Effect of the Mediterranean Diet (MeDi) on the Progression of Retinal Disease: A Narrative Review
Abstract
1. Introduction
2. Visual System and Conditions Leading to Retinal Diseases
3. The Mediterranean Diet (MeDi)
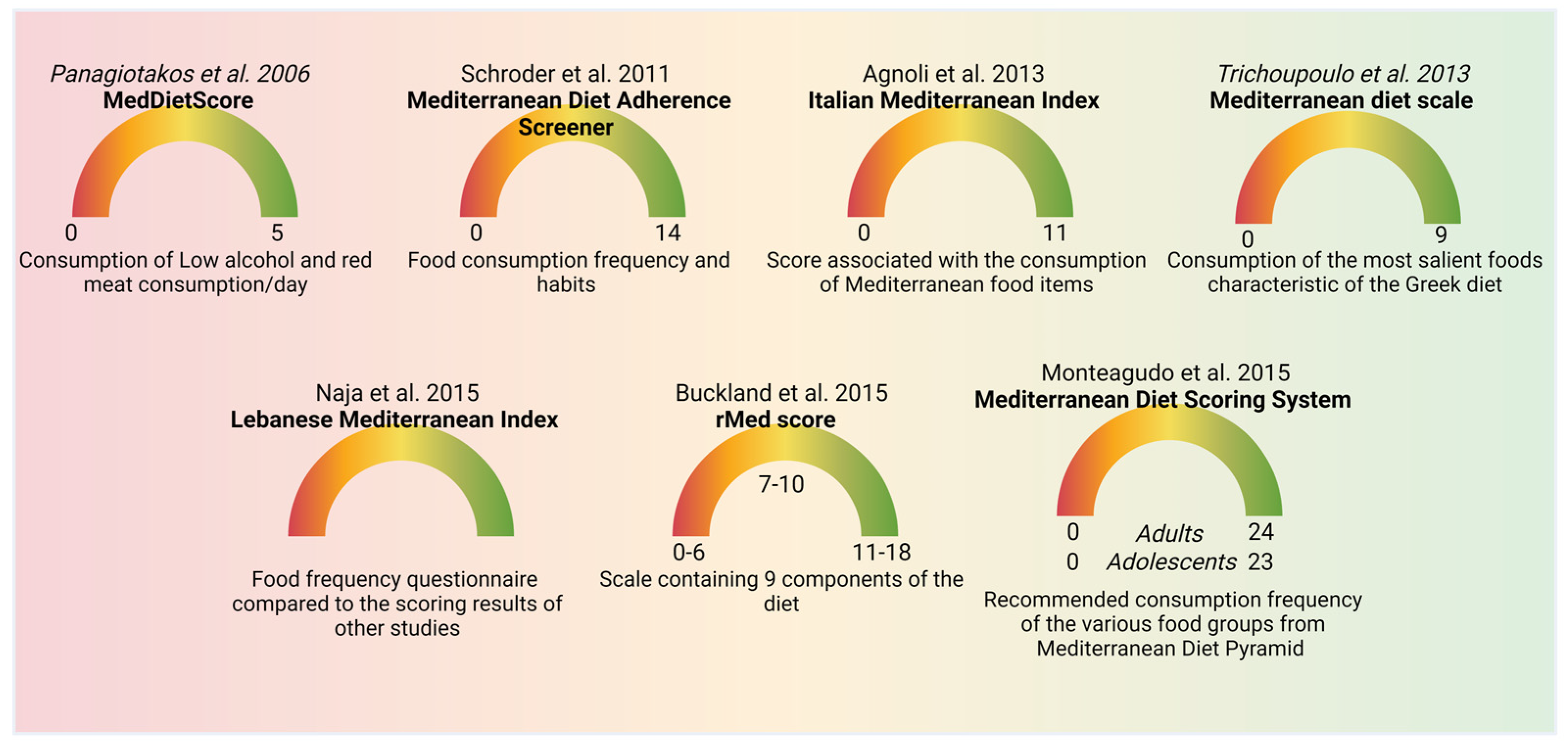
4. MeDi Scoring
5. Natural Molecules Enriched in the MeDi
5.1. Phenolic Compounds
5.2. Isoprenoids
5.3. Carbohydrates
5.4. Proteins
5.5. Lipids and Fatty Acids
5.6. Vitamins
5.7. Melatonin
5.8. Saffron
5.9. Taurine
5.10. Palmitoyethanolamide (PEA)
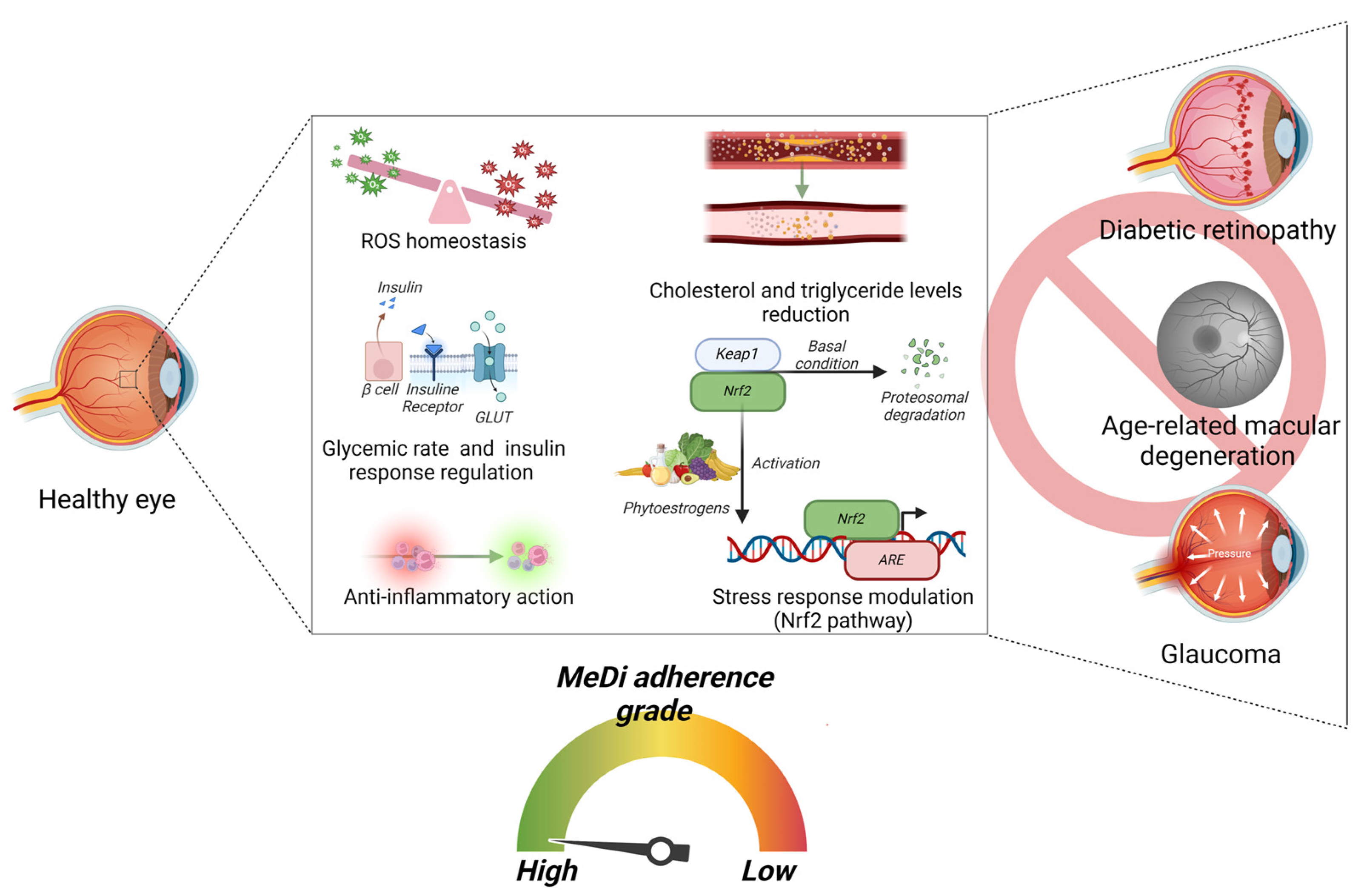
6. MeDi and Stress Response Involved in Retinal Diseases: Focus on Nrf-2 Pathway
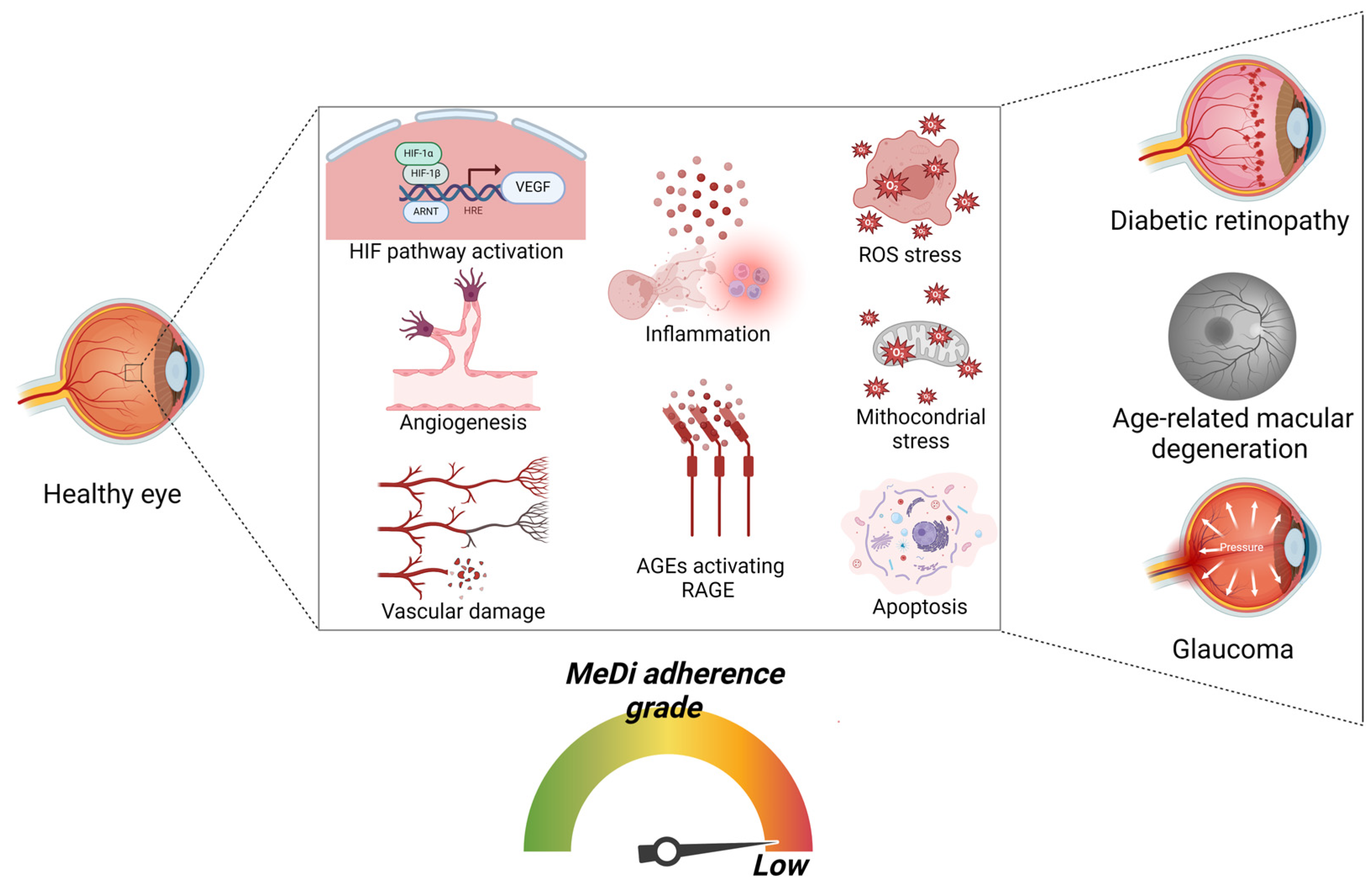
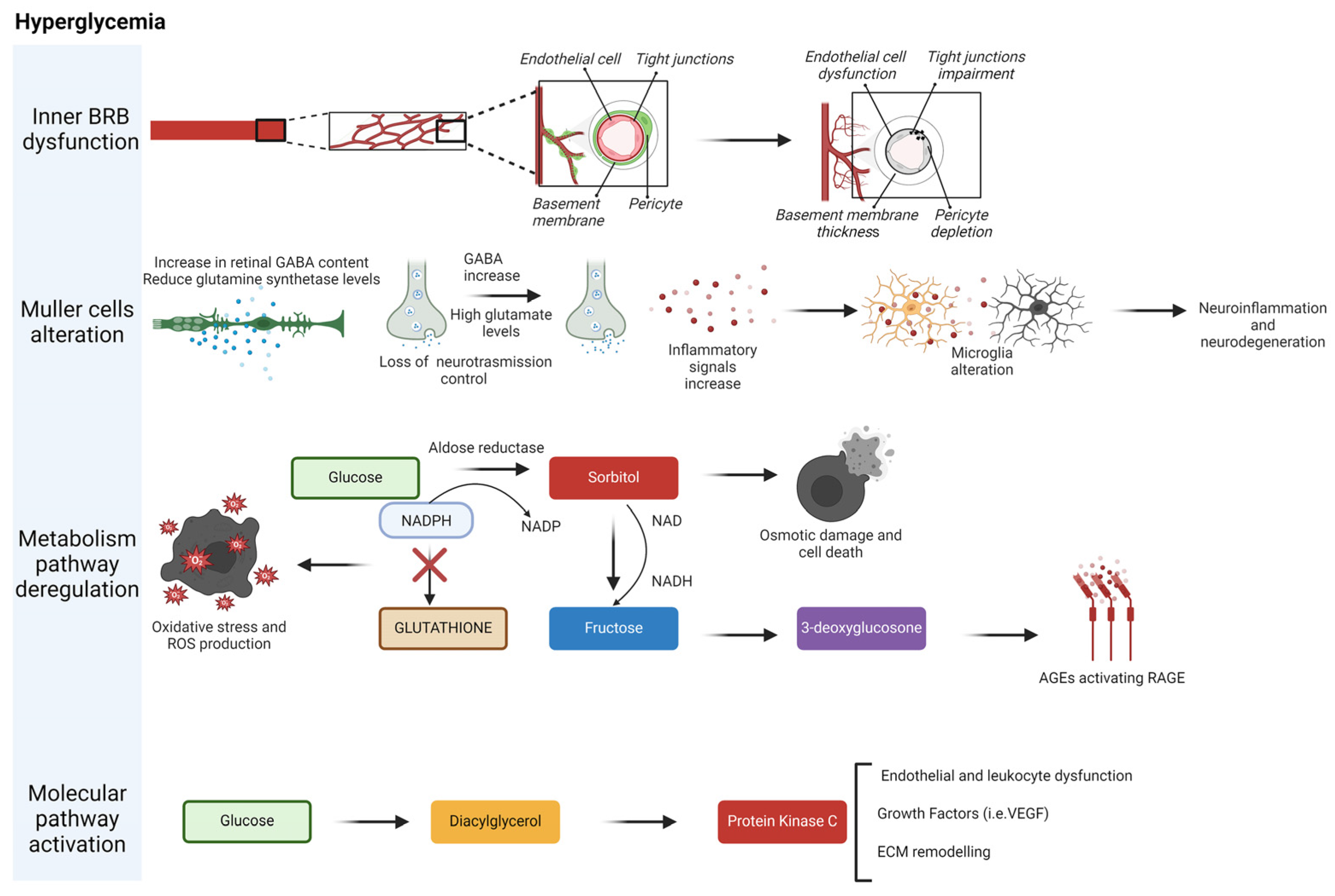
7. MeDi’s Role in the Prevention and Amelioration of Diabetic Retinopathy
8. MeDi’s Role in the Prevention and Amelioration of Age-Related Macular Degeneration (AMD)
9. MeDi’s Role in the Prevention and Amelioration of Glaucoma
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- French, S. Visual Impairment and Work: Experiences of Visually Impaired People, 1st ed.; Routledge: London, UK, 2017. [Google Scholar]
- Bernabei, V.; Morini, V.; Moretti, F.; Marchiori, A.; Ferrari, B.; Dalmonte, E.; De Ronchi, D.; Atti, A.R. Vision and hearing impairments are associated with depressive–anxiety syndrome in Italian elderly. Aging Ment. Health 2011, 15, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef]
- Gropper, S.S. The Role of Nutrition in Chronic Disease. Nutrients 2023, 15, 664. [Google Scholar] [CrossRef] [PubMed]
- Dobroslavska, P.; Silva, M.L.; Vicente, F.; Pereira, P. Mediterranean Dietary Pattern for Healthy and Active Aging: A Narrative Review of an Integrative and Sustainable Approach. Nutrients 2024, 16, 1725. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Bringmann, A. New functions of Müller cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.O.; Reh, T.A. Regenerative medicine for retinal diseases: Activating endogenous repair mechanisms. Trends Mol. Med. 2010, 16, 193–202. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Falsini, B.; Placidi, G.; De Siena, E.; Savastano, M.C.; Minnella, A.M.; Maceroni, M.; Midena, G.; Ziccardi, L.; Parisi, V.; Bertelli, M.; et al. USH2A-Related Retinitis Pigmentosa: Staging of disease severity and morphofunctional studies. Diagnostics 2021, 11, 213. [Google Scholar] [CrossRef]
- Falsini, B.; Placidi, G.; De Siena, E.; Chiurazzi, P.; Minnella, A.M.; SAvastano, M.C.; Ziccardi, L.; Parosi, V.; Iarossi, G.; Percio, M.; et al. Genetic characteristics of 234 Italian patients with macular and cone/cone-rod dystrophy. Sci. Rep. 2022, 12, 3774. [Google Scholar] [CrossRef]
- Colombo, L.; Maltese, P.E.; Castori, M.; El Shamieh, S.; Zeitz, C.; Audo, I.; Zulian, A.; Marinelli, C.; Benedetti, S.; Costantini, A.; et al. Molecular epidemiology in 591 italian probands with nonsyndromic retinitis pigmentosa and usher syndrome. Investig. Ophthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef]
- Sohocki, M.M.; Daiger, S.P.; Bowne, S.J.; Rodriquez, J.A.; Northrup, H.; Heckenlively, J.R.; Birch, D.G.; Mintz-Hittner, H.; Ruiz, R.S.; Lewis, R.A.; et al. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum. Mutat. 2001, 17, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, N.; Michaelides, M.; Smith, A.J.; Ali, R.R.; Bainbridge, J.W.B. Retinal gene therapy. Br. Med. Bull. 2018, 126, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Saaddine, J.B.; Honeycutt, A.A.; Narayan, K.M.; Zhang, X.; Klein, R.; Boyle, J.P. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005–2050. Arch. Ophthalmol. 2008, 126, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Devi, T.S.; Hosoya, K.C.; Terasaki, T.; Singh, L.P. Thioredoxin Interacting Protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J. Cell Physiol. 2009, 221, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Devi, T.S.; Hosoya, K.C.; Terasaki, T.; Singh, L.P. Inhibition of TXNIP Expression In Vivo Blocks Early Pathologies of Diabetic Retinopathy. Cell Death Dis. 2010, 1, e65. [Google Scholar] [CrossRef]
