Antineoplastic Activity of Sodium Caseinate in a Cytarabine-Resistant Mouse Acute Myeloid Leukemia Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
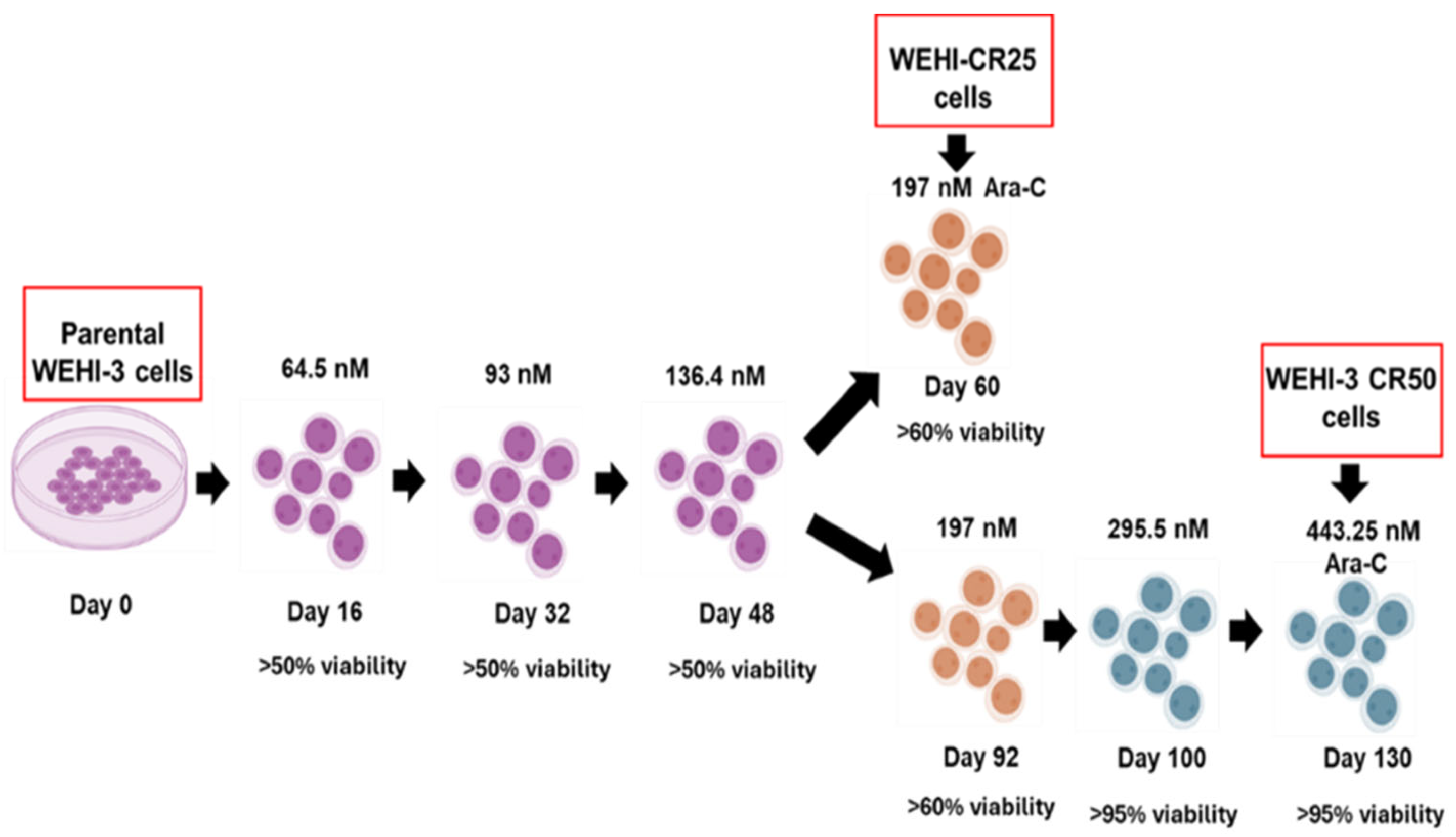
2.2. Development of the Cytarabine-Resistant Subline
2.3. Cell Proliferation and Viability Curves
2.4. Reverse Transcriptase–Polymerase Chain Reaction
2.5. Immunofluorescence
2.6. In Vitro SC Assays
2.7. Apoptosis Assay
2.8. Survival Curves
2.9. Statistical Analysis
3. Results
3.1. Sensitivity of Parental WEHI-3 Cells to Cytarabine
3.2. Development of Cytarabine-Resistant Cells
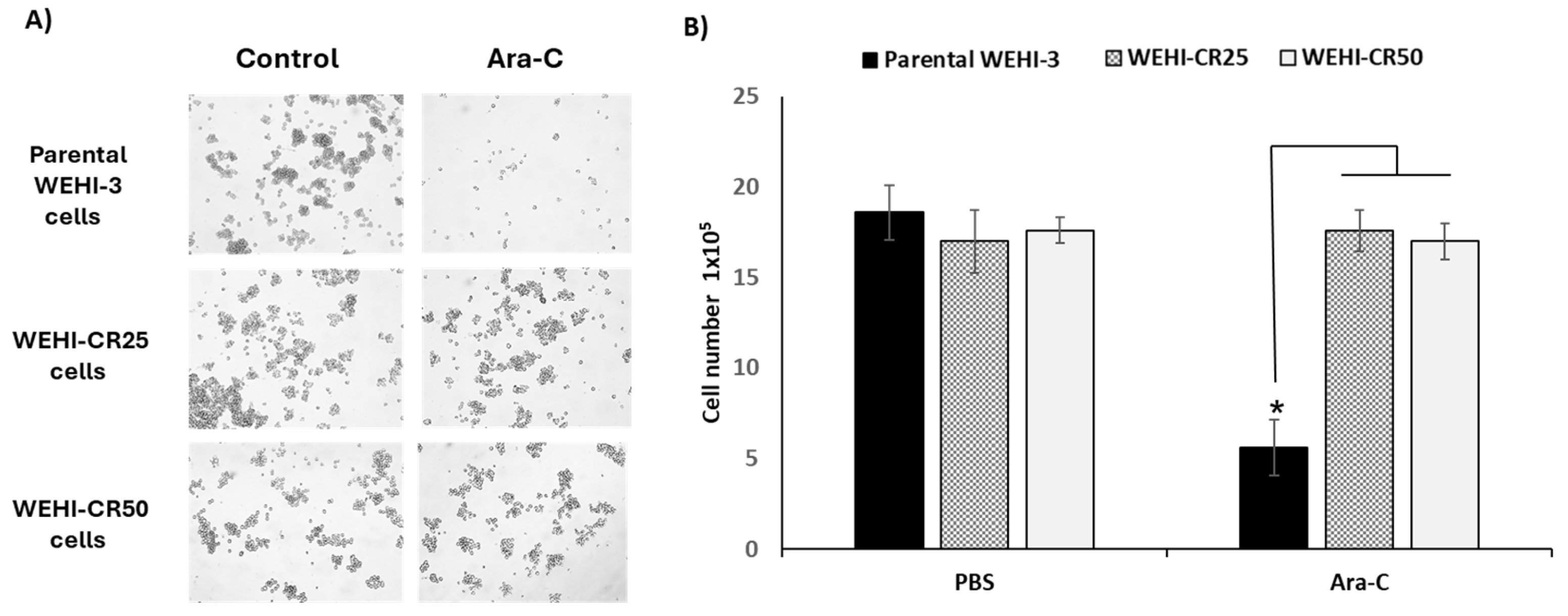
3.3. Cytarabine Inhibits the Proliferation of Parental WEHI-3 Cells but Does Not Affect the Proliferation of WEHI-CR25 and WEHI-CR50 Cells
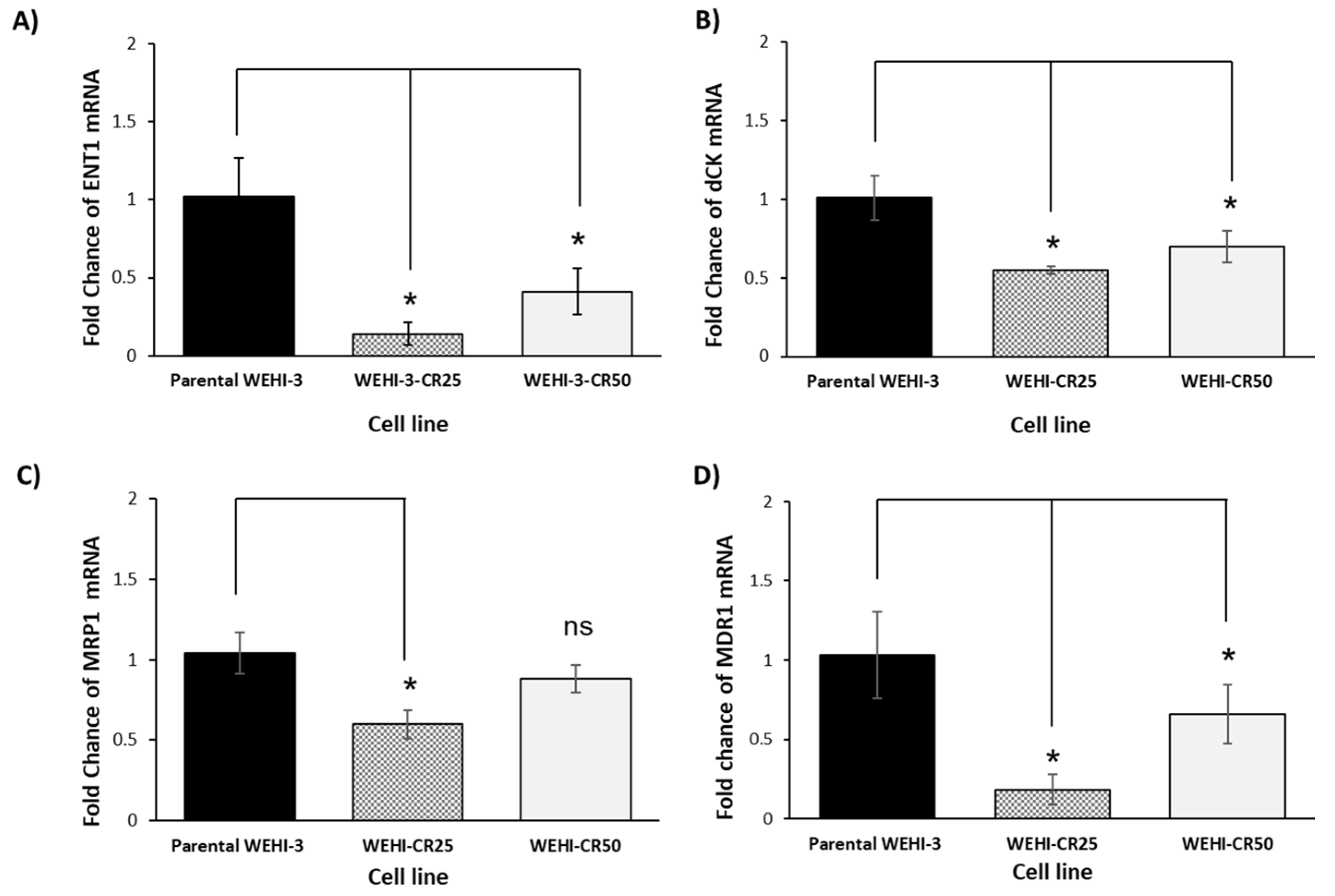
3.4. The Resistance of the WEHI-CR25 and WEHI-CR50 Cells to Cytarabine Modulates the Expression of Genes Involved in Chemoresistance
