Yoyo Dieting, Post-Obesity Weight Loss, and Their Relationship with Gut Health
Abstract
1. Introduction
1.1. Yoyo Dieting: Cyclic Weight Regain after Weight Loss
1.2. The Relationship between Obesity, the Gut, and the Susceptibility to Weight Regain Is Potentially Due to Changes in the Gut Microbiota
2. The Influence of the Gut in Weight Regain after Weight Loss
2.1. Gut Peptide Hormones That Regulate Energy Balance
2.2. Sympathetic Nervous System Regulates Energy Expenditure and Thermogenesis as a Metabolic Adaptation to Weight Loss
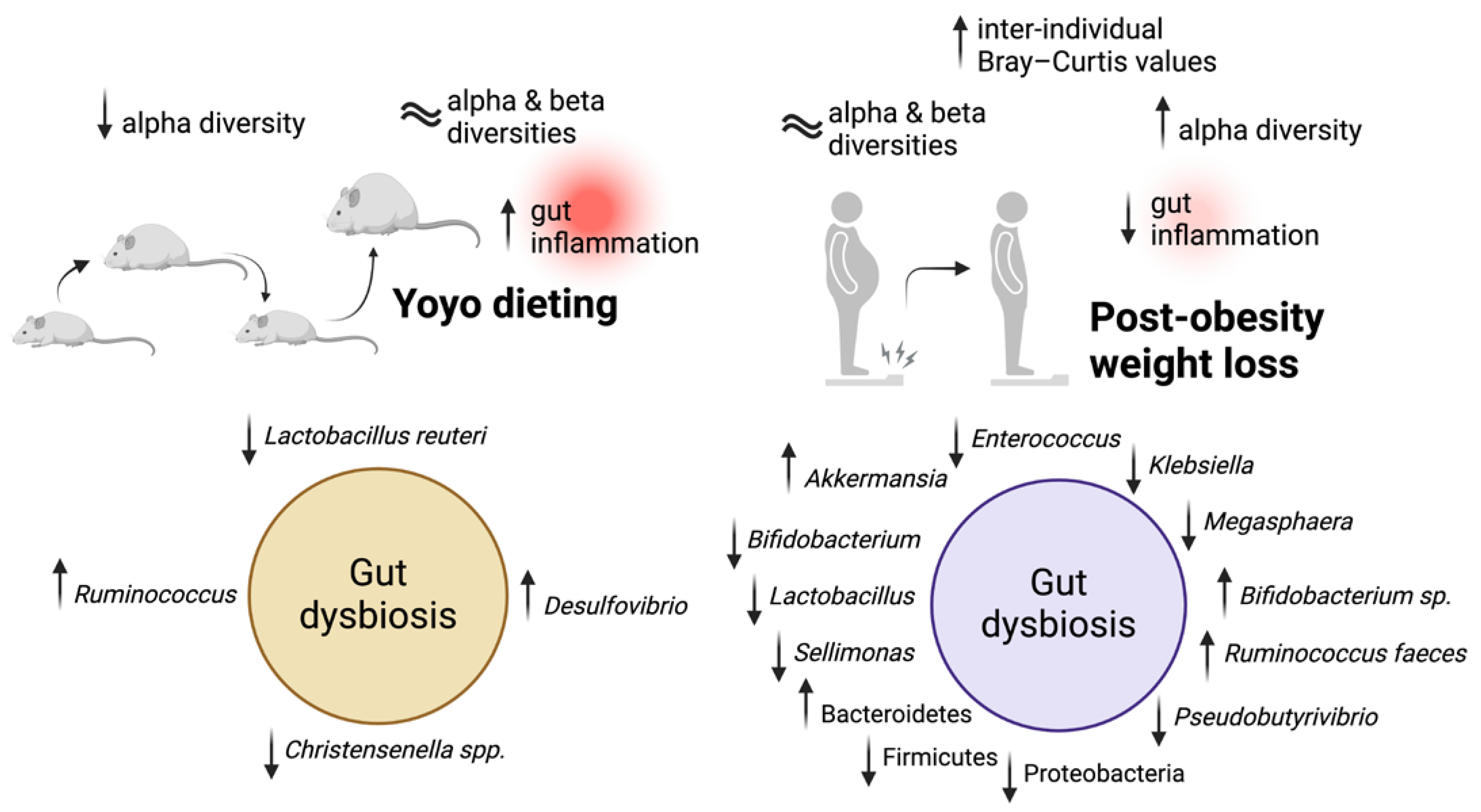
3. Yoyo Dieting and Gut Inflammation
3.1. Links between Yoyo Dieting and Gut Inflammation
3.2. Post-Obesity Weight Loss as a Potential Approach to Reduce Gut Inflammation in Humans
4. Yoyo Dieting and Gut Microbiome
4.1. Different Gut Microbiota Profiles in Yoyo Dieting
4.2. Will the Gut Microbiome Be the Next Target to Prevent Weight Regain?
4.2.1. Dietary Interventions Enhance Weight Loss and Alter Gut Microbiota in Animal Studies
4.2.2. Post-Obesity Weight Loss May Be Beneficial in Improving Gut Microbiota in Humans
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, V.S.; Willet, W.C.; Hu, F.B. Nearly a decade on—Trends, risk factors and policy implications in global obesity. Nat. Rev. Endocrinol. 2020, 16, 615–616. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Obesity and Overweight; World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- Barber, T.M. Is obesity a disease? Expert Rev. Endocrinol. Metab. 2018, 13, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.M.; Campbell-Scherer, D.L. Redefining obesity: Beyond the numbers. Obesity 2017, 25, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Bixby, H.; Bentham, J.; Zhou, B.; Di Cesare, M.; Paciorek, C.J.; Bennett, J.E.; Taddei, C.; Stevens, G.A.; Rodriguez-Martinez, A.; Carrillo-Larco, R.M.; et al. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature 2019, 569, 260–264. [Google Scholar]
- World Health Organization. Obesity; World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2017, 34, 575–584. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and nonalcoholic fatty liver disease: Current perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 533–542. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing The Global Epidemic; World Health Organisation: Geneva, Switzerland, 2000. [Google Scholar]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Júnior, W.S.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhu, L.; Yang, L. Gut and obesity/metabolic disease: Focus on microbiota metabolites. Medcomm 2022, 3, e171. [Google Scholar] [CrossRef]
- Velapati, S.R.; Shah, M.; Kuchkuntla, A.R.; Abu-Dayyeh, B.; Grothe, K.; Hurt, R.T.; Mundi, M.S. Weight Regain After Bariatric Surgery: Prevalence, Etiology, and Treatment. Curr. Nutr. Rep. 2018, 7, 329–334. [Google Scholar] [CrossRef]
- Kraschnewski, J.L.; Boan, J.; Esposito, J.; E Sherwood, N.; Lehman, E.B.; Kephart, D.K.; Sciamanna, C.N. Long-term weight loss maintenance in the United States. Int. J. Obes. 2010, 34, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Neumark-Sztainer, D.; Rock, C.L.; Thornquist, M.D.; Cheskin, L.J.; Neuhouser, M.L.; Barnett, M.J. Weight-Control Behaviors among Adults and Adolescents: Associations with Dietary Intake. Prev. Med. 2000, 30, 381–391. [Google Scholar] [CrossRef]
- Jeffery, R.W.; Adlis, S.A.; Forster, J.L. Prevalence of dieting among working men and women: The healthy worker project. Health Psychol. 1991, 10, 274. [Google Scholar] [CrossRef]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579–584. [Google Scholar] [CrossRef]
- Weiss, E.C.; Galuska, D.A.; Khan, L.K.; Gillespie, C.; Serdula, M.K. Weight regain in US adults who experienced substantial weight loss, 1999–2002. Am. J. Prev. Med. 2007, 33, 34–40. [Google Scholar] [CrossRef]
- Bacon, L.; Aphramor, L. Weight Science: Evaluating the Evidence for a Paradigm Shift. Nutr. J. 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Tomiyama, A.J.; Westling, E.; Lew, A.-M.; Samuels, B.; Chatman, J. Medicare’s search for effective obesity treatments: Diets are not the answer. Am. Psychol. 2007, 62, 220. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, C.A.; Karfopoulou, E.; Yannakoulia, M. Weight regaining: From statistics and behaviors to physiology and metabolism. Metabolism 2015, 64, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef]
- Jimenez, L.S.; Chaim, F.H.M.; Chaim, F.D.M.; Utrini, M.P.; Gestic, M.A.; Chaim, E.A.; Cazzo, E. Impact of Weight Regain on the Evolution of Non-alcoholic Fatty Liver Disease After Roux-en-Y Gastric Bypass: A 3-Year Follow-up. Obes. Surg. 2018, 28, 3131–3135. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Zulet, M.A.; Lopez-Legarrea, P.; de la Iglesia, R.; Pardo, M.; Carreira, M.C.; Martínez, J.A.; Casanueva, F.F. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism 2014, 63, 520–531. [Google Scholar] [CrossRef]
- Lien, L.F.; Haqq, A.M.; Arlotto, M.; Slentz, C.A.; Muehlbauer, M.J.; McMahon, R.L.; Rochon, J.; Gallup, D.; Bain, J.R.; Ilkayeva, O.; et al. The STEDMAN project: Biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. OMICS J. Integr. Biol. 2009, 13, 21–35. [Google Scholar] [CrossRef]
- Wang, P.; Holst, C.; Wodzig, W.K.W.H.; Andersen, M.R.; Astrup, A.; van Baak, M.A.; Larsen, T.M.; Jebb, S.A.; Kafatos, A.; Pfeiffer, A.F.H.; et al. Circulating ACE is a predictor of weight loss maintenance not only in overweight and obese women, but also in men. Int. J. Obes. 2012, 36, 1545–1551. [Google Scholar] [CrossRef]
- Linna, M.S.; Borg, P.; Kukkonen-Harjula, K.; Fogelholm, M.; Nenonen, A.; Ahotupa, M.; Vasankari, T.J. Successful weight maintenance preserves lower levels of oxidized LDL achieved by weight reduction in obese men. Int. J. Obes. 2006, 31, 245–253. [Google Scholar] [CrossRef]
- Thomas, T.R.; Warner, S.O.; Dellsperger, K.C.; Hinton, P.S.; Whaley-Connell, A.T.; Rector, R.S.; Liu, Y.; Linden, M.A.; Chockalingam, A.; Thyfault, J.P.; et al. Exercise and the metabolic syndrome with weight regain. J. Appl. Physiol. 2010, 109, 3–10. [Google Scholar] [CrossRef]
- Delbridge, E.A.; Prendergast, L.A.; Pritchard, J.E.; Proietto, J. One-year weight maintenance after significant weight loss in healthy overweight and obese subjects: Does diet composition matter? Am. J. Clin. Nutr. 2009, 90, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Kato, Y.; Murotake, Y.; Kim, M.-K.; Unno, H.; Tanaka, K. An increase in high-density lipoprotein cholesterol after weight loss intervention is associated with long-term maintenance of reduced visceral abdominal fat. Int. J. Obes. 2010, 34, 1742–1751. [Google Scholar] [CrossRef]
- Brunner, K.T.; Henneberg, C.J.; Wilechansky, R.M.; Long, M.T. Nonalcoholic Fatty Liver Disease and Obesity Treatment. Curr. Obes. Rep. 2019, 8, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, S.J.; Gershuni, V.M.; Hazbun, T.L.; Athinarayanan, S.J. Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients 2019, 11, 766. [Google Scholar] [CrossRef]
