” | I: 96 | Off-pump 64 ± 10 On-pump 63 ± 10 | Off-pump 37 (82%) On-pump: 39 (76%) | 2 g/day | 1:2 | Pre-OD 5 to discharge | (=) Length of hospital stay (p = 0.75) |
| C: 105 | Off-pump (G1) 63 ± 10 On-pump (G2) 63 ± 9 | G1) 42 (87%) (G2) 46 (80%) | No intervention | |||||||
| OPERA 2012 (Mozaffarian) [45] | US, Italy, Argentina (28 centers) | Multinational RCT, DB | CABG, valve repair or replacement, other open cardiac surgery | I: 758 | 63.8 SD (12.6) | 551 (72.7%) | Pre-operatively 10 g loading over 3–5 days or 8 g over 2 days Post-operatively 2 g ≥840 g/capsule 465 mg EPA: 375 mg DHA | Not specified | Pre-OD 3–5 to Post-OD 10 or discharge | (=) ICU stay (p = 0.38) (=) Hospital stay (p = 0.48) |
| C: 758 | 63.6 SD (12.4) | 543 (71.6%) | Olive oil | |||||||
| Joss, 2017 [47] | US | RCT, DB | CABG, valve replacement/repair surgery | I: 284 | 66.6 ± 10.72 | 212 (74.7%) | 2 g/day | 2:3 DHA: EPA | Pre-OD 5 or 24 h Post-operatively to Post-operative week 4 75% of pts started and 25% post-OD1 | (=) Length of hospital stay (p = 0.834) |