- Amerasinghe, N.; Zhang, J.; Thalamuthu, A.; He, M.; Vithana, E.N.; Viswanathan, A.; Wong, T.Y.; Foster, P.J.; Aung, T. The heritability and sibling risk of angle closure in Asians. Ophthalmology 2011, 118, 480–485. [Google Scholar] [CrossRef]
- Lanzetta, P.; Sarao, V.; Scanlon, P.H.; Barratt, J.; Porta, M.; Bandello, F.; Loewenstein, A. Vision Academy. Fundamental principles of an effective diabetic retinopathy screening program. Acta Diabetol. 2020, 57, 785–798. [Google Scholar] [CrossRef]
- Pondorfer, S.G.; Terheyden, J.H.; Heinemann, M.; Wintergerst, M.W.M.; Holz, F.G.; Finger, R.P. Association of vision-related quality of life with visual function in age-related macular degeneration. Sci. Rep. 2019, 9, 15326. [Google Scholar] [CrossRef]
- Dernini, S.; Berry, E.M. Mediterranean Diet: From a Healthy Diet to a Sustainable Dietary Pattern. Front. Nutr. 2015, 2, 15. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Real, H.; Dias, R.R.; Graça, P. Mediterranean Diet Conceptual Model and Future Trends of Its Use in Portugal. Health Promot. Int. 2021, 36, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Serra-Majem, L.; Carrasco, J.L.; Roman, B.; Ngo, J.; Bertomeu, I.; Obrador, B. The use of indexes evaluating the adherence to the Mediterranean diet in epidemiological studies: A review. Public Health Nutr. 2006, 9, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Naja, F.; Hwalla, N.; Itani, L.; Baalbaki, S.; Sibai, A.; Nasreddine, L. A novel Mediterranean diet index from Lebanon: Comparison with Europe. Eur. J. Nutr. 2015, 54, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Buckland, G.; Agudo, A.; Luján, L.; Jakszyn, P.; Bueno-de-Mesquita, H.B.; Palli, D.; Boeing, H.; Carneiro, F.; Krogh, V.; Sacerdote, C.; et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am. J. Clin. Nutr. 2010, 91, 381–390. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Agnoli, C.; Grioni, S.; Sieri, S.; Palli, S.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Giurdanella, M.C.; Pala, V.; et al. Italian Mediterranean Index and risk of colorectal cancer in the Italian section of the EPIC cohort. Int. J. Cancer 2013, 132, 1404–1411. [Google Scholar] [CrossRef]
- Monteagudo, C.; Mariscal-Arcas, M.; Rivas, A.; LOrenzo-Tovar, M.L.; Tur, J.A.; Olea-Serrano, F. Proposal of a Mediterranean Diet Serving Score. PLoS ONE 2015, 10, e0128594. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a literature-based adherence score to Mediterranean diet: The MEDI-LITE score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Obeid, C.A.; Gubbels, J.S.; Jaalouk, D.; Kremers, S.P.J.; Oenema, A. Adherence to the Mediterranean diet among adults in Mediterranean countries: A systematic literature review. Eur. J. Nutr. 2022, 61, 3327–3344. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Grant, W.B. Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer’s disease incidence and prevalence. J. Alzheimers Dis. 2015, 45, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Remesy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Cucciolla, V.; Della Ragione, F.; Galletti, P. Dietary polyphenols: Focus on resveratrol, a promising agent in the prevention of cardiovascular diseases and control of glucose homeostasis. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sundquist, A.R. Antioxidant functions of vitamins. Vitamins E and C.; beta-carotene, and other carotenoids. Ann. N. Y. Acad. Sci. 1992, 669, 7–20. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernandez, J. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Palozza, P.; Catalano, A.; Simone, R.; Cittadini, A. Lycopene as a guardian of redox signalling. Acta Biochim. Pol. 2012, 59, 21–25. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.A.; Qin, J.J.; Wang, W.; Wang, M.-H.; Wang, H.; Zhang, R. Ginsenosides as Anticancer Agents: In vitro and in vivo Activities, Structure-Activity Relationships, and Molecular Mechanisms of Action. Front. Pharmacol. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Dongowski, G.; Huth, M.; Gebhardt, E.; Flamme, W. Dietary fiber-rich barley products beneficially affect the intestinal tract of rats. J. Nutr. 2002, 132, 3704–3714. [Google Scholar] [CrossRef] [PubMed]