- Jeong, S.; Choi, S.; Chang, J.; Kim, K.; Kim, S.M.; Hwang, S.Y.; Son, J.S.; Lee, G.; Park, S.M. Association of weight fluctuation with cardiovascular disease risk among initially obese adults. Sci. Rep. 2021, 11, 10152. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Enderle, J.; Bosy-Westphal, A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr. Obes. Rep. 2016, 5, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Brownell, K.D.; Rodin, J. Medical, Metabolic, and Psychological Effects of Weight Cycling. Arch. Intern. Med. 1994, 154, 1325–1330. [Google Scholar] [CrossRef]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2017, 102, 183–197. [Google Scholar] [CrossRef]
- MacLean, P.S.; Higgins, J.A.; Johnson, G.C.; Fleming-Elder, B.K.; Peters, J.C.; Hill, J.O. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R288–R297. [Google Scholar] [CrossRef]
- Field, A.; Wing, R.; Manson, J.; Spiegelman, D.; Willett, W. Relationship of a large weight loss to long-term weight change among young and middle-aged US women. Int. J. Obes. 2001, 25, 1113–1121. [Google Scholar] [CrossRef][Green Version]
- Sarlio-Lähteenkorva, S.; Rissanen, A.; Kaprio, J. A descriptive study of weight loss maintenance: 6 and 15 year follow-up of initially overweight adults. Int. J. Obes. 2000, 24, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Strohacker, K.; Carpenter, K.C.; McFarlin, B.K. Consequences of Weight Cycling: An Increase in Disease Risk? Int. J. Exerc. Sci. 2009, 2, 191–201. [Google Scholar] [CrossRef]
- Simonds, S.E.; Pryor, J.T.; Cowley, M.A. Repeated weight cycling in obese mice causes increased appetite and glucose intolerance. Physiol. Behav. 2018, 194, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Strandberg, T.; Pentti, J.; Nyberg, S.T.; Frank, P.; Jokela, M.; Ervasti, J.; Suominen, S.B.; Vahtera, J.; Sipilä, P.N.; et al. Body-mass index and risk of obesity-related complex multimorbidity: An observational multicohort study. Lancet Diabetes Endocrinol. 2022, 10, 253–263. [Google Scholar] [CrossRef]
- Mikolajczyk, R.T.; E Maxwell, A.; El Ansari, W.; Stock, C.; Petkeviciene, J.; Guillen-Grima, F. Relationship between perceived body weight and body mass index based on self- reported height and weight among university students: A cross-sectional study in seven European countries. BMC Public Health 2010, 10, 40. [Google Scholar] [CrossRef]
- Haakstad, L.A.H.; Stensrud, T.; Rugseth, G.; Gjestvang, C. Weight Cycling and Dieting Behavior in Fitness Club Members. Front. Endocrinol. 2022, 13, 851887. [Google Scholar] [CrossRef] [PubMed]
- Lakicevic, N.; Mani, D.; Paoli, A.; Roklicer, R.; Bianco, A.; Drid, P. Weight cycling in combat sports: Revisiting 25 years of scientific evidence. BMC Sports Sci. Med. Rehabil. 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Alwan, N.; Moss, S.L.; Davies, I.G.; Elliott-Sale, K.J.; Enright, K. Weight loss practices and eating behaviours among female physique athletes: Acquiring the optimal body composition for competition. PLoS ONE 2022, 17, e0262514. [Google Scholar] [CrossRef]
- Miles-Chan, J.L.; Isacco, L. Weight cycling practices in sport: A risk factor for later obesity? Obes. Rev. 2021, 22 (Suppl. S2), e13188. [Google Scholar] [CrossRef]
- Rhee, E.-J. Weight Cycling and Its Cardiometabolic Impact. J. Obes. Metab. Syndr. 2017, 26, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Kakinami, L.; Knäuper, B.; Brunet, J. Weight cycling is associated with adverse cardiometabolic markers in a cross-sectional representative US sample. J. Epidemiol. Community Health 2020, 74, 662–667. [Google Scholar] [CrossRef]
- Zou, H.; Yin, P.; Liu, L.; Duan, W.; Li, P.; Yang, Y.; Li, W.; Zong, Q.; Yu, X. Association between weight cycling and risk of developing diabetes in adults: A systematic review and meta-analysis. J. Diabetes Investig. 2021, 12, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Park, K.Y.; Hwang, H.S.; Cho, K.H.; Han, K.; Nam, G.E.; Kim, Y.H.; Kwon, Y.; Park, Y.G. Body Weight Fluctuation as a Risk Factor for Type 2 Diabetes: Results from a Nationwide Cohort Study. J. Clin. Med. 2019, 8, 950. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.J.; Moon, J.H.; Choi, S.H.; Lim, S.; Park, K.S.; Cho, N.H.; Jang, H.C. Body-Weight Fluctuation and Incident Diabetes Mellitus, Cardiovascular Disease, and Mortality: A 16-Year Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2018, 104, 639–646. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Kim, M.-H.; Yun, K.E.; Kim, J.; Park, E.; Chang, Y.; Ryu, S.; Kim, H.-L.; Kim, H.-N. Gut microbiota and metabolic health among overweight and obese individuals. Sci. Rep. 2020, 10, 19417. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Itav, S.; Rothschild, D.; Meijer, M.T.; Levy, M.; Moresi, C.; Dohnalová, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Humblot, C.; Seyoum, Y.; Turpin, W.; Mrabt, R.; List, E.O.; Berryman, D.E.; Jensen, E.A.; Sustarsic, E.G.; Kopchick, J.J.; Ricort, J. Long Term Weight Cycling Affects Fecal Microbiota of Mice. Mol. Nutr. Food Res. 2022, 66, 2200439. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Chanda, D.; De, D. Meta-analysis reveals obesity associated gut microbial alteration patterns and reproducible contributors of functional shift. Gut Microbes 2024, 16, 2304900. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef]
- Ayogu, R.N.B.; Oshomegie, H.; Udenta, E.A. Energy intake, expenditure and balance, and factors associated with energy balance of young adults (20–39 years): A retrospective cross-sectional community-based cohort study. BMC Nutr. 2022, 8, 142. [Google Scholar] [CrossRef]
- Mietlicki-Baase, E.G.; Hayes, M.R. Gut Hormones and Obesity. In Metabolic Syndrome: A Comprehensive Textbook; Ahima, R.S., Ed.; Springer International Publishing: Cham, Switerzland, 2014; pp. 1–28. [Google Scholar]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef]
- Méquinion, M.; Foldi, C.J.; Andrews, Z.B. The Ghrelin-AgRP Neuron Nexus in Anorexia Nervosa: Implications for Metabolic and Behavioral Adaptations. Front. Nutr. 2019, 6, 190. [Google Scholar] [CrossRef]
- Ye, L.; Liddle, R.A. Gastrointestinal hormones and the gut connectome. Curr. Opin. Endocrinol. Diabetes 2017, 24, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.M.; Park, S.J.; Valinsky, W.C.; Beyak, M.J. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J. Physiol. 2011, 589, 2857–2870. [Google Scholar] [CrossRef]
- Egerod, K.L.; Engelstoft, M.S.; Grunddal, K.V.; Nøhr, M.K.; Secher, A.; Sakata, I.; Pedersen, J.; Windeløv, J.A.; Füchtbauer, E.-M.; Olsen, J.; et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 2012, 153, 5782–5795. [Google Scholar] [CrossRef]
- Dockray, G.J. Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 8–12. [Google Scholar] [CrossRef]