| C: 275 | 66.4 ± 10.72 | 199 (72.4%) | Mineral oil with a trace amount of wheat germ oil | |||||||
| Bernabe-Garcia, 2014 [51] | Mexico | RCT, DB | CABG | I: 12 | 58.7 ± 2.7 | 100% | 2.4 g/day omega-3 in 4 capsules (1.6 g/day EPA + 0.8 g DHA) | Not specified | Pre-OD 1 to Post-OD 6 | (=) ICU stay, days (p= 0.440) (-) Total hospital stays, days (p = 0.038) |
| C: 11 | 66.0 ± 2.5 | 100% | Corn starch |
3.2.1. IV Administration
3.2.2. Oral Supplementation
3.3. Internal Validity (Risk-of-Bias Assessment)
3.4. External Validity
3.5. Source of Funding and Conflicts of Interest
3.6. Meta-Analysis (Quantitative Assessment)
Synthesis of Meta-Analysis
3.7. Forest Plot of the Effect of Omega-3 PUFA on ICU Stay
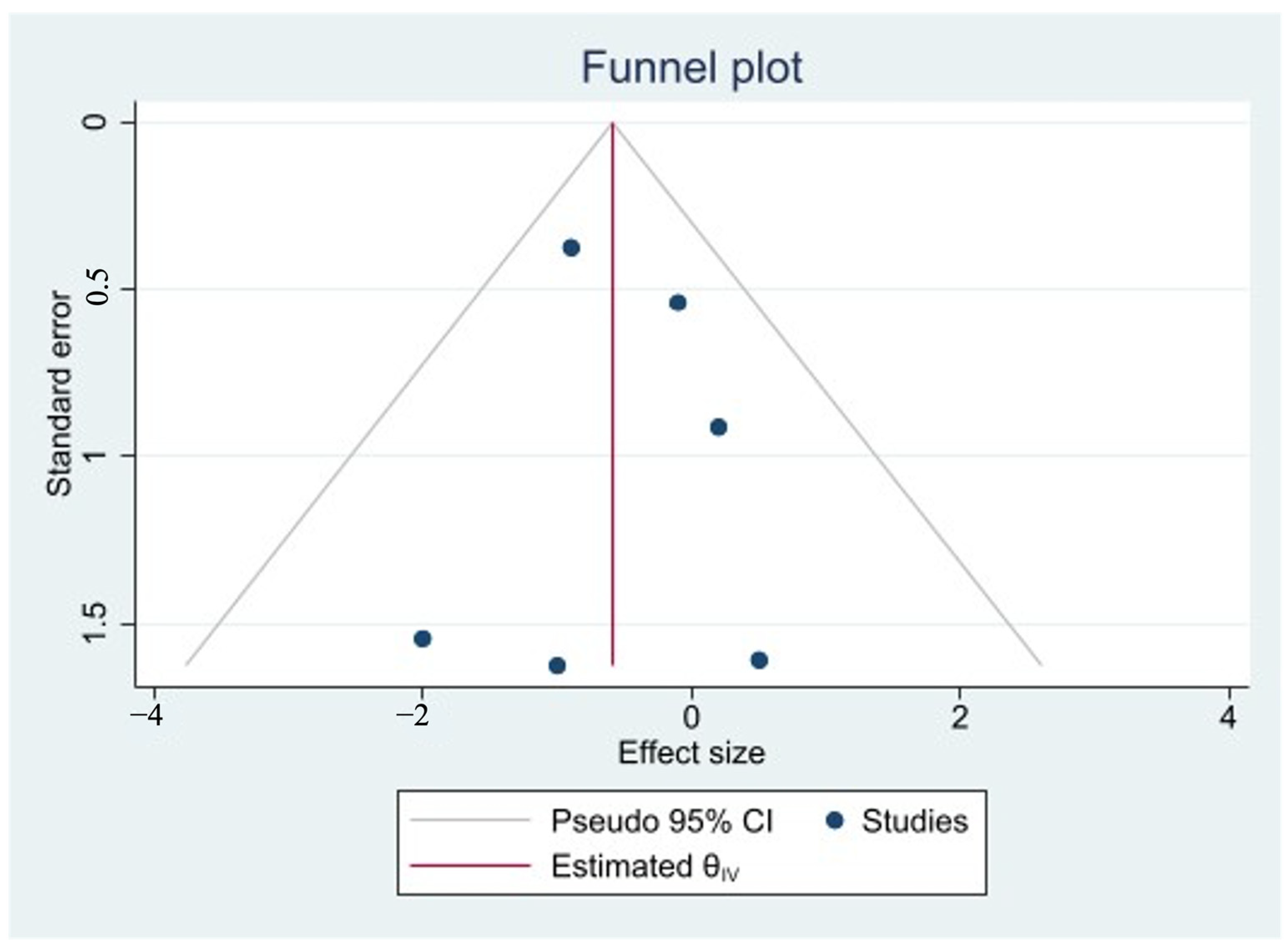
3.8. Assessment of Publication Bias
3.8.1. Effect of Omega-3 Polyunsaturated Fatty Acids (PUFA) on Hospital Length of Stay after Coronary Artery Bypass Graft (CABG) Surgery
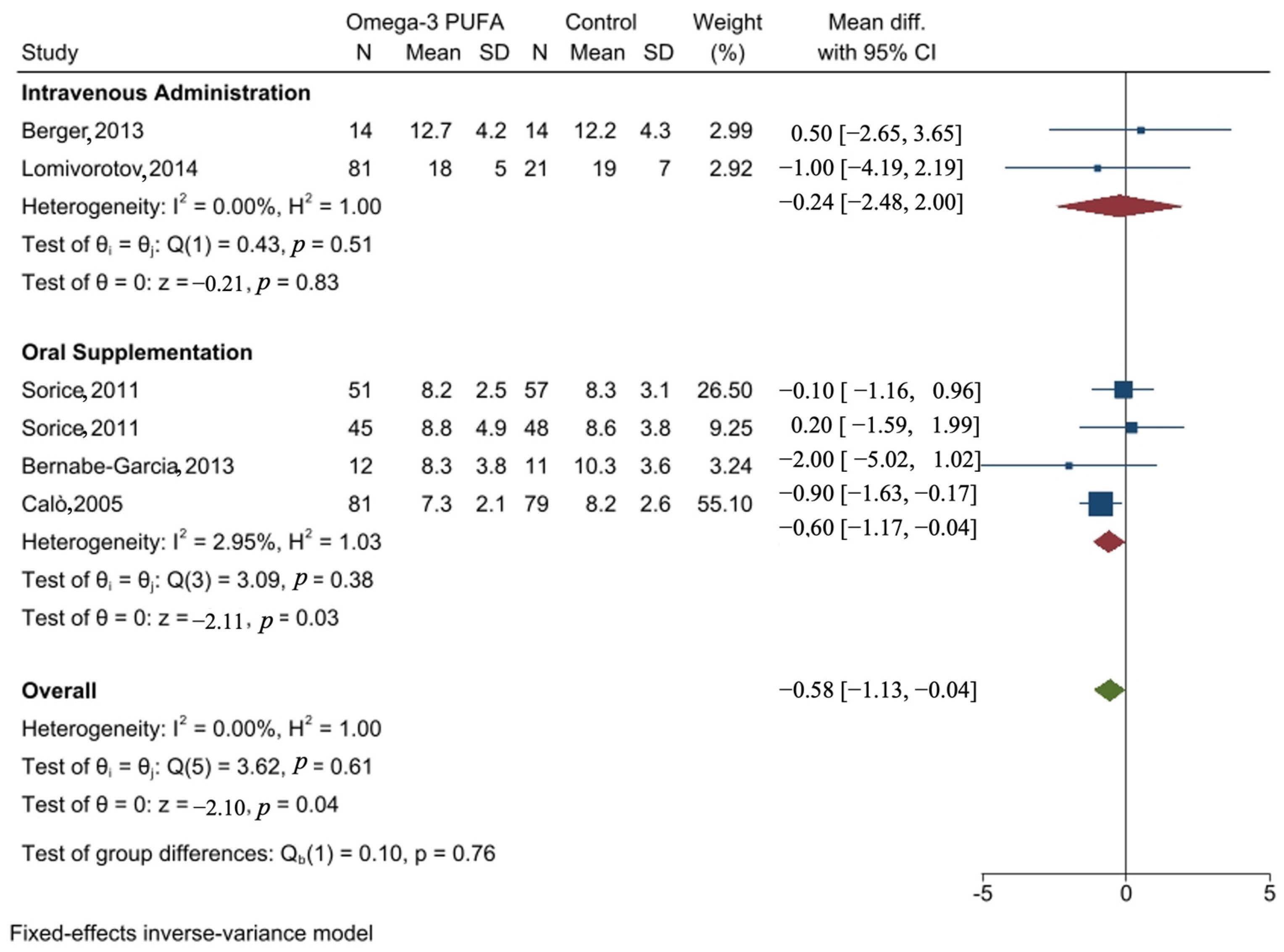
3.8.2. Forest Plot of the Effect of Omega-3 PUFA on the Length of Hospital Stay
4. Discussion
4.1. The Effect of Omega-3 PUFA on ICU Length of Stay Post-CABG Surgery
4.2. Omega-3 and Hospital Length of Stay Post-CABG Surgery
4.3. Implications for Future Research
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekhri, T.; Kanwar, R.; Wilfred, R.; Chugh, P.; Chhillar, M.; Aggarwal, R.; Sharma, Y.; Sethi, J.; Sundriyal, J.; Bhadra, K. Prevalence of risk factors for coronary artery disease in an urban Indian population. BMJ Open 2014, 4, e005346. [Google Scholar] [CrossRef]
- Indicators, O. Health at a Glance 2009. Health Workers: Psychiatrists. Available online: http://www.oecd-ilibrary.org/docserver/download/fulltext/8109111ec030.pdf2009 (accessed on 20 January 2023).
- Weiss, A.J.; Elixhauser, A. Trends in Operating Room Procedures in US Hospitals, 2001–2011: Statistical Brief# 171. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]; Agency for Healthcare Research and Quality: Rockville, MA, USA, 2014. [Google Scholar]
- NIH. What Is Coronary Artery Bypass Grafting? 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507836/ (accessed on 20 January 2023).