3.5. Sodium Caseinate Inhibits the Proliferation of WEHI-CR25 and WEHI-CR50 Cells, Similarly to Parental WEHI-3 Cells
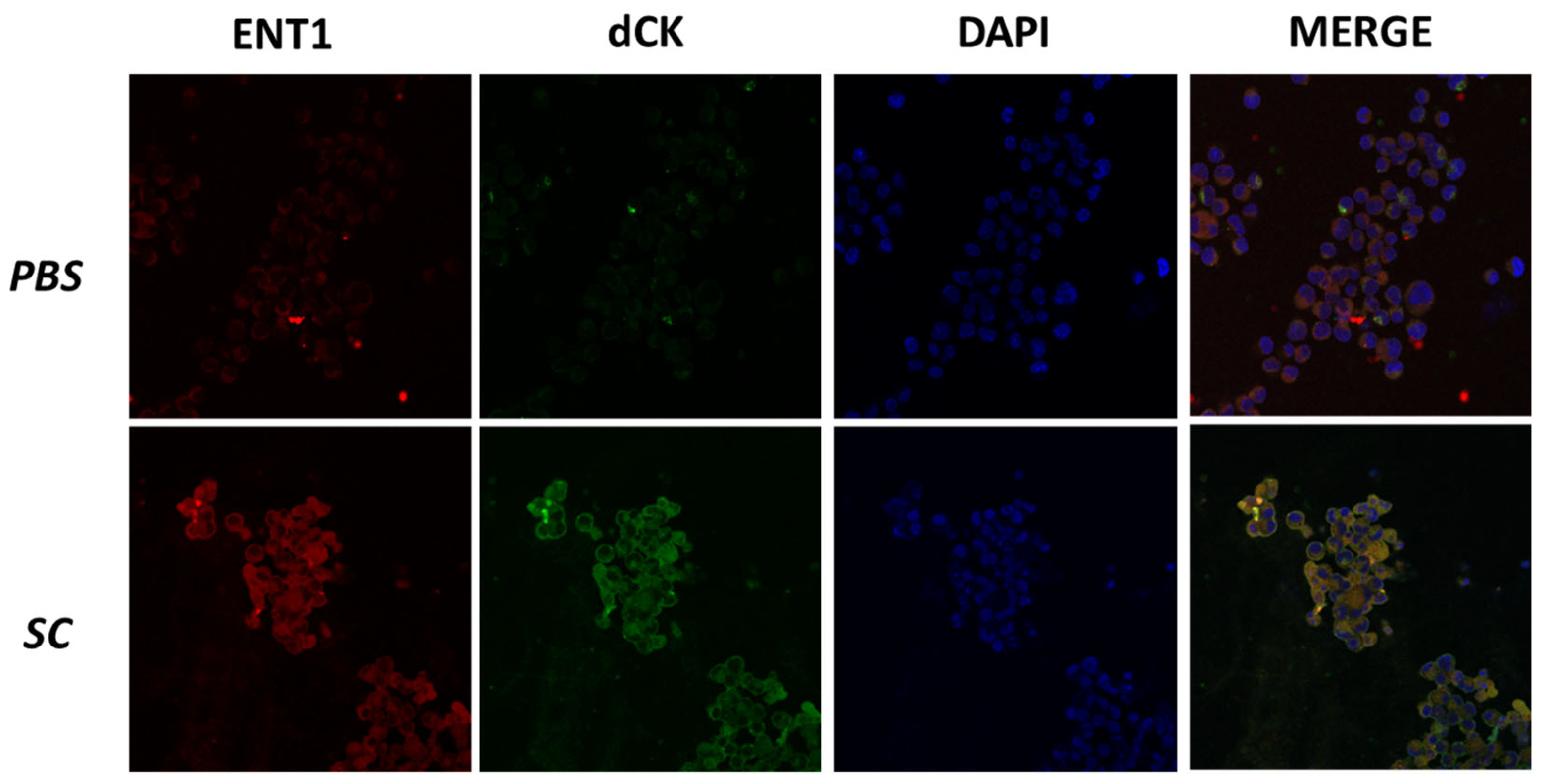
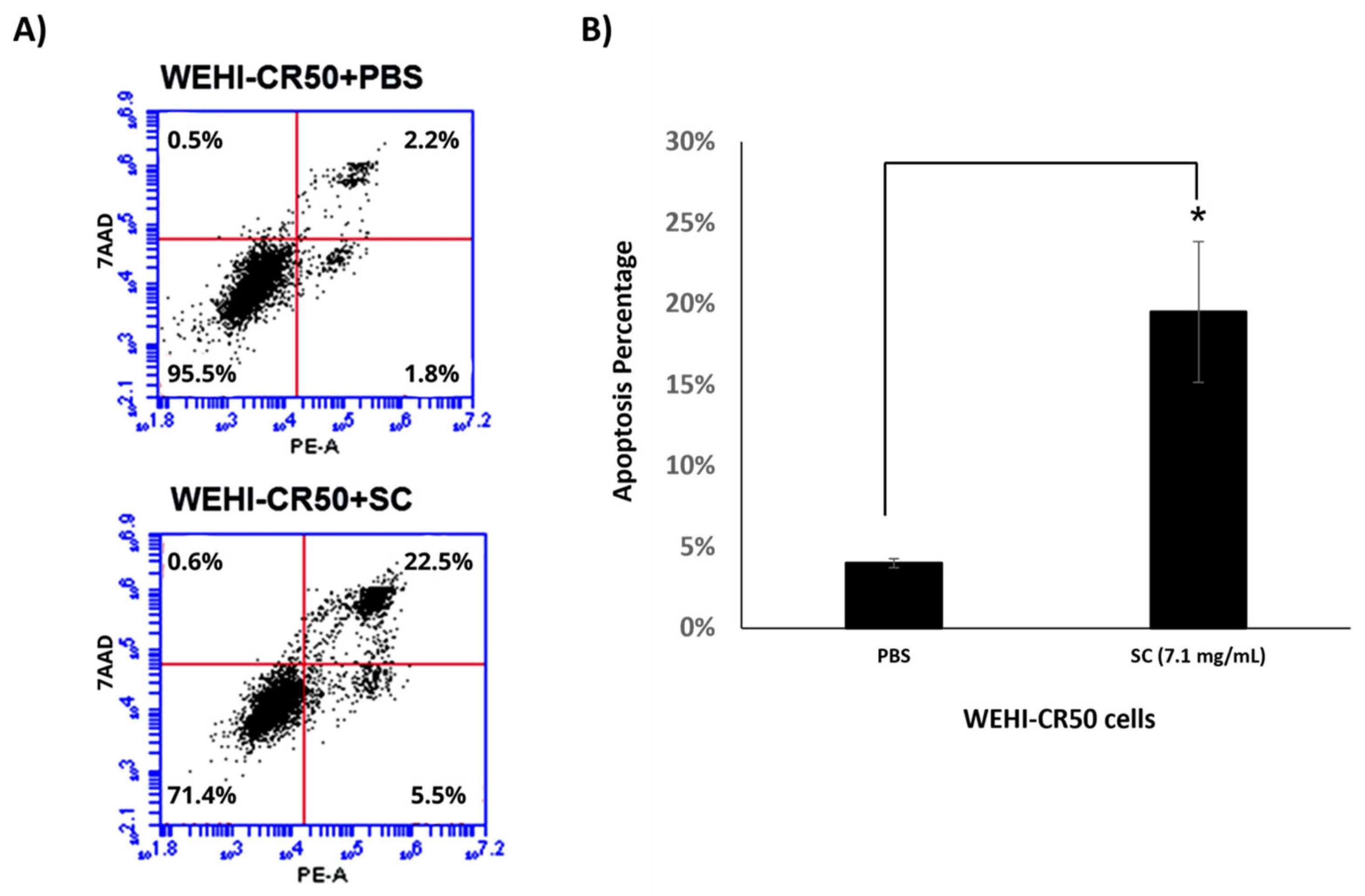
3.6. Sodium Caseinate Induces Apoptosis and Modulates the Expression of Genes Associated with Resistance in WEHI-CR50 Cells
3.7. Sodium Caseinate Increases the Survival of Leukemic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, J.A.; Pmerratz, K.W. Acute myeloid leukemia in the elderly: Therapeutic options and choice. Leuk. Lymphoma 2018, 59, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Kloss, R.; Ahmed-khan, M.; Carmona-Pires, F.; Okam, N.; Weeraddana, P.; Dharmaratna, D.; Dandwani, M.; Moin, K. A review of treatment options employed in relapsed/refractory AML. Hematology 2023, 28, 2196482. [Google Scholar] [CrossRef] [PubMed]