- Drzikova, B.; Dongowski, G.; Gebhardt, E. Dietary fibre-rich oat-based products affect serum lipids, microbiota, formation of short-chain fatty acids and steroids in rats. Br. J. Nutr. 2005, 94, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y.; Nyberg, L.; Bjorck, I. Muesli with 4 g oat beta-glucans lowers glucose and insulin responses after a bread meal in healthy subjects. Eur. J. Clin. Nutr. 2008, 62, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, H.; Jung, M.H.; Hong, S.; Song, J. Consumption of barley betaglucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010, 54, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Gibson, G.R.; Rastell, R.A. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J.G.; Lijeberg-Elmstahl, H.G. Consumption of both resistant starch and beta-glucan improves postprandial plasma glucose and insulin in women. Diabetes Care 2006, 29, 976–981. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Mahaling, B.; Low, S.W.Y.; Beck, M.; Kumar, D.; Ahmed, S.; Connor, T.B.; Ahmad, B.; Chaurasia, S.S. Damage-Associated Molecular Patterns (DAMPs) in Retinal Disorders. Int. J. Mol. Sci. 2022, 23, 2591. [Google Scholar] [CrossRef] [PubMed]
- Riemersma, R.A.; Wood, D.A.; Butler, S.; Elton, R.A.; Oliver, M.; Salo, M.; Nikkari, T.; Vartiainen, E.; Puska, P.; Gey, F.; et al. Linoleic acid content in adipose tissue and coronary heart disease. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Folsom, A.R.; Province, M.A.; Hunt, S.C.; Ellison, R.C. Dietary linolenic acid and carotid atherosclerosis: The National Heart, Lung, and Blood Institute Family Heart Study. Am. J. Clin. Nutr. 2003, 77, 819–825. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K.C. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef]
- Burdge, G. Alpha-linolenic acid metabolism in men and women: Nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The antiinflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Micek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune function and Micronutrient requirements change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Sharma, Y.; Saxena, S.; Mishra, A.; Saxena, A. Nutrition for diabetic retinopathy: Plummeting the inevitable threat of diabetic vision loss. Eur. J. Nutr. 2017, 56, 2013–2027. [Google Scholar] [CrossRef]
- Cammalleri, M.; Dal Monte, M.; Amato, R.; Bagnoli, P.; Rusciano, D. A dietary combination of forskolin with homotaurine, spearmint and b vitamins protects injured retinal ganglion cells in a rodent model of hypertensive glaucoma. Nutrients 2020, 12, 1189. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary sources and bioactivities of melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Maccarone, R.; Di Marco, S.; Bisti, S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Investig. Ophthal Vis. Sci. 2008, 49, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Rashid, R.; Saroya, S.; Deverapalli, M.; Brim, H.; Ashktorab, H. Saffron as a Promising Therapy for Inflammatory Bowel Disease. Nutrients 2024, 16, 2353. [Google Scholar] [CrossRef]
- Castelli, V.; Paladini, A.; d’Angelo, M.; Allegretti, M.; Mantelli, F.; Brandolini, L.; Cocchiaro, P.; Cimini, A.; Varrassi, G. Taurine and oxidative stress in retinal health and disease. CNS Neurosci. Ther. 2021, 27, 403–412. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Di Pierdomenico, J.; Hadj-Said, W.; Marie, M.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Picaud, S.; Villegas-Perez, M.P. Taurine depletion causes ipRGC loss and increases light-induced photoreceptor degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1396. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Giovannucci, E.L.; Rosner, B.A.; Sastry, S.M.; Willett, W.C.; Schaumberg, D.A. Dietary intakes of eicosapentaenoic acid and docosahexaenoic acid and risk of age-related macular degeneration. Ophthalmology 2017, 124, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tuo, J.; Wang, Z.; Zhu, A.; Machalinska, A.; Long, Q. Pathogenesis of Common Ocular Diseases. J. Ophthalmol. 