- Buchman, A.L.; Katz, S.; Fang, J.C.; Bernstein, C.N.; Abou-Assi, S.G.; Group, T.S. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Tang-Christensen, M.; Vrang, N.; Larsen, P. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int. J. Obes. 2001, 25, S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Malkova, D. Altered gut and adipose tissue hormones in overweight and obese individuals: Cause or consequence? Int. J. Obes. 2016, 40, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Akalu, Y.; Molla, M.D.; Dessie, G.; Ayelign, B. Physiological Effect of Ghrelin on Body Systems. Int. J. Endocrinol. 2020, 2020, 1385138. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Woodward, O.R.M.; Gribble, F.M.; Reimann, F.; Lewis, J.E. Gut peptide regulation of food intake—Evidence for the modulation of hedonic feeding. J. Physiol. 2022, 600, 1053–1078. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the endocrine control of energy balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef]
- Chan, J.L.; Heist, K.; DePaoli, A.M.; Veldhuis, J.D.; Mantzoros, C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 2003, 111, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Zurita-Cruz, J.N.; Villasís-Keever, M.A.; Manuel-Apolinar, L.; Damasio-Santana, L.; Garrido-Magaña, E.; Rivera-Hernández, A.d.J. Leptin/adiponectin ratio as a prognostic factor for increased weight gain in girls with central precocious puberty. Front. Endocrinol. 2023, 14, 1101399. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K.; Fath, M.A.; Seo, S.; Thedens, D.R.; Berry, C.J.; Weiss, R.; Nishimura, D.Y.; Sheffield, V.C. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J. Clin. Investig. 2008, 118, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Kos, K.; Harte, A.L.; da Silva, N.F.; Tonchev, A.; Chaldakov, G.; James, S.; Snead, D.R.; Hoggart, B.; O’hare, J.P.; McTernan, P.G.; et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J. Clin. Endocrinol. Metab. 2007, 92, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Alkaabi, J.; Yasin, J.; Al Essa, A. Total adiponectin in overweight and obese subjects and its response to visceral fat loss. BMC Endocr. Disord. 2019, 19, 55. [Google Scholar] [CrossRef]
- Reinehr, T.; de Sousa, G.; Niklowitz, P.; Roth, C.L. Amylin and its relation to insulin and lipids in obese children before and after weight loss. Obesity 2007, 15, 2006–2011. [Google Scholar] [CrossRef]
- Strohacker, K.; McCaffery, J.M.; MacLean, P.S.; Wing, R.R. Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: A review of current literature. Int. J. Obes. 2013, 38, 388–396. [Google Scholar] [CrossRef]
- Zhang, Q.; Delessa, C.T.; Wolfrum, C. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 2021, 33, 833–844.e5. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Weiss, E.P.; Albert, S.G.; Reeds, D.N.; Kress, K.S.; Ezekiel, U.R.; McDaniel, J.L.; Patterson, B.W.; Klein, S.; Villareal, D.T. Calorie Restriction and Matched Weight Loss From Exercise: Independent and Additive Effects on Glucoregulation and the Incretin System in Overweight Women and Men. Diabetes Care 2015, 38, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Batterham, R.L.; Aylwin, S.J.B.; Patterson, M.; Borg, C.M.; Wynne, K.J.; Kent, A.; Vincent, R.P.; Gardiner, J.; Ghatei, M.A.; et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 2006, 147, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Chearskul, S.; Delbridge, E.; Shulkes, A.; Proietto, J.; Kriketos, A. Effect of weight loss and ketosis on postprandial cholecystokinin and free fatty acid concentrations. Am. J. Clin. Nutr. 2008, 87, 1238–1246. [Google Scholar] [CrossRef]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The role of diet in shaping human gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101828. [Google Scholar] [CrossRef]
- Ojo, O.; Feng, Q.-Q.; Ojo, O.O.; Wang, X.-H. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-T.; Tsai, Y.-H.; Liou, C.-W.; Fan, C.-H.; Hou, Y.-T.; Yao, T.-H.; Chuang, H.-L.; Wu, W.-L. The gut microbiota modulate locomotion via vagus-dependent glucagon-like peptide-1 signaling. NPJ Biofilms Microbiomes 2024, 10, 2. [Google Scholar] [CrossRef]
- Degen, L.; Drewe, J.; Piccoli, F.; Gräni, K.; Oesch, S.; Bunea, R.; D’Amato, M.; Beglinger, C. Effect of CCK-1 receptor blockade on ghrelin and PYY secretion in men. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R1391–R1399. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Zinina, V.V.; Ruehle, F.; Winkler, P.; Rebmann, L.; Lukas, H.; Möckel, S.; Diefenbach, A.; Mendez-Lago, M.; Soshnikova, N. ID2 controls differentiation of enteroendocrine cells in mouse small intestine. Acta Physiol. 2022, 234, e13773. [Google Scholar] [CrossRef]
- Frias, A.B.; Hyzny, E.J.; Buechel, H.M.; Beppu, L.Y.; Xie, B.; Jurczak, M.J.; D’cruz, L.M. The Transcriptional Regulator Id2 Is Critical for Adipose-Resident Regulatory T Cell Differentiation, Survival, and Function. J. Immunol. 2019, 203, 658–664. [Google Scholar] [CrossRef]
- Hou, T.Y.; Ward, S.M.; Murad, J.M.; Watson, N.P.; Israel, M.A.; Duffield, G.E. ID2 (inhibitor of DNA binding 2) is a rhythmically expressed transcriptional repressor required for circadian clock output in mouse liver. J. Biol. Chem. 2009, 284, 31735–31745. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Moran, A.W.; Burdyga, G.; Meyer-Gerspach, A.C.; Peterli, R.; Manz, M.; Thumshirn, M.; Daly, K.; Beglinger, C.; Shirazi-Beechey, S.P. Deregulation of transcription factors controlling intestinal epithelial cell differentiation; a predisposing factor for reduced enteroendocrine cell number in morbidly obese individuals. Sci. Rep. 2017, 7, 8174. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef]
- Ducastel, S.; Touche, V.; Trabelsi, M.-S.; Boulinguiez, A.; Butruille, L.; Nawrot, M.; Peschard, S.; Chávez-Talavera, O.; Dorchies, E.; Vallez, E.; et al. The nuclear receptor FXR inhibits Glucagon-Like Peptide-1 secretion in response to microbiota-derived Short-Chain Fatty Acids. Sci. Rep. 2020, 10, 174. [Google Scholar] [CrossRef]
- Cawthon, C.R.; de La Serre, C.B. The critical role of CCK in the regulation of food intake and diet-induced obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- Anderson, E.M.; Rozowsky, J.M.; Fazzone, B.J.; Schmidt, E.A.; Stevens, B.R.; O’malley, K.A.; Scali, S.T.; Berceli, S.A. Temporal Dynamics of the Intestinal Microbiome Following Short-Term Dietary Restriction. Nutrients 2022, 14, 2785. [Google Scholar] [CrossRef]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Büttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D.; et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef]
- Valenzano, A.; Tartaglia, N.; Ambrosi, A.; Tafuri, D.; Monda, M.; Messina, A.; Sessa, F.; Campanozzi, A.; Monda, V.; Cibelli, G.; et al. The Metabolic Rearrangements of Bariatric Surgery: Focus on Orexin-A and the Adiponectin System. J. Clin. Med. 2020, 9, 3327. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.P.; Miras, A.D.; Scholtz, S.; Jackson, S.; Neff, K.J.; Pénicaud, L.; Geoghegan, J.; Chhina, N.; Durighel, G.; Bell, J.D.; et al. Link Between Increased Satiety Gut Hormones and Reduced Food Reward After Gastric Bypass Surgery for Obesity. J. Clin. Endocrinol. Metab. 2016, 101, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Laferrère, B.; Pattou, F. Weight-Independent Mechanisms of Glucose Control After Roux-en-Y Gastric Bypass. Front. Endocrinol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Kim, K.-S.; Peck, B.C.; Hung, Y.-H.; Koch-Laskowski, K.; Wood, L.; Dedhia, P.H.; Spence, J.R.; Seeley, R.J.; Sethupathy, P.; Sandoval, D.A. Vertical sleeve gastrectomy induces enteroendocrine cell differentiation of intestinal stem cells through bile acid signaling. J. Clin. Investig. 2022, 7, e154302. [Google Scholar] [CrossRef]