- Demir, D. Insights into the New Molecular Updates in Acute Myeloid Leukemia Pathogenesis. Genes 2023, 14, 1424. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, O.K.; Porwit, A.; Orazi, A.; Hasserjian, R.P.; Foucar, K.; Duncavage, E.J.; Arber, D.A. The International Consensus Classification of acute myeloid leukemia. Virchows Arch. 2023, 482, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Tao, Q.; Wang, J.; Zhang, Q.; Dong, Y. Cladribine, cytarabine, and filgrastim based regimen in relapsed or refractory acute myeloid leukemia: A systematic review and meta-analysis. Medicine 2023, 102, e34949. [Google Scholar] [CrossRef]
- Fajardo-Orduña, G.R.; Ledesma-Martínez, E.; Aguiñiga-Sánchez, I.; Mora-García, M.D.L.; Weiss-Steider, B.; Santiago-Osorio, E. Inhibitors of Chemoresistance Pathways in Combination with Ara-C to Overcome Multidrug Resistance in AML. A Mini Review. Int. J. Mol. Sci. 2021, 22, 4955. [Google Scholar] [CrossRef]
- Stelmach, P.; Trumpp, A. Leukemic stem cells and therapy resistance in acute myeloid leukemia. Hematology 2023, 108, 353–366. [Google Scholar] [CrossRef]