2015, 2015, 734527. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.; Gallego-Pinazo, R.; García-Medina, J.J.; Zanon-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martinez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramirez, C.; Lopez-Galvez, M.I.; et al. Oxidative stress in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Zanón-Moreno, V.; García-Medina, J.J.; Arevalo, J.F.; Gallego-Pinazo, R.; Nucci, C. Eclectic Ocular Comorbidities and Systemic Diseases with Eye Involvement: A Review. BioMed Res. Int. 2016, 2016, 6215745. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, F.; D‘Aprile, S.; Denaro, S.; Pavone, A.M.; Alberghina, C.; Zappalà, A.; Giuffrida, R.; Salvatorelli, L.; Broggi, G.; Magro, G.G.; et al. Epigenetics and Metabolism Reprogramming Interplay into Glioblastoma: Novel Insights on Immunosuppressive Mechanisms. Antioxidants 2023, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.R. Life event, stress and illness. Malays. J. Med. Sci. 2008, 15, 9–18. [Google Scholar] [PubMed]
- Zarkovic, N. Roles and Functions of ROS and RNS in Cellular Physiology and Pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef]
- Yau, Y.H.C.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099–e00200. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; Dos Santos, N.B.; Scavone, C.; Demarchi Munhoz, C. Nrf2/ARE Pathway Modulation by Dietary Energy Regulation in Neurological Disorders. Front. Pharmacol. 2019, 10, 33. [Google Scholar] [CrossRef]
- Zhao, F.; Ci, X.; Man, X.; Li, J.; Wie, Z.; Zhang, S. Food-Derived Pharmacological Modulators of the Nrf2/ARE Pathway: Their Role in the Treatment of Diseases. Molecules 2021, 26, 1016. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Brunetti, K.; Del Quondam, S.; Cervia, D. Targeting Mitochondrial Dysfunction and Oxidative Stress to Prevent the Neurodegeneration of Retinal Ganglion Cells. Antioxidants 2023, 12, 2011. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Zeng, S.; Zhang, X.; Zhou, F.; Gillies, M.V.; Zhu, L. The Role of Nrf2/sMAF Signalling in Retina Ageing and Retinal Diseases. Biomedicines 2023, 11, 1512. [Google Scholar] [CrossRef]
- Liu, X.-F.; Zhou, D.-D.; Xie, T.; Hao, J.-L.; Malik, T.H.; Lu, C.-B.; Qi, J.; Pant, O.P.; Lu, C.-W. The Nrf2 Signaling in Retinal Ganglion Cells under Oxidative Stress in Ocular Neurodegenerative Diseases. Int. J. Biol. Sci. 2018, 14, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.-M.; Jiang, L.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef]
- Pérez, R.; Burgos, V.; Marín, V.; Camins, A.; Olloquequi, J.; Gonzales-Chavarria, I.; Ulrich, H.; Wyneke, U.; Luarte, A.; Ortiz, L.; et al. Caffeic Acid Phenethyl Ester (CAPE): Biosynthesis, Derivatives and Formulations with Neuroprotective Activities. Antioxidants 2023, 12, 1500. [Google Scholar] [CrossRef]
- Lecomte, S.; Demay, F.; Ferrière, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.Y.; Zhang, H.; He, J.; Yu, F. The Nrf2-Keap1 pathway is activated by steroid hormone signaling to govern neuronal remodeling. Cell Rep. 2021, 36, 109466. [Google Scholar] [CrossRef]
- Pontelli, R.C.N.; Souza, M.C.O.; Fantucci, M.Z.; de Andrade, M.; Rocha, E.M. The role of endocrine disruptors in ocular surface diseases. Med. Hypotheses 2019, 122, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, R.; Scalabrin, S.; Becco, A.; Panzica, G. Gonadal Hormones and Retinal Disorders: A Review. Front. Endocrinol. 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, A.; Ceklic, L.; Dysli, C.; Munk, M.R. Gender differences in retinal diseases: A review. Clin. Exp. Ophthalmol. 2024, 52, 317–333. [Google Scholar] [CrossRef]
- Wang, M.; Ren, Y.; Zheng, H.; Zhang, J.; Zhou, X. Correlative study on retinal microvascular changes and sex hormones in male patients with central serous chorioretinopathy. J. Men’s Health 2023, 19, 71–76. [Google Scholar]
- Ishii, T.; Warabi, E. Mechanism of Rapid Nuclear Factor-E2-Related Factor 2 (Nrf2) Activation via Membrane-Associated Estrogen Receptors: Roles of NADPH Oxidase 1, Neutral Sphingomyelinase 2 and Epidermal Growth Factor Receptor (EGFR). Antioxidants 2019, 8, 69. [Google Scholar] [CrossRef]