- Abdeen, G.; le Roux, C. Mechanism Underlying the Weight Loss and Complications of Roux-en-Y Gastric Bypass. Rev. Obes. Surg. 2015, 26, 410–421. [Google Scholar] [CrossRef]
- le Roux, C.W.; Welbourn, R.; Werling, M.; Osborne, A.; Kokkinos, A.; Laurenius, A.; Lönroth, H.; Fändriks, L.; Ghatei, M.A.; Bloom, S.R. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007, 246, 780–785. [Google Scholar] [CrossRef]
- Guida, C.; Stephen, S.D.; Watson, M.; Dempster, N.; Larraufie, P.; Marjot, T.; Cargill, T.; Rickers, L.; Pavlides, M.; Tomlinson, J.; et al. PYY plays a key role in the resolution of diabetes following bariatric surgery in humans. eBioMedicine 2019, 40, 67–76. [Google Scholar] [CrossRef]
- Alexiadou, K.; Tan, T.M.-M. Gastrointestinal Peptides as Therapeutic Targets to Mitigate Obesity and Metabolic Syndrome. Curr. Diabetes Rep. 2020, 20, 26. [Google Scholar] [CrossRef]
- Alruwaili, H.; Dehestani, B.; le Roux, C.W. Clinical Impact of Liraglutide as a Treatment of Obesity. Clin. Pharmacol. Adv. Appl. 2021, 13, 53–60. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.W.; Le Roux, C.W.; Ortiz, R.V.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Singh, G.; Krauthamer, M.; Bjalme-Evans, M. Wegovy (Semaglutide): A New Weight Loss Drug for Chronic Weight Management. J. Investig. Med. 2022, 70, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Landó, L.F.; Mao, H.; Cui, X.; A Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.R.; Lee, C.P.; Lam, K.S.L.; Ho, P.C. 31—Anti-Obesity Drugs for Obese Women Planning Pregnancy. In Obesity; Mahmood, T., Arulkumaran, S., Eds.; Elsevier: Oxford, UK, 2013; pp. 423–430. [Google Scholar]
- Kwon, Y.-J.; Kwon, G.E.; Lee, H.S.; Choi, M.H.; Lee, J.-W. The Effect of Orlistat on Sterol Metabolism in Obese Patients. Front. Endocrinol. 2022, 13, 824269. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, J. Orlistat in the long-term treatment of obesity in primary care settings. Arch. Fam. Med. 2000, 9, 160–167. [Google Scholar] [CrossRef]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological management of obesity: An endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef]
- Lee, C.; Dixon, J. Pharmacotherapy for obesity. Aust. Fam. Physician 2017, 46, 472–477. [Google Scholar]
- Colbourne, J.R.M.; Fisher, O.M.; Mo, S.; Rigas, G.S.; Talbot, M.L. The role of adjuvant pharmacotherapy with liraglutide for patients with inadequate weight loss following bariatric surgery. Langenbeck’s Arch. Surg. 2023, 408, 115. [Google Scholar] [CrossRef]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar]
- Ghusn, W.; De la Rosa, A.; Sacoto, D.; Cifuentes, L.; Campos, A.; Feris, F.; Hurtado, M.D.; Acosta, A. Weight Loss Outcomes Associated With Semaglutide Treatment for Patients with Overweight or Obesity. JAMA Netw. Open 2022, 5, e2231982. [Google Scholar] [CrossRef]
- Landsberg, L. Insulin-mediated sympathetic stimulation: Role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J. Hypertens. 2001, 19, 523–528. [Google Scholar] [CrossRef]
- Cero, C.; Lea, H.J.; Zhu, K.Y.; Shamsi, F.; Tseng, Y.-H.; Cypess, A.M. β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. J. Clin. Investig. 2021, 6, e139160. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.M.; Ahmadian, M.; Keinan, O.; Abu-Odeh, M.; Zhao, P.; Zhou, X.; Keller, M.P.; Gao, H.; Yu, R.T.; Liddle, C.; et al. β3-Adrenergic receptor downregulation leads to adipocyte catecholamine resistance in obesity. J. Clin. Investig. 2022, 132, e153357. [Google Scholar] [CrossRef] [PubMed]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011, 214, 242–253. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Virtanen, K.A.; Richard, D.; Haman, F.; Turcotte, É.E. Brown Adipose Tissue Energy Metabolism in Humans. Front. Endocrinol. 2018, 9, 447. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; You, Y.; Meng, M.; Zheng, Z.; Dong, M.; Lin, J.; Zhao, Q.; Zhang, C.; Yuan, X.; et al. Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology 2015, 156, 2461–2469. [Google Scholar] [CrossRef]
- Goele, K.; Bosy-Westphal, A.; Rümcker, B.; Lagerpusch, M.; Müller, M.J. Influence of changes in body composition and adaptive thermogenesis on the difference between measured and predicted weight loss in obese women. Obes. Facts 2009, 2, 6. [Google Scholar] [CrossRef]
- Byrne, N.M.; Wood, R.E.; Schutz, Y.; Hills, A.P. Does metabolic compensation explain the majority of less-than-expected weight loss in obese adults during a short-term severe diet and exercise intervention? Int. J. Obes. 2012, 36, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Spiegelman, B.M. Towards a molecular understanding of adaptive thermogenesis. Nature 2000, 404, 652–660. [Google Scholar] [CrossRef]
- Müller, M.; Bosy-Westphal, A. Adaptive thermogenesis with weight loss in humans. Obesity 2013, 21, 218–228. [Google Scholar] [CrossRef]
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell death and inflammation during obesity: “Know my methods, WAT(son)”. Cell Death Differ. 2022, 30, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-E.; Kehlenbrink, S.; Lee, H.; Hawkins, M.; Yudkin, J.S. Getting the message across: Mechanisms of physiological cross talk by adipose tissue. Am. J. Physiol. Metab. 2009, 296, E1210–E1229. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef]
- Trayhurn, P.; Bing, C.; Wood, I.S. Adipose Tissue and Adipokines—Energy Regulation from the Human Perspective. J. Nutr. 2006, 136, 1935S–1939S. [Google Scholar] [CrossRef]
- Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.J.; Magness, S.; Jobin, C.; Lund, P.K. High-Fat Diet: Bacteria Interactions Promote Intestinal Inflammation Which Precedes and Correlates with Obesity and Insulin Resistance in Mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef]
- Alzamil, H. Elevated Serum TNF-α Is Related to Obesity in Type 2 Diabetes Mellitus and Is Associated with Glycemic Control and Insulin Resistance. J. Obes. 2020, 2020, 5076858. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines IL-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.K.; Bunkin, D.A.; Greenberg, A.S. Omental and Subcutaneous Adipose Tissues of Obese Subjects Release Interleukin-6: Depot Difference and Regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 1998, 83, 847–850. [Google Scholar] [CrossRef]
- El-Mikkawy, D.M.E.; El-Sadek, M.A.; El-Badawy, M.A.; Samaha, D. Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egypt. Rheumatol. Rehabil. 2020, 47, 7. [Google Scholar] [CrossRef]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.-P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef]