- Swaminathan, M.; Wang, E.S. Novel therapies for AML: A round-up for clinicians. Expert Rev. Clin. Pharmacol. 2020, 13, 1389–1400. [Google Scholar] [CrossRef]
- Molica, M.; Breccia, M.; Foa, R.; Jabbour, E.; Kadia, T.M. Maintenance therapy in AML: The past, the present and the future. Am. J. Hematol. 2019, 94, 1254–1265. [Google Scholar] [CrossRef]
- Li, K.; Du, Y.; Cai, Y.; Liu, W.; Lv, Y.; Huang, B.; Zhang, L.; Wang, Z.; Liu, P.; Sun, Q.; et al. Single-cell analysis reveals the chemotherapy-induced cellular reprogramming and novel therapeutic targets in relapsed/refractory acute myeloid leukemia. Leukemia 2023, 37, 308–325. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Bhise, N.S.; Lamba, V.; Lamba, J. Microrna Expression and Drug-Induced Changes in Gene Expression Correlate with Ara-C Chemosensitivity in AML Cell Lines. Blood 2014, 124, 3623. [Google Scholar] [CrossRef]
- Wu, B.; Mao, Z.J.; Wang, Z.; Wu, P.; Huang, H.; Zhao, W.; Zhang, L.; Zhang, Z.; Yin, H.; Gale, R.P.; et al. Deoxycytidine Kinase (DCK) Mutations in Human Acute Myeloid Leukemia Resistant to Cytarabine. Acta Haematol. 2021, 144, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Fanciullino, R.; Farnault, L.; Donnette, M.; Imbs, D.-C.; Roche, C.; Venton, G.; Berda-Haddad, Y.; Ivanov, V.; Ciccolini, J.; Ouafik, L.; et al. CDA as a predictive marker for life-threatening toxicities in patients with AML treated with cytarabine. Blood Adv. 2018, 2, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Negoro, E.; Yamauchi, T.; Urasaki, Y.; Nishi, R.; Hori, H.; Ueda, T. Characterization of Cytarabine-Resistant Leukemic Cell Lines Established from Five Different Blood Cell Lineages Using Gene Expression and Proteomic Analyses. Int. J. Oncol. 2011, 38, 911–919. Available online: https://www.spandidos-publications.com/ijo/38/4/911 (accessed on 13 August 2024).