- Montrief, T.; AKoyfman Long, B. Coronary artery bypass graft surgery complications: A review for emergency clinicians. Am. J. Emerg. Med. 2018, 36, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, J.; Hosseini, E.; Kargar, F.; Maghsudlu, M.; Ghasemzadeh, M. Platelet transfusion enhances pro-aggregatory status shortly after coronary artery bypass grafting (CABG while modulating platelet pro-inflammatory state 1-week post-surgery. J. Cell. Mol. Med. 2024, 28, e18573. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.; Ahmadi, J.; Kargar, F.; Ghasemzadeh, M. Coronary artery bypass grafting (CABG) induces pro-inflammatory and immunomodulatory phenotype of platelets in the absence of a pro-aggregatory state. Microvasc. Res. 2024, 153, 104669. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, J.; Hosseini, E.; Kargar, F.; Ghasemzadeh, M. Stable CAD patients show higher levels of platelet-borne TGF-β1 associated with a superior pro-inflammatory state than the pro-aggregatory status; evidence highlighting the importance of platelet-derived TGF-β1 in atherosclerosis. J. Thromb. Thrombolysis 2023, 55, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, P.H.; Kim, H.J.; Kim, J.B.; Park, S.; Kyoung, D.-S.; Kang, S.-J.; Lee, S.-W.; Kim, Y.-H.; Lee, C.W. Incidence and predictors of intracranial bleeding after coronary artery bypass graft surgery. Front. Cardiovasc. Med. 2022, 9, 863590. [Google Scholar] [CrossRef]
- Marteinsson, S.A.; Heimisdóttir, A.A.; Axelsson, T.A.; Johannesdottir, H.; Arnadottir, L.O.; Gardarsdottir, H.R.; Johnsen, A.; Sigurdsson, M.I.; Helgadottir, S.; Gudbjartsson, T. Reoperation for bleeding following coronary artery bypass surgery with special focus on long-term outcomes. Scand. Cardiovasc. J. 2020, 54, 265–273. [Google Scholar] [CrossRef]
- Peng, W.; Yang, B.; Qiao, H.; Liu, Y.; Lin, Y. Prediction of acute kidney injury following coronary artery bypass graft surgery in elderly Chinese population. J. Cardiothorac. Surg. 2023, 18, 287. [Google Scholar] [CrossRef]
- Lin, H.; Hou, J.; Tang, H.; Chen, K.; Sun, H.; Zheng, Z.; Hu, S. A novel nomogram to predict perioperative acute kidney injury following isolated coronary artery bypass grafting surgery with impaired left ventricular ejection fraction. BMC Cardiovasc. Disord. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Pryor, D.B.; Shaw, L.; Harrell, F.E., Jr.; Lee, K.L.; Hlatky, M.A.; Mark, D.B.; Muhlbaier, L.H.; Califf, R.M. Estimating the likelihood of severe coronary artery disease. Am. J. Med. 1991, 90, 553–562. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Nakamura, T.; Shimomura, I.; Kotani, K. Visceral fat accumulation and cardiovascular disease. Obes. Res. 1995, 3 (Suppl. S5), 645s–647s. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Kim, N.H.; Kim, D.H.; Kim, Y.H.; Park, Y.K.; Kim, S.M. Associations between Body Mass Index, Waist Circumference, and Myocardial Infarction in Older Adults Aged over 75 Years: A Population-Based Cohort Study. Medicina 2022, 58, 1768. [Google Scholar] [CrossRef] [PubMed]
- Kuhail, M.; Shab-Bidar, S.; Yaseri, M.; Djafarian, K. Major Dietary Patterns Relationship with Severity of Coronary Artery Disease in Gaza-Strip, Palestine: A Cross-Sectional Study. Ethiop. J. Health Sci. 2021, 31, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Redberg, R.F.; Meier, P. Saturated fat does not clog the arteries: Coronary heart disease is a chronic inflammatory condition, the risk of which can be effectively reduced from healthy lifestyle interventions. Br. J. Sports Med. 2017, 51, 1111–1112. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Saita, E.; Taguchi, C.; Aoyama, M.; Ikegami, Y.; Ohmori, R.; Kondo, K.; Momiyama, Y. Associations between Green Tea Consumption and Coffee Consumption and the Prevalence of Coronary Artery Disease. J. Nutr. Sci. Vitaminol. 2020, 66, 237–245. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Hu, E.A.; Steffen, L.M.; Coresh, J.; Appel, L.J.; Rebholz, C.M. Adherence to the Healthy Eating Index-2015 and Other Dietary Patterns May Reduce Risk of Cardiovascular Disease, Cardiovascular Mortality, and All-Cause Mortality. J. Nutr. 2020, 150, 312–321. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Yang, Z.; Duan, M.L. Dietary approach to stop hypertension diet and risk of coronary artery disease: A meta-analysis of prospective cohort studies. Int. J. Food. Sci. Nutr. 2019, 70, 668–674. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Pradelli, L.; Mayer, K.; Klek, S.; Omar Alsaleh, A.J.; Clark, R.A.C.; Rosenthal, M.D.; Heller, A.R.; Muscaritoli, M. omega-3 Fatty-Acid Enriched Parenteral Nutrition in Hospitalized Patients: Systematic Review With Meta-Analysis and Trial Sequential Analysis. JPEN J. Parenter. Enter. Nutr. 2020, 44, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.O.; Basun, H.; et al. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-beta42 by human microglia and decrease inflammatory markers. J. Alzheimers Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.E.; Willett, W.C.; Ma, J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef]
- Berger, M.M.; Delodder, F.; Liaudet, L.; Tozzi, P.; Schlaepfer, J.; Chiolero, R.L.; Tappy, L. Three short perioperative infusions of n-3 PUFAs reduce systemic inflammation induced by cardiopulmonary bypass surgery: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 246–254. [Google Scholar] [CrossRef]
- Lombardi, M.; Carbone, S.; Del Buono, M.G.; Chiabrando, J.G.; Vescovo, G.M.; Camilli, M.; Montone, R.A.; Vergallo, R.; Abbate, A.; Biondi-Zoccai, G.; et al. Omega-3 fatty acids supplementation and risk of atrial fibrillation: An updated meta-analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, e69–e70. [Google Scholar] [CrossRef]
- Gencer, B.; Djousse, L.; Al-Ramady, O.T.; Cook, N.R.; Manson, J.E.; Albert, C.M. Effect of Long-Term Marine ω-3 Fatty Acids Supplementation on the Risk of Atrial Fibrillation in Randomized Controlled Trials of Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Circulation 2021, 144, 1981–1990. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Sandesara, C.M.; Metcalf, R.G.; Latini, R.; Libby, P.; Lombardi, F.; O’Gara, P.T.; Page, R.L.; et al. Fish Oil and Post-Operative Atrial Fibrillation: A Meta-Analysis of Randomized Controlled Trials. J. Am. Coll. Cardiol. 2013, 61, 2194–2196. [Google Scholar] [CrossRef]
- Zhang, B.; Zhen, Y.; Tao, A.; Bao, Z.; Zhang, G. Polyunsaturated fatty acids for the prevention of atrial fibrillation after cardiac surgery: An updated meta-analysis of randomized controlled trials. J. Cardiol. 2014, 63, 53–59. [Google Scholar] [CrossRef]
- Langlois, P.L.; Hardy, G.; Manzanares, W. Omega-3 polyunsaturated fatty acids in cardiac surgery patients: An updated systematic review and meta-analysis. Clin. Nutr. 2017, 36, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Qian, J.; Si, W.; Cheng, H.; Ji, F.; Shen, Z. The clinical benefits of perioperative antioxidant vitamin therapy in patients undergoing cardiac surgery: A meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- National Health Service (NHS). Resources for Advanced Searching. 2023. Available online: https://library.nhs.uk/knowledgehub/resources-for-advanced-searching/.