- Sougiannis, A.T.; VanderVeen, B.N.; Cranford, T.L.; Enos, R.T.; Velazquez, K.T.; McDonald, S.; Bader, J.E.; Chatzistamou, I.; Fan, D.; Murphy, E.A. Impact of weight loss and partial weight regain on immune cell and inflammatory markers in adipose tissue in male mice. J. Appl. Physiol. 2020, 129, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Tareen, S.H.K.; Kutmon, M.; de Kok, T.M.; Mariman, E.C.M.; van Baak, M.A.; Evelo, C.T.; Adriaens, M.E.; Arts, I.C.W. Stratifying cellular metabolism during weight loss: An interplay of metabolism, metabolic flexibility and inflammation. Sci. Rep. 2020, 10, 1651. [Google Scholar] [CrossRef]
- Caslin, H.L.; Cottam, M.A.; Piñon, J.M.; Boney, L.Y.; Hasty, A.H. Weight cycling induces innate immune memory in adipose tissue macrophages. Front. Immunol. 2023, 13, 984859. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Lund, P.K. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Fuchs, R.; Müller, R.L.; Keller, L.; Baumann, Z.; Bosch, A.J.T.; Schneider, R.; Labes, D.; Langer, I.; Pilz, J.B.; et al. Obesity in Humans Is Characterized by Gut Inflammation as Shown by Pro-Inflammatory Intestinal Macrophage Accumulation. Front. Immunol. 2021, 12, 668654. [Google Scholar] [CrossRef]
- Khan, S.; Luck, H.; Winer, S.; Winer, D.A. Emerging concepts in intestinal immune control of obesity-related metabolic disease. Nat. Commun. 2021, 12, 2598. [Google Scholar] [CrossRef]
- Volpato, E.; Bosio, C.; Previtali, E.; Leone, S.; Armuzzi, A.; Pagnini, F.; Graffigna, G. The evolution of IBD perceived engagement and care needs across the life-cycle: A scoping review. BMC Gastroenterol. 2021, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.B.d.L.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Li, X.; Wei, X.; Sun, Y.; Du, J.; Li, X.; Xun, Z.; Li, Y.C. High-fat diet promotes experimental colitis by inducing oxidative stress in the colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G453–G462. [Google Scholar] [CrossRef]
- Pavelock, N.; Masood, U.; Minchenberg, S.; Heisig, D. Effects of obesity on the course of inflammatory bowel disease. Bayl. Univ. Med. Cent. Proc. 2019, 32, 14–17. [Google Scholar] [CrossRef]
- Weissman, S.; Patel, K.; Kolli, S.; Lipcsey, M.; Qureshi, N.; Elias, S.; Walfish, A.; Swaminath, A.; Feuerstein, J.D. Obesity in Inflammatory Bowel Disease Is Associated with Early Readmissions Characterised by an Increased Systems and Patient-level Burden. J. Crohn’s Colitis 2021, 15, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.M.; Chen, Y.; Casey, K.; Olen, O.; Ludvigsson, J.F.; Carbonnel, F.; Oldenburg, B.; Gunter, M.J.; Tjønneland, A.; Grip, O.; et al. Obesity is Associated With Increased Risk of Crohn’s disease, but not Ulcerative Colitis: A Pooled Analysis of Five Prospective Cohort Studies. Clin. Gastroenterol. Hepatol. 2022, 20, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Burstein, E.; Cipher, D.J.; Feagins, L.A. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig. Dis. Sci. 2015, 60, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Crandall, W.V.; Leibowitz, I.H.; Duffy, L.; del Rosario, F.; Kim, S.C.; Integlia, M.J.; Berman, J.; Grunow, J.; Colletti, R.B.; et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm. Bowel. Dis. 2011, 17, 2162–2168. [Google Scholar] [CrossRef]
- Li, X.; Jiang, L.; Yang, M.; Wu, Y.W.; Sun, J.Z. Impact of weight cycling on CTRP3 expression, adipose tissue inflammation and insulin sensitivity in C57BL/6J mice. Exp. Ther. Med. 2018, 16, 2052–2059. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Li, G.; Feng, Y.; Xian, L.; Bakhsh, F.; Xu, D.; Xu, C.; Vong, T.; Wu, B.; et al. Adipokine C1q/Tumor Necrosis Factor- Related Protein 3 (CTRP3) Attenuates Intestinal Inflammation via Sirtuin 1/NF-κB Signaling. Cell. Mol. Gastroenterol. Hepatol. 2022, 15, 1000–1015. [Google Scholar] [CrossRef]
- Wolf, R.M.; Steele, K.E.; Peterson, L.A.; Magnuson, T.H.; Schweitzer, M.A.; Wong, G.W. Lower Circulating C1q/TNF-Related Protein-3 (CTRP3) Levels Are Associated with Obesity: A Cross-Sectional Study. PLoS ONE 2015, 10, e0133955. [Google Scholar] [CrossRef]
- Reinecker, H.-C.; Loh, E.Y.; Ringler, D.J.; Mehta, A.; Rombeau, J.L.; MacDermott, R.P. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology 1995, 108, 40–50. [Google Scholar] [CrossRef]
- Futagami, S.; Hiratsuka, T.; Tatsuguchi, A.; Suzuki, K.; Kusunoki, M.; Shinji, Y.; Shinoki, K.; Iizumi, T.; Akamatsu, T.; Nishigaki, H.; et al. Monocyte chemoattractant protein 1 (MCP-1) released from Helicobacter pylori stimulated gastric epithelial cells induces cyclooxygenase 2 expression and activation in T cells. Gut 2003, 52, 1257–1264. [Google Scholar] [CrossRef]
- Khan, W.I.; Motomura, Y.; Wang, H.; El-Sharkawy, R.T.; Verdu, E.F.; Verma-Gandhu, M.; Rollins, B.J.; Collins, S.M. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am. J. Physiol. Liver Physiol. 2006, 291, G803–G811. [Google Scholar] [CrossRef]
- Grimm, M.C.; Elsbury, S.K.; Pavli, P.; Doe, W.F. Interleukin 8: Cells of origin in inflammatory bowel disease. Gut 1996, 38, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.C.; O Elsbury, S.K.; Pavli, P.; Doe, W.F. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J. Leukoc. Biol. 1996, 59, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Lissner, D.; Schumann, M.; Batra, A.; Kredel, L.-I.; Kühl, A.A.; Erben, U.; May, C.; Schulzke, J.-D.; Siegmund, B. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm. Bowel Dis. 2015, 21, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, W.; Wang, S.; Zhang, P.; Wang, Q.; Xiao, J.; Zhang, C.; Zheng, X.; Xu, X.; Xue, S.; et al. YAP Aggravates Inflammatory Bowel Disease by Regulating M1/M2 Macrophage Polarization and Gut Microbial Homeostasis. Cell Rep. 2019, 27, 1176–1189.e5. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, Q.; Pan, L.-L.; Sun, J. Macrophage immunometabolism in inflammatory bowel diseases: From pathogenesis to therapy. Pharmacol. Ther. 2022, 238, 108176. [Google Scholar] [CrossRef]
- Pendyala, S.; Neff, L.M.; Suárez-Fariñas, M.; Holt, P.R. Diet-induced weight loss reduces colorectal inflammation: Implications for colorectal carcinogenesis. Am. J. Clin. Nutr. 2011, 93, 234–242. [Google Scholar] [CrossRef]
- Walana, W.; Ye, Y.; Li, M.; Wang, J.; Wang, B.; Cheng, J.-W.; Gordon, J.R.; Li, F. IL-8 antagonist, CXCL8(3-72)K11R/G31P coupled with probiotic exhibit variably enhanced therapeutic potential in ameliorating ulcerative colitis. Biomed. Pharmacother. 2018, 103, 253–261. [Google Scholar] [CrossRef]
- Nishitani, Y.; Zhang, L.; Yoshida, M.; Azuma, T.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. Intestinal Anti-Inflammatory Activity of Lentinan: Influence on IL-8 and TNFR1 Expression in Intestinal Epithelial Cells. PLoS ONE 2013, 8, e62441. [Google Scholar] [CrossRef]
- Casado-Bedmar, M.; Heil, S.D.S.; Myrelid, P.; Söderholm, J.D.; Keita, V. Upregulation of intestinal mucosal mast cells expressing VPAC1 in close proximity to vasoactive intestinal polypeptide in inflammatory bowel disease and murine colitis. Neurogastroenterol. Motil. 2018, 31, e13503. [Google Scholar] [CrossRef]