- Chu, C.; Gao, X.; Li, X.; Zhang, X.; Ma, R.; Jia, Y.; Li, D.; Wang, D.; Xu, F. Involvement of Estrogen Receptor-α in the Activation of Nrf2-Antioxidative Signaling Pathways by Silibinin in Pancreatic β-Cells. Biomol. Ther. 2020, 28, 163–171. [Google Scholar] [CrossRef]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.-Q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.-M.; Zhang, H.; Liu, Z.-D. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, X.; Shi, L.; Wang, L.; Chen, J.; Kang, C.; Zhu, J.; Mi, M. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS ONE 2013, 8, e79075. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Hsih, W.H.; Lin, Y.B.; Wen, C.Y.; Chang, T.J.J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. Diabetes Investig. 2021, 12, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simo’, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Serikbaeva, A.; Li, Y.; Ma, S.; Yi, D.; Kazlauskas, A. Resilience to diabetic retinopathy. Prog. Retin. Eye Res. 2024, 101, 101271. [Google Scholar] [CrossRef]
- Ahmed, T.S.; Shah, J.; Zhen, Y.N.B.; Chua, J.; Wong, D.W.K.; Nusinovici, S.; Tan, R.; Tan, G.; Schmetterer, L.; Tan, B. Ocular microvascular complications in diabetic retinopathy: Insights from machine learning. BMJ Open Diabetes Res. Care 2024, 12, e003758. [Google Scholar] [CrossRef] [PubMed]
- Kadłubowska, J.; Malaguarnera, L.; Wąż, P.; Zorena, K. Neurodegeneration and Neuroinflammation in Diabetic Retinopathy: Potential Approaches to Delay Neuronal Loss. Curr. Neuropharmacol. 2016, 14, 831–839. [Google Scholar] [CrossRef]
- Dow, C.; Mancini, F.; Rajaobelina, K.; Boutron-Ruault, M.C.; Balkau, B.; Bonnet, F.; Fagherazzi, G. Diet and risk of diabetic retinopathy: A systematic review. Eur. J. Epidemiol. 2018, 33, 141–156. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Díaz-López, A.; Valls-Pedret, C.; Cofan, M.; Garcia-Layana, A.; Lamuela-Raventos, R.M.; Castaner, O.; Zanon-Moreno, V.; Martinez-Gonzales, M.A.; Toledo, E.; et al. Prevención con Dieta Mediterránea (PREDIMED) Investigators. JAMA Ophthalmol. 2016, 134, 1142–1149. [Google Scholar] [CrossRef]
- Valero-Vello, M.; Peris-Martínez, C.; García-Medina, J.J.; Sanz-Gonzalez, S.M.; Ramirez, A.I.; Fernandez-Albarral, J.A.; Galarreta-Mira, D.; Zanon-Moreno, V.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D. Searching for the antioxidant, anti-inflammatory, and neuroprotective potential of natural food and nutritional supplements for ocular health in the mediterranean population. Foods 2021, 10, 1231. [Google Scholar] [CrossRef]
- Díaz-López, A.; Babio, N.; Martínez-González, M.A.; Corella, D.; Amor, A.J.; Fito’, M.; Estruch, R.; Aros, F.; Gomez-Gracia, E.; Fiol, M.; et al. Mediterranean Diet, Retinopathy, Nephropathy, and Microvascular Diabetes Complications: A Post Hoc Analysis of a Randomized Trial. Diabetes Care 2015, 38, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y. Dietary Intake of Omega-3 Fatty Acids From Fish and Risk of Diabetic Retinopathy. JAMA 2017, 317, 2226–2227. [Google Scholar] [CrossRef] [PubMed]
- Bryl, A.; Mrugacz, M.; Falkowski, M.; Zorena, K. A Mediterranean Diet May Be Protective in the Development of Diabetic Retinopathy. Int. J. Mol. Sci. 2023, 24, 11145. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M. Dual antiplatelet therapy may lead to hemorrhagic complications in vitrectomy of proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 425–426. [Google Scholar] [CrossRef]
- Yuan, D.; Xu, Y.; Xue, L.; Zhang, W.; Gu, L.; Liu, Q. Resveratrol protects against diabetic retinal ganglion cell damage by activating the Nrf2 signaling pathway. Heliyon 2024, 10, e30786. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and Ophthalmic Diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef]
- Mirmiran, P.; Hosseinpour-Niazi, S.; Naderi, Z.; Bahadoran, Z.; Sadeghi, M.; Azizi, F. Association between interaction and ratio of omega-3 and omega-6 polyunsaturated fatty acid and the metabolic syndrome in adults. Nutrition 2012, 28, 856–863. [Google Scholar] [CrossRef]