- Abraham, A.; Varatharajan, S.; Karathedath, S.; Philip, C.; Lakshmi, K.M.; Jayavelu, A.K.; Mohanan, E.; Janet, N.B.; Srivastava, V.M.; Shaji, R.V.; et al. RNA Expression of Genes Involved in Cytarabine Metabolism and Transport Predicts Cytarabine Response in Acute Myeloid Leukemia. Pharmacogenomics 2015, 16, 877–890. [Google Scholar] [CrossRef]
- Gabra, M.M.; Salmena, L. microRNAs and Acute Myeloid Leukemia Chemoresistance: A Mechanistic Overview. Front. Oncol. 2017, 7, 255. [Google Scholar] [CrossRef]
- Galmarini, C.M.; Thomas, X.; Calvo, F.; Rousselot, P.; Jafaari, A.E.; Cros, E.; Dumontet, C. Potential mechanisms of resistance to cytarabine in AML patients. Leuk. Res. 2002, 26, 621–629. [Google Scholar] [CrossRef]
- Levin, M.; Stark, M.; Berman, B.; Assaraf, Y.G. Surmounting Cytarabine-resistance in acute myeloblastic leukemia cells and specimens with a synergistic combination of hydroxyurea and azidothymidine. Cell Death Dis. 2019, 10, 390. [Google Scholar] [CrossRef]
- Illangeswaran, R.S.S.; Jebanesan, D.Z.P.; Sivakumar, K.K.; Vidhyadharan, R.T.; Rajamani, B.M.; Janet, N.B.; David, E.; Velayudhan, S.R.; Mathews, V.; Balasubramanian, P. Chemotherapeutic drugs elicit stemness and metabolic alteration to mediate acquired drug-resistant phenotype in acute myeloid leukemia cell lines. Leuk. Res. 2023, 128, 107054. [Google Scholar] [CrossRef]
- Kanno, S.; Hiura, T.; Ohtake, T.; Koiwai, K.; Suzuki, H.; Ujibe, M.; Ishikawa, M. Characterization of resistance to cytosine arabinoside (Ara-C) in NALM-6 human B leukemia cells. Clin. Chim. Acta 2007, 377, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Romero-Trejo, D.; Aguiñiga-Sanchez, I.; Ledesma-Martínez, E.; Weiss-Steider, B.; Sierra-Mondragón, E.; Santiago-Osorio, E. Anti-cancer potential of casein and its derivatives: Novel strategies for cancer treatment. Med. Oncol. 2024, 41, 200. [Google Scholar] [CrossRef] [PubMed]
- Aguiñiga-Sánchez, I.; Meléndez-Ibarra, F.M.; Ledesma-Martínez, E.; Weiss-Steider, B.; Fajardo-Orduña, G.R.; Rangel-Corona, R.; García-Gervasio, S.-N.; Ramírez-Padilla, M.G.; Lara-Castañeda, J.L.; Santiago-Osorio, E. Improved Survival of Leukemic Mice Treated with Sodium Caseinate in Combination with Daunorubicin without Toxicity. J. Oncol. 2021, 2021, 6635650. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Martínez, E.; Pérez-Cordero, C.; Córdova-Galaviz, Y.; Sánchez-Tellez, G.; Huerta-Yepez, S.; Aguiñiga-Sánchez, I.; Miranda-Peralta, E.; Monroy-García, A.; Weiss-Steider, B.; Santiago-Osorio, E. Casein induces the proliferation of bone marrow mononuclear cells, apoptosis of WEHI-3 leukaemic cells and increased survival in a leukaemia mouse model. Oncol. Lett. 2012, 4, 461–466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Córdova-Galaviz, Y.; Ledesma-Martínez, E.; Aguíñiga-Sánchez, I.; Soldevila-Melgarejo, G.; Soto-Cruz, I.; Weiss-Steider, B.; Santiago-Osorio, E. Sodium caseinate induces increased survival in leukaemic mouse J774 model. In Vivo 2014, 28, 819–825. [Google Scholar]
- Aguiñiga-Sanchez, I.; Ledesma-Martínez, E.; Lara-Castañeda, J.L.; Melendez-Ibarra, F.; Weiss-Steider, B.; Soto-Cruz, I.; Fajardo-Orduña, G.; Santiago-Osorio, E. Sodium Caseinate in Combination with Daunorubicin or Cytarabine Improves Survival of Mice with Long-established Leukemia. CDP 2022, 2, 496–502. [Google Scholar] [CrossRef]
- Santiago-Osorio, E.; Ledesma-Martínez, E.; Aguiñiga-Sánchez, I.; Poblano-Pérez, I.; Weiss-Steider, B.; Montesinos-Montesinos, J.J.; de L. Mora-García, M. Sodium Caseinate (CasNa) Induces Mobilization of Hematopoietic Stem Cells in a BALB/c Mouse Model. Med. Sci. Monit. Basic Res. 2015, 21, 206–212. [Google Scholar] [CrossRef]
- Lambarry, B.J. Eje SIRT1-P53 en Resistencia a Citarabina en la Línea de Leucemia Mieloide Aguda WEHI-3; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2020. [Google Scholar]
- NOM-062-ZOO-1999; Specifications for the Care and Handling of Laboratory Animals. Secretary of Health: Mexico City, Mexico, 1999.