- Abad, C.; Martinez, C.; Juarranz, M.G.; Arranz, A.; Leceta, J.; Delgado, M.; Gomariz, R.P. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology 2003, 124, 961–971. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Mazzawi, T.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. The role of peptide YY in gastrointestinal diseases and disorders (review). Int. J. Mol. Med. 2013, 31, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A. Factors affecting circulating levels of peptide YY in humans: A comprehensive review. Nutr. Res. Rev. 2014, 27, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Karra, E.; Chandarana, K.; Batterham, R.L. The role of peptide YY in appetite regulation and obesity. J. Physiol. 2009, 587, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Inczefi, O.; Bacsur, P.; Resál, T.; Keresztes, C.; Molnár, T. The Influence of Nutrition on Intestinal Permeability and the Microbiome in Health and Disease. Front. Nutr. 2022, 9, 718710. [Google Scholar] [CrossRef]
- Ahmad, R.; Sorrell, M.; Batra, S.; Dhawan, P.; Singh, A. Gut permeability and mucosal inflammation: Bad, good or context dependent. Mucosal Immunol. 2017, 10, 307–317. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef]
- Kawashima, T.; Ogata, M.; Fujita, N.; Takahashi, R. Daisaikoto Prevents Post-dieting Weight Regain by Reversing Dysbiosis and Reducing Serum Corticosterone in Mice. Front. Physiol. 2019, 10, 1483. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A taxonomic signature of obesity in a large study of American adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Toyama, M.; Banno, T.; Chonan, O.; Benno, Y.; Watanabe, K. Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 2016, 16, 284. [Google Scholar] [CrossRef]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.M.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Xu, W.; Ma, Y.; Wang, Q.; Eatman, D.; You, S.; Zou, J.; Champion, J.; Zhao, L.; et al. C-Reactive Protein Causes Adult-Onset Obesity Through Chronic Inflammatory Mechanism. Front. Cell Dev. Biol. 2020, 8, 18. [Google Scholar] [CrossRef]
- Mazier, W.; Le Corf, K.; Martinez, C.; Tudela, H.; Kissi, D.; Kropp, C.; Coubard, C.; Soto, M.; Elustondo, F.; Rawadi, G.; et al. A New Strain of Christensenella minuta as a Potential Biotherapy for Obesity and Associated Metabolic Diseases. Cells 2021, 10, 823. [Google Scholar] [CrossRef]
- Relizani, K.; Le Corf, K.; Kropp, C.; Martin-Rosique, R.; Kissi, D.; Déjean, G.; Bruno, L.; Martinez, C.; Rawadi, G.; Elustondo, F.; et al. Selection of a novel strain of Christensenella minuta as a future biotherapy for Crohn’s disease. Sci. Rep. 2022, 12, 6017. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Kropp, C.; Le Corf, K.; Relizani, K.; Tambosco, K.; Martinez, C.; Chain, F.; Rawadi, G.; Langella, P.; Claus, S.P.; Martin, R. The Keystone commensal bacterium Christensenella minuta DSM 22607 displays anti-inflammatory properties both in vitro and in vivo. Sci. Rep. 2021, 11, 11494. [Google Scholar] [CrossRef]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteribiofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef]
- Giraffa, G.; Chanishvili, N.; Widyastuti, Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010, 161, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-X.; He, F.; Guo, Y.-X.; Mo, L.-Z.; Zhu, X. Lactobacillus reuteri Biofilms Inhibit Pathogens and Regulate Microbiota in In Vitro Fecal Fermentation. J. Agric. Food Chem. 2022, 70, 11935–11943. [Google Scholar] [CrossRef] [PubMed]
- Ahl, D.; Liu, H.; Schreiber, O.; Roos, S.; Phillipson, M.; Holm, L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol. 2016, 217, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, S.; Cai, S.; Yu, H.; Wang, Y.; Zeng, X.; Qiao, S. Lactobacillus reuteri Ameliorates Intestinal Inflammation and Modulates Gut Microbiota and Metabolic Disorders in Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2020, 12, 2298. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, J.; Liu, Y.; Meng, Q.; Liu, H.; Yao, Q.; Song, W.; Ren, X.; Chen, X. The role of potential probiotic strains Lactobacillus reuteri in various intestinal diseases: New roles for an old player. Front. Microbiol. 2023, 14, 1095555. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- La Reau, A.J.; Meier-Kolthoff, J.P.; Suen, G. Sequence-based analysis of the genus Ruminococcus resolves its phylogeny and reveals strong host association. Microb. Genom. 2016, 2, e000099. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Grahnemo, L.; Nethander, M.; Coward, E.; Gabrielsen, M.E.; Sree, S.; Billod, J.-M.; Engstrand, L.; Abrahamsson, S.; Langhammer, A.; Hveem, K.; et al. Cross-sectional associations between the gut microbe Ruminococcus gnavus and features of the metabolic syndrome: The HUNT study. Lancet Diabetes Endocrinol. 2022, 10, 481–483. [Google Scholar] [CrossRef]
- Goldstein, E.J.C.; Citron, D.M.; Peraino, V.A.; Cross, S.A. Desulfovibrio desulfuricans bacteremia and review of human Desulfovibrio infections. J. Clin. Microbiol. 2003, 41, 2752–2754. [Google Scholar] [CrossRef] [PubMed]
- Rowan, F.; Docherty, N.G.; Murphy, M.; Murphy, B.; Coffey, J.C.; O’connell, P.R. Desulfovibrio Bacterial Species Are Increased in Ulcerative Colitis. Dis. Colon Rectum 2010, 53, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Verstreken, I.; Laleman, W.; Wauters, G.; Verhaegen, J. Desulfovibrio desulfuricans bacteremia in an immunocompromised host with a liver graft and ulcerative colitis. J. Clin. Microbiol. 2012, 50, 199–201. [Google Scholar] [CrossRef]
- Murros, K.E.; Huynh, V.A.; Takala, T.M.; Saris, P.E.J. Desulfovibrio Bacteria Are Associated With Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2021, 11, 652617. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, A.; Zorzi, F.; Del Chierico, F.; Altomare, A.; Cocca, S.; Avola, A.; De Biasio, F.; Russo, A.; Cella, E.; Reddel, S.; et al. Fecal and Mucosal Microbiota Profiling in Irritable Bowel Syndrome and Inflammatory Bowel Disease. Front. Microbiol. 2019, 10, 1655. [Google Scholar] [CrossRef]
- Laval, L.; Martin, R.; Natividad, J.; Chain, F.; Miquel, S.; de Maredsous, C.D.; Capronnier, S.; Sokol, H.; Verdu, E.; Vlieg, J.v.H.; et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Jang, H.R.; Park, H.-J.; Kang, D.; Chung, H.; Nam, M.H.; Lee, Y.; Park, J.-H.; Lee, H.-Y. A protective mechanism of probiotic Lactobacillus against hepatic steatosis via reducing host intestinal fatty acid absorption. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Chung, H.-J.; Yu, J.G.; Lee, I.-A.; Liu, M.-J.; Shen, Y.-F.; Sharma, S.P.; Jamal, M.A.H.M.; Yoo, J.-H.; Kim, H.-J.; Hong, S.-T. Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity. FEBS Open Bio 2016, 6, 64–76. [Google Scholar] [CrossRef]
- Curciarello, R.; Canziani, K.E.; Salto, I.; Romero, E.B.; Rocca, A.; Doldan, I.; Peton, E.; Brayer, S.; Sambuelli, A.M.; Goncalves, S.; et al. Probiotic Lactobacilli Isolated from Kefir Promote Down-Regulation of Inflammatory Lamina Propria T Cells from Patients with Active IBD. Front. Pharmacol. 2021, 12, 658026. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Park, H.J.; Cha, M.G.; Park, E.; Won, S.-M.; Ganesan, R.; Gupta, H.; Gebru, Y.A.; Sharma, S.P.; Lee, S.B.; et al. The Lactobacillus as a Probiotic: Focusing on Liver Diseases. Microorganisms 2022, 10, 288. [Google Scholar] [CrossRef]