- Kawasaki, R.; Tanaka, S.; Tanaka, S.; Yamamoto, T.; Sone, H.; Ohashi, Y.; Akanuma, Y.; Yamada, N.; Yamashita, H.; Japan Diabetes Complications Study Group. Incidence and progression of diabetic retinopathy in Japanese adults with type 2 diabetes: 8 year follow-up study of the Japan Diabetes Complications Study (JDCS). Diabetologia 2011, 54, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Brazionis, L.; Rowley, K.; Itsiopoulos, C.; O’Dea, K. Plasma carotenoids and diabetic retinopathy. Br. J. Nutr. 2009, 101, 270–277. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Pinazo-Duran, M.D.; Garcia-Medina, M.; Zanon-Moreno, V.; Pons-Vazquez, S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011, 21, 637–643. [Google Scholar] [CrossRef]
- Tanaka, S.; Yoshimura, Y.; Kawasaki, R.; Kamada, C.; Tanaka, S.; Horikawa, C.; Ohashi, Y.; Araki, A.; Ito, H.; Akamura, Y.; et al. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology 2013, 24, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Post, R.E.; Mainous, A.G., 3rd; King, D.E.; Simpson, K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board. Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef]
- Bryl, A.; Mrugacz, M.; Mariak, Z. Blood flow in vessels supplying the eye in persons with degenerative myopia. Part II. Blood flow in the central retinal artery. Klin. Ocz. 2013, 115, 222–225. [Google Scholar]
- Chatziralli, I.P.; Theodossiadis, G.; Dimitriadis, P.; Charalambidis, M.; Agorastos, A.; Migkos, Z.; Platogiannis, N.; Moschos, M.M.; Theodossiadis, P.; Keryttopoulos, P. The Effect of Vitamin E on Oxidative Stress Indicated by Serum Malondialdehyde in Insulin-dependent Type 2 Diabetes Mellitus Patients with Retinopathy. Open Ophthalmol. J. 2017, 11, 51–58. [Google Scholar] [CrossRef]
- Bursell, S.E.; Clermont, A.C.; Aiello, L.P.; Aiello, L.M.; Schlossman, D.K.; Feener, E.P.; Laffel, L.; King, G.L. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care 1999, 22, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Hernandez, C. What else can we do to prevent diabetic retinopathy? Diabetologia 2023, 66, 1614–1621. [Google Scholar] [CrossRef]
- Barba, I.; Garcia-Ramirez, M.; Hernandez, C.; Alonso, M.A.; Masmiquel, L.; Garcia-Dorado, D.; Simo, R. Metabolic fingerprints of proliferative diabetic retinopathy: An 1H-NMR-based metabonomic approach using vitreous humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4416–4421. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Ghim, W.; Oh, S.; Kim, Y.; Park, U.C.; Kang, J.; Yu, H.G. Association of vitreous vitamin C depletion with diabetic macular ischemia in proliferative diabetic retinopathy. PLoS ONE 2019, 14, e0218433. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Cheong, Z.Y.; Tan, B.; Wong, D.; Liu, X.; Chua, J. Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature. Nutrients 2022, 14, 5021. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Cukras, C.A.; Chew, E.Y. Age-Related Macular Degeneration: Epidemiology and Clinical Aspects. Adv. Exp. Med. Biol. 2021, 1256, 1–31. [Google Scholar]
- Hogg, R.E.; Woodside, J.V.; McGrath, A.; Young, I.S.; Vioque, J.L.; Chakravarthy, U.; de Jong, P.T.; Rahu, M.; Seland, J.; Soubrane, G.; et al. Mediterranean Diet Score and Its Association with Age-Related Macular Degeneration: The European Eye Study. Ophthalmology 2017, 124, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Mares, J.A.; Voland, R.P.; Sondel, S.A.; Millen, A.E.; Larowe, T.; Moeller, S.M.; Klein, M.L.; Blodi, B.A.; Chappell, R.J.; Tinker, L.; et al. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch. Ophthalmol. 2011, 129, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Colijn, J.M.; Cougnard-Grégoire, A.; de KOnig-Backus, A.P.M.; Delyfer, M.-N.; Kiefte-de Jong, J.C.; Meester-Smoor, M.; Feart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean Diet and Incidence of Advanced Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Rosner, B.; Seddon, J.M. Genetic Susceptibility, Diet Quality, and Two-Step Progression in Drusen Size. Investig. Ophthalmol. Vis. Sci. 2020, 61, 17. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Agrón, E.; Mares, J.; Chew, E.Y.; Keenan, T.D.L.; AREDS2 Research Group. Adherence to a Mediterranean Diet and Geographic Atrophy Enlargement Rate: Age-Related Eye Disease Study 2 Report 29. Ophthalmol. Retin. 2022, 6, 762–770. [Google Scholar] [CrossRef]