- Kuendgen, A.; Germing, U. Emerging treatment strategies for acute myeloid leukemia (AML) in the elderly. Cancer Treat. Rev. 2009, 35, 97–120. [Google Scholar] [CrossRef]
- Fukuda, T.; Kamishima, T.; Kakihara, T.; Ohnishi, Y.; Suzuki, T. Characterization of newly established human myeloid leukemia cell line (KF-19) and its drug resistant sublines. Leuk. Res. 1996, 20, 931–939. [Google Scholar] [CrossRef]
- Legrand, O.; Zittoun, R.; Marie, J.-P. Role of MRP1 in multidrug resistance in acute myeloid leukemia. Leukemia 1999, 13, 578–584. [Google Scholar] [CrossRef]
- McDermott, M.; Eustace, A.J.; Busschots, S.; Breen, L.; Crown, J.; Clynes, M.; O’Donovan, N.; Stordal, B. In Vitro Development of Chemotherapy and Targeted Therapy Drug-Resistant Cancer Cell Lines: A Practical Guide with Case Studies. Front. Oncol. 2014, 4, 40. Available online: https://journal.frontiersin.org/article/10.3389/fonc.2014.00040/abstract (accessed on 13 August 2024). [CrossRef] [PubMed]
- Funato, T.; Harigae, H.; Abe, S.; Sasaki, T. Assessment of drug resistance in acute myeloid leukemia. Expert Rev. Mol. Diagn. 2004, 4, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Ling, V.Y.; Straube, J.; Godfrey, W.; Haldar, R.; Janardhanan, Y.; Cooper, L.; Bruedigam, C.; Cooper, E.; Tavakoli Shirazi, P.; Jacquelin, S.; et al. Targeting cell cycle and apoptosis to overcome chemotherapy resistance in acute myeloid leukemia. Leukemia 2023, 37, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Macanas-Pirard, P.; Broekhuizen, R.; González, A.; Oyanadel, C.; Ernst, D.; García, P.; Montecinos, V.P.; Court, F.; Ocqueteau, M.; Ramirez, P.; et al. Resistance of leukemia cells to cytarabine chemotherapy is mediated by bone marrow stroma, involves cell-surface equilibrative nucleoside transporter-1 removal and correlates with patient outcome. Oncotarget 2017, 8, 23073–23086. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hou, J.; Newman, E.; Kim, Y.; Donohue, C.; Liu, X.; Thomas, S.H.; Forman, S.J.; Kane, S.E. CD30 Downregulation, MMAE Resistance, and MDR1 Upregulation Are All Associated with Resistance to Brentuximab Vedotin. Mol. Cancer Ther. 2015, 14, 1376–1384. [Google Scholar] [CrossRef]
- Yi, Y.; Jia, X.; Zhu, C.; Wang, J.; Chen, J.; Wang, H.; Li, Y. Solanine Reverses Multidrug Resistance in Human Myelogenous Leukemia K562/ADM Cells by Downregulating MRP1 Expression. Oncol. Lett. 2018, 15, 10070–10076. Available online: https://www.spandidos-publications.com/10.3892/ol.2018.8563 (accessed on 13 August 2024). [CrossRef]
- Ling, S.; Li, J.; Shan, Q.; Dai, H.; Lu, D.; Wen, X.; Song, P.; Xie, H.; Zhou, L.; Liu, J.; et al. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol. Oncol. 2017, 11, 682–695. [Google Scholar] [CrossRef]
- Chu, F.; Chou, P.M.; Zheng, X.; Mirkin, B.L.; Rebbaa, A. Control of Multidrug Resistance Gene mdr1 and Cancer Resistance to Chemotherapy by the Longevity Gene sirt1. Cancer Res. 2005, 65, 10183–10187. [Google Scholar] [CrossRef]
- Lotem, J.; Sachs, L. Independent regulation of myeloid cell growth and differentiation inducing proteins: In vivo regulation by compounds that induce inflammation. Int. J. Cancer 1985, 35, 93–100. [Google Scholar] [CrossRef]
- Metcalf, D.; Robb, L.; Dunn, A.R.; Misfud, S.; Di Rago, L. Role of Granulocyte-Macrophage Colony-Stimulating Factor and Granulocyte Colony-Stimulating Factor in the Development of an Acute Neutrophil Inflammatory Response in Mice. Blood 1996, 88, 3755–3764. [Google Scholar] [CrossRef]
- Hatzoglou, A.; Bakogeorgou, E.; Kampa, M.; Panagiotou, S.; Martin, P.M.; Loukas, S.; Castanas, E. Somatostatin and Opioid Receptors in Mammary Tissue. In Biology of the Mammary Gland; Mol, J.A., Clegg, R.A., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2002; Volume 480, pp. 55–63. Available online: http://link.springer.com/10.1007/0-306-46832-8_6 (accessed on 4 September 2024).