- Varshney, R.; Mishra, R.; Das, N.; Sircar, D.; Roy, P. A comparative analysis of various flavonoids in the regulation of obesity and diabetes: An in vitro and in vivo study. J. Funct. Foods 2019, 59, 194–205. [Google Scholar] [CrossRef]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124 086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef]
- Vernarelli, J.A.; Lambert, J.D. Flavonoid intake is inversely associated with obesity and C-reactive protein, a marker for inflammation, in US adults. Nutr. Diabetes 2017, 7, e276. [Google Scholar] [CrossRef] [PubMed]
- Hoek-van den Hil, E.F.; van Schothorst, E.M.; van der Stelt, I.; Swarts, H.J.M.; van Vliet, M.; Amolo, T.; Vervoort, J.J.M.; Venema, D.; Hollman, P.C.H.; Rietjens, I.M.C.M.; et al. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. 2015, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Dayem, A.A.; Han, J.; Yin, Y.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef]
- Baky, M.H.; Elshahed, M.S.; Wessjohann, L.A.; Farag, M.A. Interactions between dietary flavonoids and the gut microbiome: A comprehensive review. Br. J. Nutr. 2021, 128, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y.; Chang, W.; Zhou, M. The Effects and Mechanisms of Flavonoids on Cancer Prevention and Therapy: Focus on Gut Microbiota. Int. J. Biol. Sci. 2022, 18, 1451–1475. [Google Scholar] [CrossRef]
- Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Järnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 2010, 139, 1844–1854.e1. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in Citrobacter rodentium-infected mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle 2018, 17, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.M.; Gu, T.; Munagala, R.; Jeyabalan, J.; Egilmez, N.K.; Gupta, R.C. Chemoprevention of Colorectal Cancer by Anthocyanidins and Mitigation of Metabolic Shifts Induced by Dysbiosis of the Gut MicrobiomeAnthos Prevent Metabolic Shifts from Microbiome and B [a] P. Cancer Prev. Res. 2020, 13, 41–52. [Google Scholar]
- Wu, J.; Tsai, M.; Lai, C.; Lo, C.; Ho, C.; Wang, Y.; Pan, M. Polymethoxyflavones prevent benzo[a]pyrene/dextran sodium sulfate-induced colorectal carcinogenesis through modulating xenobiotic metabolism and ameliorate autophagic defect in ICR mice. Int. J. Cancer 2018, 142, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, Y.; Deng, B.; Li, J.; Cao, H.; Qu, Y.; Qian, X.; Zhong, G. Isoliquiritigenin decreases the incidence of colitis-associated colorectal cancer by modulating the intestinal microbiota. Oncotarget 2016, 7, 85318–85331. [Google Scholar] [CrossRef]
- Qi, J.; Yu, J.; Li, Y.; Luo, J.; Zhang, C.; Ou, S.; Zhang, G.; Yang, X.; Peng, X. Alternating consumption of β-glucan and quercetin reduces mortality in mice with colorectal cancer. Food Sci. Nutr. 2019, 7, 3273–3285. [Google Scholar] [CrossRef]
- Bian, S.; Wan, H.; Liao, X.; Wang, W. Inhibitory effects of apigenin on tumor carcinogenesis by altering the gut microbiota. Mediat. Inflamm. 2020, 2020, 7141970. [Google Scholar] [CrossRef]
- Mcfadden, R.M.T.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, B.; Zhong, C.; Guo, J.; Zhang, L.; Mu, T.; Zhang, Q.; Bi, X. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis 2018, 39, 471–481. [Google Scholar] [CrossRef]
- Horiba, Y.; Yoshino, T.; Watanabe, K. Daisaikoto for menstrual pain: A lesson from a case with menstrual pain successfully treated with daisaikoto. Case Rep. Med. 2015, 2015, 929514. [Google Scholar] [CrossRef]
- Morita, S.; Sakamaki, A.; Koyama, K.; Shibata, O.; Owaki, T.; Oda, C.; Kimura, A.; Nakaya, T.; Ohbuchi, K.; Nahata, M.; et al. Daisaikoto improves fatty liver and obesity in melanocortin-4 receptor gene-deficient mice via the activation of brown adipose tissue. Sci. Rep. 2022, 12, 10105. [Google Scholar] [CrossRef]
- Qian, W.; Cai, X.; Zhang, X.; Wang, Y.; Qian, Q.; Hasegawa, J. Effect of daisaikoto on expressions of SIRT1 and NF-kappaB of diabetic fatty liver rats induced by high-fat diet and streptozotocin. Yonago Acta Medica 2016, 59, 149. [Google Scholar] [PubMed]
- Ishizawa, S.; Nishi, A.; Kaifuchi, N.; Shimobori, C.; Nahata, M.; Yamada, C.; Iizuka, S.; Ohbuchi, K.; Nishiyama, M.; Fujitsuka, N.; et al. Integrated analysis of effect of daisaikoto, a traditional Japanese medicine, on the metabolome and gut microbiome in a mouse model of nonalcoholic fatty liver disease. Gene 2022, 846, 146856. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Mirshekar-Syahkal, B.; Kennish, L.; Karachalias, N.; Babaei-Jadidi, R.; Thornalley, P.J. Assay of advanced glycation endproducts in selected beverages and food by liquid chromatography with tandem mass spectrometric detection. Mol. Nutr. Food Res. 2005, 49, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in lactobacillus in obese patients and methanogens in anorexic patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2017, 53, 95–106. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, H.; Yang, Y.; Zhang, Y.; Lai, H.; Cheng, Y.; Yu, H.; Feng, N.; Huang, R.; Liu, S.; et al. High-protein diet prevents fat mass increase after dieting by counteracting Lactobacillus-enhanced lipid absorption. Nat. Metab. 2022, 4, 1713–1731. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Hu, Y.; Brown, I.L.; Woodman, R.J.; Young, G.P. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis 2010, 31, 246–251. [Google Scholar] [CrossRef]
- Sekine, K.; Toida, T.; Saito, M.; Kuboyama, M.; Kawashima, T.; Hashimoto, Y. A new morphologically characterized cell wall preparation (whole peptidoglycan) from Bifidobacterium infantis with a higher efficacy on the regression of an established tumor in mice. Cancer Res. 1985, 45, 1300–1307. [Google Scholar] [PubMed]
- Pool-Zobel, B.; Neudecker, C.; Domizlaff, I.; Ji, S.; Schillinger, U.; Rumney, C.; Moretti, M.; Vilarini, I.; Scassellati-Sforzolini, R.; Rowland, I. Lactobacillus-and Bifidobacterium-mediated antigenotoxicity in the colon of rats. Nutr. Cancer 1996, 26, 365–380. [Google Scholar] [CrossRef]
- Tavan, E.; Cayuela, C.; Antoine, J.-M.; Cassand, P. Antimutagenic activities of various lactic acid bacteria against food mutagens: Heterocyclic amines. J. Dairy Res. 2002, 69, 335–341. [Google Scholar] [CrossRef]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.-L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N.; et al. The Intestinal Microbiome Predicts Weight Loss on a Calorie-Restricted Diet and Is Associated With Improved Hepatic Steatosis. Front. Nutr. 2021, 8, 718661. [Google Scholar] [CrossRef]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar]
- Wand, M.E.; Baker, K.S.; Benthall, G.; McGregor, H.; McCowen, J.W.I.; Deheer-Graham, A.; Sutton, J.M. Characterization of pre-antibiotic era klebsiella pneumoniae isolates with respect to antibiotic/disinfectant susceptibility and virulence in galleria mellonella. Antimicrob. Agents Chemother. 2015, 59, 3966–3972. [Google Scholar] [CrossRef]