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hong Kong Baptist University. Estimating the Sample Mean and Standard Deviation (SD) from the Five-Number Summary and Its Application in Meta-Analysis. Available online: https://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html (accessed on 18 September 2023).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Heidt, M.C.; Vician, M.; Stracke, S.K.; Stadlbauer, T.; Grebe, M.T.; Boening, A.; Vogt, P.R.; Erdogan, A. Beneficial effects of intravenously administered N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A prospective randomized study. Thorac. Cardiovasc. Surg. 2009, 57, 276–280. [Google Scholar] [CrossRef]
- Lomivorotov, V.V.; Efremov, S.M.; Pokushalov, E.A.; Romanov, A.B.; Ponomarev, D.N.; Cherniavsky, A.M.; Shilova, A.N.; Karaskov, A.M.; Lomivorotov, V.N. Randomized trial of fish oil infusion to prevent atrial fibrillation after cardiac surgery: Data from an implantable continuous cardiac monitor. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1278–1284. [Google Scholar] [CrossRef]
- Saravanan, P.; O’Neill, S.C.; Bridgewater, B.; Davidson, N.C. Fish oils supplementation does not reduce risk of atrial fibrillation following coronary artery bypass surgery. Heart Rhythm 2009, 6, S283. [Google Scholar]
- Mozaffarian, D.; Marchioli, R.; Macchia, A.; Silletta, M.G.; Ferrazzi, P.; Gardner, T.J.; Latini, R.; Libby, P.; Lombardi, F.; O Gara, P.T.; et al. Fish oil and postoperative atrial fibrillation: The Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. JAMA 2012, 308, 2001–2011. [Google Scholar] [CrossRef]
- Farquharson, A.L.; Metcalf, R.G.; Sanders, P.; Stuklis, R.; Edwards, J.R.; Gibson, R.A.; Cleland, L.G.; Sullivan, T.R.; James, M.J.; Young, G.D. Effect of dietary fish oil on atrial fibrillation after cardiac surgery. Am. J. Cardiol. 2011, 108, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Joss, J.D.; Hernan, J.; Collier, R.; Cardenas, A. Perioperative supplementation of polyunsaturated omega-3 fatty acid for the prevention of atrial fibrillation after cardiothoracic surgery. Am. J. Health-Syst. Pharm. 2017, 74, e17–e23. [Google Scholar] [CrossRef] [PubMed]
- Sandesara, C.M.; Chung, M.K.; Van Wagoner, D.R.; Barringer, T.A.; Allen, K.; Ismail, H.M.; Zimmerman, B.; Olshansky, B. A Randomized, Placebo-Controlled Trial of Omega-3 Fatty Acids for Inhibition of Supraventricular Arrhythmias After Cardiac Surgery: The FISH Trial. J. Am. Heart Assoc. 2012, 1, e000547. [Google Scholar] [CrossRef] [PubMed]