- Yiu, G.; Chiu, S.J.; Petrou, P.A.; Stinnett, S.; Sarin, N.; Farsiu, S.; Chew, E.Y.; Wong, W.T.; Toth, C.A. Relationship of central choroidal thickness with age-related macular degeneration status. Am. J. Ophthalmol. 2015, 159, 617–626. [Google Scholar] [CrossRef]
- Zheng, Y.; Lamoureux, E.L.; Lavanya, R.; Wu, R.; Ikram, M.K.; Wang, J.J.; Mitchell, P.; Cheung, N.; Aung, T.; saw, S.-M.; et al. Prevalence and risk factors of diabetic retinopathy in migrant Indians in an urbanized society in Asia: The Singapore Indian eye study. Ophthalmology 2012, 119, 2119–2124. [Google Scholar] [CrossRef]
- Nonyane, B.A.S.; Nitsch, D.; Whittaker, J.C.; Sofat, R.; Smeeth, L.; Chakravarthy, U.; Fletcher, A.E. An ecological correlation study of late age-related macular degeneration and the complement factor H Y402H polymorphism. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2393–2402. [Google Scholar] [CrossRef][Green Version]
- Wang, J.J.; Buitendijk, G.H.; Rochtchina, E.; Lee, K.E.; Klein, B.E.K.; van Duijn, C.M.; Flood, V.M.; Meuer, S.M.; Attia, J.; Myers, C.; et al. Genetic susceptibility, dietary antioxidants, and long-term incidence of age-related macular degeneration in two populations. Ophthalmology 2014, 121, 667–675. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; den Hollander, A.I.; Demirkan, A.; Cougnard-Gregoire, A.; Verzijden, T.; Kersten, E.; Meester-Smoor, M.A.; Merle, B.M.J.; Papageorgiou, G.; Ahmad, S.; et al. Increased High-Density Lipoprotein Levels Associated with Age-Related Macular Degeneration: Evidence from the EYE-RISK and European Eye Epidemiology Consortia. Ophthalmology 2019, 126, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shi, X.; Fan, Y.; Wang, D.; Li, B.; Zhou, J.; Pei, C.; Ma, L. Dietary omega-3 polyunsaturated fatty acids and fish intake and risk of age-related macular degeneration. Clin. Nutr. 2021, 40, 5662–5673. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Rosner, B.; Sperduto, R.D.; Yannuzzi, L.; Haller, J.A.; Blair, N.P.; Willett, W. Dietary fat and risk for advanced age-related macular degeneration. Arch. Ophthalmol. 2001, 119, 1191–1199. [Google Scholar] [CrossRef]
- Augood, C.; Chakravarthy, U.; Young, I.; Vioque, J.; de Jong, P.T.V.M.; Bentham, G.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am. J. Clin. Nutr. 2008, 88, 398–406. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef]
- Anekonda, T.S.; Adamus, G. Resveratrol prevents antibody-induced apoptotic death of retinal cells through upregulation of Sirt1 and Ku70. BMC Res. Notes 2008, 1, 122. [Google Scholar] [CrossRef]
- Bryl, A.; Falkowski, M.; Zorena, K.; Mrugacz, M. The Role of Resveratrol in Eye Diseases—A Review of the Literature. Nutrients 2022, 14, 2974. [Google Scholar] [CrossRef]
- Richer, S.; Stiles, W.; Ulanski, L.; Carroll, D.; Podella, C. Observation of human retinal remodeling in octogenarians with a resveratrol based nutritional supplement. Nutrients 2013, 5, 1989–2005. [Google Scholar] [CrossRef]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T.J.M. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef]
- Koushan, K.; Rusovici, R.; Li, W.; Ferguson, L.R.; Chalam, K.V. The role of lutein in eye-related disease. Nutrients 2013, 5, 1823–1839. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.M., 2nd; Snodderly, D.M.; Johnson, E.J.; Schalch, W.; Koepcke, W.; Gerss, J.; Neuringer, M. Nutritional manipulation of primate retinas, V: Effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3934–3942. [Google Scholar] [CrossRef] [PubMed]
- Woodall, A.A.; Lee, S.W.; Weesie, R.J.; Jackson, M.J.; Britton, G. Oxidation of carotenoids by free radicals: Relationship between structure and reactivity. Biochim. Biophys. Acta 1997, 1336, 33–42. [Google Scholar] [CrossRef]