- Sahna, K.O.; Cakir, B.; Tunali-Akbay, T. Antiproliferative Activity of Whey and Casein Bioactive Peptides on Breast Cancer: An In Vitro and In Silico Study. Int. J. Pept. Res. Ther. 2022, 28, 128. [Google Scholar] [CrossRef]
- Mori, S.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; Ohmori, H.; Goto, K.; Nakashima, C.; Nishiguchi, Y.; Kawahara, I.; Luo, Y.; et al. Enhancement of Anti-Tumoral Immunity by β-Casomorphin-7 Inhibits Cancer Development and Metastasis of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 8232. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.-T.; Tian, H.; Sun, J.; Zou, J.-B.; Zhang, X.-F.; Cheng, J.-X.; Shi, Y.-J.; Fan, Y.; Guo, D.-Y. Urokinase-type plasminogen activator receptor (uPAR) as a therapeutic target in cancer. J. Transl. Med. 2022, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Wohlfromm, F.; Kähne, T.; Bongartz, H.; Seyrek, K.; Kit, Y.; Chinak, O.; Richter, V.A.; Koval, O.A.; Lavrik, I.N. The Recombinant Fragment of Human κ-Casein Induces Cell Death by Targeting the Proteins of Mitochondrial Import in Breast Cancer Cells. Cancers 2020, 12, 1427. [Google Scholar] [CrossRef]
- Ovcherenko, S.S.; Chinak, O.A.; Chechushkov, A.V.; Dobrynin, S.A.; Kirilyuk, I.A.; Krumkacheva, O.A.; Richter, V.A.; Bagryanskaya, E.G. Uptake of Cell-Penetrating Peptide RL2 by Human Lung Cancer Cells: Monitoring by Electron Paramagnetic Resonance and Confocal Laser Scanning Microscopy. Molecules 2021, 26, 5442. [Google Scholar] [CrossRef]




| Gen | Forward Sequences (5′→3′) | Reverse Sequences (5′→3′) |
|---|---|---|
| ENT1 | 5′-CTGGAAAGGCGTAGAGGCTG-3′ | 5′-CTTCCCTTCGCAGACTGCTT-3′ |
| dCK | 5′-AGCAGTGAGTCTGGAGGTAG-3′ | 5′-GAGAAGGCAGAGAAGGCTGG-3′ |
| SIRT1 | 5′-CGGCTACCGAGGTCCATATAC-3′ | 5′-CAGCTCAGGTGGAGGAATTGT-3′ |
| MDR1 | 5′-GTGGTGTCATTGTGGAGCAAG-3′ | 5′-GCATCAGTGTCACTCTGGGATC-3′ |
| MRP1 | 5′-CAGTGGTTCAGGGAAGGATTTA-3′ | 5′-CACTGTGGGAAGACGAGTTGCT-3′ |
| β-actin | 5′-CACTGTCGAGTCGCGTCC-3′ | 5′-CGCAGCGATATCGTCATCCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiñiga-Sánchez, I.; Ledesma-Martínez, E.; Vázquez-Guerrero, M.; Hernández-Álvarez, D.; Velasco-García, A.; Rodríguez-Terán, K.M.; Romero-Trejo, D.; Mendoza-Núñez, V.M.; Macías-Zaragoza, V.M.; Santiago-Osorio, E. Antineoplastic Activity of Sodium Caseinate in a Cytarabine-Resistant Mouse Acute Myeloid Leukemia Cell Line. Nutrients 2024, 16, 3190. https://doi.org/10.3390/nu16183190
Aguiñiga-Sánchez I, Ledesma-Martínez E, Vázquez-Guerrero M, Hernández-Álvarez D, Velasco-García A, Rodríguez-Terán KM, Romero-Trejo D, Mendoza-Núñez VM, Macías-Zaragoza VM, Santiago-Osorio E. Antineoplastic Activity of Sodium Caseinate in a Cytarabine-Resistant Mouse Acute Myeloid Leukemia Cell Line. Nutrients. 2024; 16(18):3190. https://doi.org/10.3390/nu16183190
Chicago/Turabian StyleAguiñiga-Sánchez, Itzen, Edgar Ledesma-Martínez, Mariana Vázquez-Guerrero, David Hernández-Álvarez, Amanda Velasco-García, Katia Michell Rodríguez-Terán, Daniel Romero-Trejo, Víctor Manuel Mendoza-Núñez, Víctor Manuel Macías-Zaragoza, and Edelmiro Santiago-Osorio. 2024. "Antineoplastic Activity of Sodium Caseinate in a Cytarabine-Resistant Mouse Acute Myeloid Leukemia Cell Line" Nutrients 16, no. 18: 3190. https://doi.org/10.3390/nu16183190
APA StyleAguiñiga-Sánchez, I., Ledesma-Martínez, E., Vázquez-Guerrero, M., Hernández-Álvarez, D., Velasco-García, A., Rodríguez-Terán, K. M., Romero-Trejo, D., Mendoza-Núñez, V. M., Macías-Zaragoza, V. M., & Santiago-Osorio, E. (2024). Antineoplastic Activity of Sodium Caseinate in a Cytarabine-Resistant Mouse Acute Myeloid Leukemia Cell Line. Nutrients, 16(18), 3190. https://doi.org/10.3390/nu16183190