- Kot, B.; Piechota, M.; Szweda, P.; Mitrus, J.; Wicha, J.; Grużewska, A.; Witeska, M. Virulence analysis and antibiotic resistance of Klebsiella pneumoniae isolates from hospitalised patients in Poland. Sci. Rep. 2023, 13, 4448. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Huang, F.; Fang, J.; Cao, Y.; Zhang, K.; Zhou, H.; Cai, J.; Cui, W.; Chen, C.; et al. Clinical characteristics, risk factors and outcomes of Klebsiella pneumoniae pneumonia developing secondary Klebsiella pneumoniae bloodstream infection. BMC Pulm. Med. 2023, 23, 102. [Google Scholar] [CrossRef]
- Verani, J.R.; Blau, D.M.; Gurley, E.S.; Akelo, V.; Assefa, N.; Baillie, V.; Bassat, Q.; Berhane, M.; Bunn, J.; Cossa, A.C.A.; et al. Child deaths caused by Klebsiella pneumoniae in sub-Saharan Africa and south Asia: A secondary analysis of Child Health and Mortality Prevention Surveillance (CHAMPS) data. Lancet Microbe 2024, 5, e131–e141. [Google Scholar] [CrossRef] [PubMed]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary probiotic treatment and vitamin B7 in depression—A randomized controlled trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.L.J.; Önnerfält, J.; Xu, J.; Molin, G.; Ahrné, S.; Thorngren-Jerneck, K. The Microbiota of the Gut in Preschool Children With Normal and Excessive Body Weight. Obesity 2012, 20, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Chao, A. Measuring and Estimating Species Richness, Species Diversity, and Biotic Similarity from Sampling Data. In Encyclopedia of Biodiversity; Elsevier: Oxford, UK, 2013; pp. 195–211. [Google Scholar] [CrossRef]
- Jian, C.; Silvestre, M.P.; Middleton, D.; Korpela, K.; Jalo, E.; Broderick, D.; de Vos, W.M.; Fogelholm, M.; Taylor, M.W.; Raben, A.; et al. Gut microbiota predicts body fat change following a low-energy diet: A PREVIEW intervention study. Genome Med. 2022, 14, 54. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Forbes, J.D. Gut Microbiome in Inflammatory Bowel Disease and Other Chronic Immune-Mediated Inflammatory Diseases. Inflamm. Intest. Dis. 2017, 2, 116–123. [Google Scholar] [CrossRef]
- Gionchetti, P.; Rizzello, F.; Venturi, A.; Campieri, M. Probiotics in infective diarrhoea and inflammatory bowel diseases. J. Gastroenterol. Hepatol. 2000, 15, 489–493. [Google Scholar] [CrossRef]
- Venturi, A.; Gionchetti, P.; Rizzello, F.; Johansson, R.; Zucconi, E.; Brigidi, P.; Matteuzzi, D.; Campieri, M. Impact on the composition of the faecal flora by a new probiotic preparation: Preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment. Pharmacol. Ther. 1999, 13, 1103–1108. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, R.; Khare, P.; Rajarammohan, S.; Bishnoi, M.; Bhadada, S.K.; Sharma, S.S.; Kaur, J.; Kondepudi, K.K. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci. Rep. 2020, 10, 18597. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhao, Z.; Wang, W.; Liu, X. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. J. Immunol. Res. 2021, 2021, 18597. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liao, W.; Tang, J.; Fei, T.; Gai, Z.; Han, M. Bifidobacterium BLa80 mitigates colitis by altering gut microbiota and alleviating inflammation. AMB Express 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.S.; Hansson, G.C. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe 2023, 31, 1087–1100. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Alcazar, M.; Escribano, J.; Ferré, N.; Closa-Monasterolo, R.; Selma-Royo, M.; Feliu, A.; Castillejo, G.; Luque, V.; Feliu-Rovira, A.; Muñoz-Hernando, J.; et al. Gut microbiota is associated with metabolic health in children with obesity. Clin. Nutr. 2022, 41, 1680–1688. [Google Scholar] [CrossRef]
- Luo, Y.; Lan, C.; Li, H.; Ouyang, Q.; Kong, F.; Wu, A.; Ren, Z.; Tian, G.; Cai, J.; Yu, B.; et al. Rational consideration of Akkermansia muciniphila targeting intestinal health: Advantages and challenges. NPJ Biofilms Microbiomes 2022, 8, 81. [Google Scholar] [CrossRef]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative Microbiome Signatures and Short-Chain Fatty Acids in Mouse, Rat, Non-human Primate, and Human Feces. Front. Microbiol. 2018, 9, 2897. [Google Scholar] [CrossRef] [PubMed]
| Overall Aims | Model | Diet Regime | Microbiota Changes and Metabolic Outcomes | Reference |
|---|---|---|---|---|
| Yoyo dieting changes microbiota composition and the administration of flavonoids might be beneficial in reduced weight regain | Male C57BL/6 mice | HFD → LFD → HFD → LFD | Yoyo mice:
| [61] |
| The effect of yoyo dieting and the use of daisaikoto | Female C57BL/6 mice | Yoyo dieting only: HFD → LFD → HFD Yoyo dieting with daisaikoto supplementation: HFD → LFD + daisaikoto → HFD | Yoyo mice:
| [196] |
| Long term effect of yoyo dieting on faecal microbiota | Male C57BL/6 mice | (1) HFD → LFD → HFD → LFD → HFD → LFD → HFD → LFD → HFD → LFD or (2) LFD → HFD → LFD → HFD → LFD → HFD → LFD → HFD → LFD → HFD | Yoyo mice a fed a yoyo diet ending with a LFD:
| [62] |
| Overall Aims | Model | Diet Regime | Microbiota Changes | Reference |
|---|---|---|---|---|
| Examining effects of one-month caloric restriction on gut permeability and microbiome in female individuals with obesity | Female individuals with obesity | 1-month low-calorie diet |
| [114] |
| The intestinal microbiome predicts weight loss on a calorie-restricted diet in overweight and obesity individuals | Male and female individuals with overweight/obesity | 16-week macronutrient standardised diet for weight loss | No gut microbiota difference at baseline. Individuals successfully lost at least 5% body weight:
| [262] |
| A PREVIEW intervention study investigating the effect of low-energy diets on gut microbiome of individuals with overweight, or obesity and prediabetes | Male and female individuals with overweight/obesity and prediabetes | 8-week low-energy diet | Individuals successfully lost at least 8% body weight
| [273] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuong-Nguyen, K.; McGee, S.L.; Aston-Mourney, K.; Mcneill, B.A.; Mahmood, M.Q.; Rivera, L.R. Yoyo Dieting, Post-Obesity Weight Loss, and Their Relationship with Gut Health. Nutrients 2024, 16, 3170. https://doi.org/10.3390/nu16183170
Phuong-Nguyen K, McGee SL, Aston-Mourney K, Mcneill BA, Mahmood MQ, Rivera LR. Yoyo Dieting, Post-Obesity Weight Loss, and Their Relationship with Gut Health. Nutrients. 2024; 16(18):3170. https://doi.org/10.3390/nu16183170
Chicago/Turabian StylePhuong-Nguyen, Kate, Sean L. McGee, Kathryn Aston-Mourney, Bryony A. Mcneill, Malik Q. Mahmood, and Leni R. Rivera. 2024. "Yoyo Dieting, Post-Obesity Weight Loss, and Their Relationship with Gut Health" Nutrients 16, no. 18: 3170. https://doi.org/10.3390/nu16183170
APA StylePhuong-Nguyen, K., McGee, S. L., Aston-Mourney, K., Mcneill, B. A., Mahmood, M. Q., & Rivera, L. R. (2024). Yoyo Dieting, Post-Obesity Weight Loss, and Their Relationship with Gut Health. Nutrients, 16(18), 3170. https://doi.org/10.3390/nu16183170