- Sorice, M.; Tritto, F.P.; Sordelli, C.; Gregorio, R.; Piazza, L. N-3 polyunsaturated fatty acids reduces post-operative atrial fibrillation incidence in patients undergoing “on-pump” coronary artery bypass graft surgery. Monaldi Arch. Chest Dis. 2011, 76, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Vasheghani Farahani, A.; Yousefi Azar, A.; Goodarzynejad, H.R.; Khorrami, E.; Hosseinzadeh-Attar, M.J.; Oshnouei, S.; Alizadeh Ghavidel, A.; Golfeshan, E.; Ghourban Pour, F. Fish oil supplementation for primary prevention of atrial fibrillation after coronary artery bypass graft surgery: A randomized clinical trial. Int. J. Surg. 2017, 42, 41–48. [Google Scholar] [CrossRef]
- Bernabe-Garcia, M.; Lopez-Alarcon, M.; Mansilla-Olivares, A.; Maldonado-Hernandez, J.; Blanco-Favela, F.; Chavez-Sanchez, L.; Chavez-Rueda, K.; Mancilla-Ramirez, J.; Arriaga-Pizano, L.; Riera-Kinkel, C. Oral administration of n-3 long-chain fatty acids reduce inflammatory response and improve clinical outcomes in patients with cardiovascular surgery. Exp. Clin. Cardiol. 2014, 20, 145–160. [Google Scholar]
- Calò, L.; Bianconi, L.; Colivicchi, F.; Lamberti, F.; Loricchio, M.L.; de Ruvo, E.; Meo, A.; Pandozi, C.; Staibano, M.; Santini, M. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A randomized, controlled trial. J. Am. Coll. Cardiol. 2005, 45, 1723–1728. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Calandria, J.M.; Serhan, C.N. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J. Lipid Res. 2010, 51, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.W.; Kwon, M.J.; Choi, A.M.; Kim, H.P.; Nakahira, K.; Hwang, D.H. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 2009, 284, 27384–27392. [Google Scholar] [CrossRef] [PubMed]
- Westphal, C.; Konkel, A.; Schunck, W.H. CYP-eicosanoids--a new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat. 2011, 96, 99–108. [Google Scholar] [CrossRef]
- Kacik, M.; Oliván-Viguera, A.; Köhler, R. Modulation of K(Ca)3.1 channels by eicosanoids, omega-3 fatty acids, and molecular determinants. PLoS ONE 2014, 9, e112081. [Google Scholar] [CrossRef]
- Ip, W.T.K.; Chandramouli, C.; Smith, J.A.; McLennan, P.L.; Pepe, S.; Delbridge, L.M.D. A Small Cohort Omega-3 PUFA Supplement Study: Implications of Stratifying According to Lipid Membrane Incorporation in Cardiac Surgical Patients. Heart Lung Circ. 2017, 26, 846–855. [Google Scholar] [CrossRef]

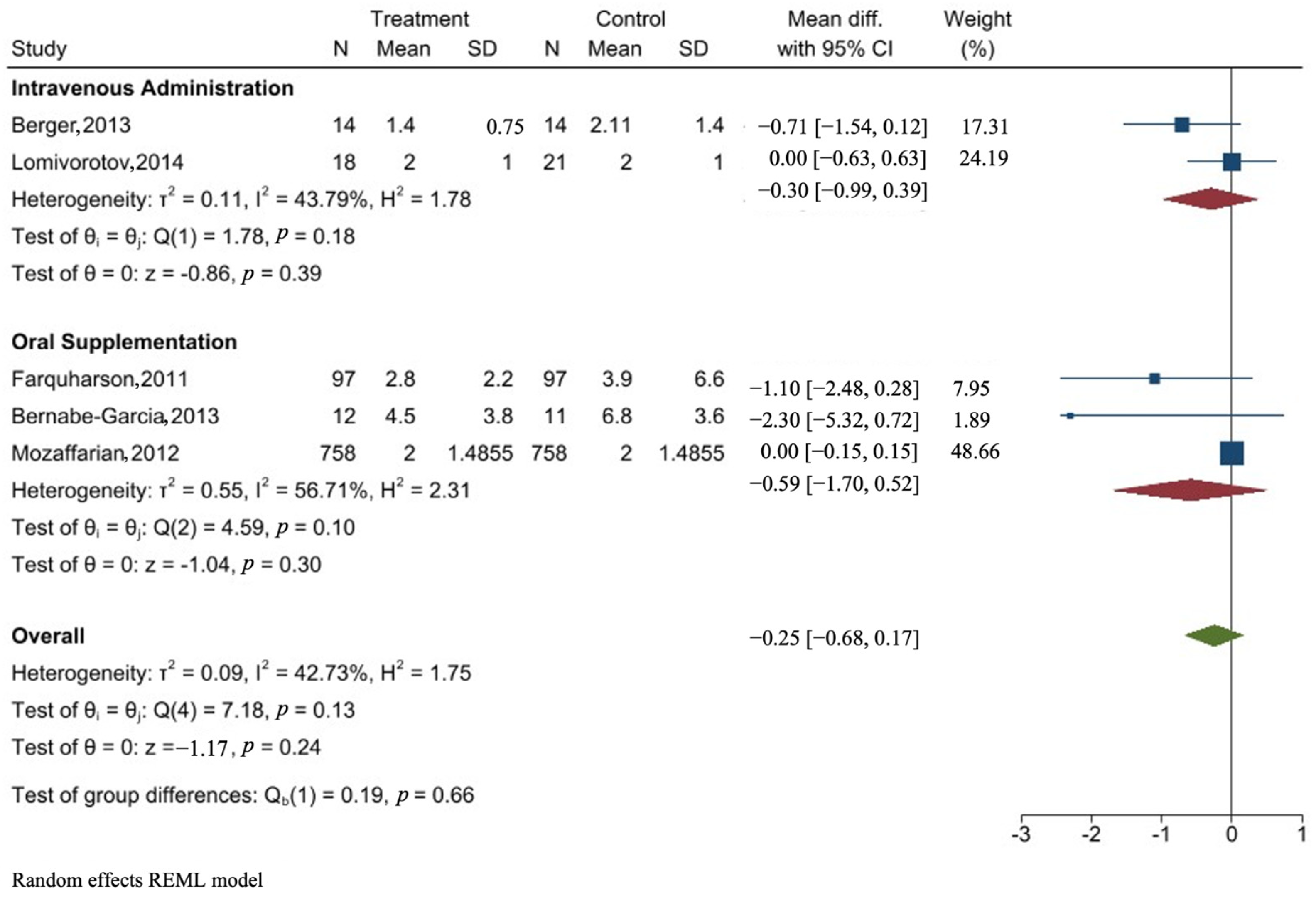
| Study | Method of Administration | Dose | Pre-Operative | Post-Operative | Mean Difference | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Berger, 2013 [29] | IV | 400 mg | Day 1 | Day 1 | −0.71 | [−1.54, 0.12] |
| Lomivorotov, 2014 [43] | IV | Pre-OP 200 mg Post-OP 100 mg | Day 1 | Days 2–7 | 0.00 | [−0.63, 0.63] |
| Farquharson, 2011 [46] | Oral | 4.6 g/day | 3 weeks | Day 6 | −1.10 | [−2.48, 0.28] |
| Bernabe-Garcia, 2014 [51] | Oral | 2.4 g/day | Day 1 | Day 6 | −2.30 | [−5.32, 0.72] |
| Mozaffarian, 2012 [45] | Oral | 8 g over 2 day Pre-OP 2 g Post-OP | Days 3–5 | Day 10 or Discharge | 0.00 | [−0.15, 0.15] |
| Study | Method of Administration | Dose | Pre-Operative | Post-Operative | Mean Difference | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Berger, 2013 [29] | IV | 400 mg | Day 1 | Day 1 | 0.5 | [−2.65, 3.65] |
| Lomivorotov, 2014 [43] | IV | Pre-OP 200 mg Post-OP 100 mg | Day 1 | Days 2–7 | −1 | [−4.19, 2.19] |
| Sorice, 2011 [49] “on-pump CABG” | Oral | 2 g/day | Day 5 | Discharge | −0.1 | [−1.16, 0.96] |
| Sorice, 2011 “on-pump CABG” [49] | Oral | 2 g/day | Day 5 | Discharge | 0.2 | [−1.59, 1.99] |
| Bernabe-Garcia, 2014 [51] | Oral | 2.4 g/day | Day 1 | Day 6 | −2 | [−5.02, 1.02] |
| Calò, 2005 [52] | Oral | 1.7 g/day | Day 5 | Discharge | −0.9 | [−1.63, −0.17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouagueni, A.; Shi, Z.; Shraim, M.; Al-Zoubi, R.M.; Zarour, A.; Al-Ansari, A.; Bawadi, H. Omega-3 Supplementation in Coronary Artery Bypass Graft Patients: Impact on ICU Stay and Hospital Stay—A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 3298. https://doi.org/10.3390/nu16193298
Ouagueni A, Shi Z, Shraim M, Al-Zoubi RM, Zarour A, Al-Ansari A, Bawadi H. Omega-3 Supplementation in Coronary Artery Bypass Graft Patients: Impact on ICU Stay and Hospital Stay—A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(19):3298. https://doi.org/10.3390/nu16193298
Chicago/Turabian StyleOuagueni, Asma, Zumin Shi, Mujahed Shraim, Raed M. Al-Zoubi, Ahmad Zarour, Abdulla Al-Ansari, and Hiba Bawadi. 2024. "Omega-3 Supplementation in Coronary Artery Bypass Graft Patients: Impact on ICU Stay and Hospital Stay—A Systematic Review and Meta-Analysis" Nutrients 16, no. 19: 3298. https://doi.org/10.3390/nu16193298
APA StyleOuagueni, A., Shi, Z., Shraim, M., Al-Zoubi, R. M., Zarour, A., Al-Ansari, A., & Bawadi, H. (2024). Omega-3 Supplementation in Coronary Artery Bypass Graft Patients: Impact on ICU Stay and Hospital Stay—A Systematic Review and Meta-Analysis. Nutrients, 16(19), 3298. https://doi.org/10.3390/nu16193298