- Sundelin, S.P.; Nilsson, S.E. Lipofuscin-formation in retinal pigment epithelial cells is reduced by antioxidants. Free Radic. Biol. Med. 2001, 31, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Schalch, W.; Cohn, W.; Barker, F.M.; Kopcke, W.; Mellerio, J.; Bird, A.C.; Robson, A.G.; Fitzke, F.F.; van Kuijk, F.J.G.M. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin—The LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch. Biochem. Biophys. 2007, 458, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.-Y.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhofer, G.; Schmidt-Erfurth, U.; et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar] [CrossRef]
- Schmidl, D.; Garhofer, G.; Schmetterer, L. Nutritional supplements in age-related macular degeneration. Acta Ophthalmol. 2015, 93, 105–121. [Google Scholar] [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and Zeaxanthin-Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, vitamins A.; C.; and E.; and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 7, CD000253. [Google Scholar] [CrossRef] [PubMed]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Davis, M.D.; Ferris, F.L., 3rd; Gensler, G.R.; Kurinij, N.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch. Ophthalmol. 2007, 125, 671–679. [Google Scholar] [PubMed]
- Wiegand, R.D.; Joel, C.D.; Rapp, L.M.; Nielsen, J.C.; Maude, M.B.; Anderson, R.E. Polyunsaturated fatty acids and vitamin E in rat rod outer segments during light damage. Investig. Ophthalmol. Vis. Sci. 1986, 27, 727–733. [Google Scholar]
- Tanito, M.; Yoshida, Y.; Kaidzu, S.; Chen, Z.-H.; Cynshi, O.; Jishage, K.-I.; Niki, E.; Ohira, A. Acceleration of age-related changes in the retina in alpha-tocopherol transfer protein null mice fed a Vitamin E-deficient diet. Investig. Ophthalmol. Vis. Sci. 2007, 48, 396–404. [Google Scholar] [CrossRef]
- West, S.; Vitale, S.; Hallfrisch, J.; Munoz, B.; Muller, D.; Bressler, S.; Bressler, N.M. Are antioxidants or supplements protective for age-related macular degeneration? Arch. Ophthalmol. 1994, 112, 222–227. [Google Scholar] [CrossRef]
- Delcourt, C.; Cristol, J.P.; Tessier, F.; Leger, C.L.; Descomps, B.; Papoz, L. Age-related macular degeneration and antioxidant status in the POLA study. POLA Study Group. Pathologies Oculaires Liees a l’Age. Arch. Ophthalmol. 1999, 117, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Tikellis, G.; Robman, L.D.; McCarty, C.A.; McNeil, J.J. Vitamin E supplementation and macular degeneration: Randomised controlled trial. BMJ 2002, 325, 11. [Google Scholar] [CrossRef] [PubMed]
- Teikari, J.M.; Laatikainen, L.; Rapola, J.M.; Virtamo, J.; Haukka, J.; Liesto, K.; Heinonen, O.P. Retinal vascular changes following supplementation with alpha-tocopherol or beta-carotene. Acta Ophthalmol. Scand. 1998, 76, 68–73. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, A.; Zou, M.; Zhang, Y.; Jin, L.; Li, Y.; Zheng, D.; Jin, G.; Congdon, N. Time trends, associations and prevalence of blindness and vision loss due to glaucoma: An analysis of observational data from the Global Burden of Disease Study 2017. BMJ Open 2022, 12, e053805. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the early manifest glaucoma trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Society, E.G. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 2021, 105, 1–169. [Google Scholar]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Neuronal death in glaucoma. Prog. Retin. Eye Res. 1999, 18, 39–57. [Google Scholar] [CrossRef]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. North. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hayashi, H.; Nakao, F.; Hayashi, F. Intraocular lens tilt and decentration after implantation in eyes with glaucoma. J. Cataract. Refract. Surg. 1999, 25, 1515–1520. [Google Scholar] [CrossRef]
- Pérez-De-Arcelus, M.; Toledo, E.; Martínez-González, M.Á.; Martin-Calvo, N.; Fernandez-Montero, A.; Moreno-Montanes, J. Smoking and incidence of glaucoma. The SUN Cohort. Medicine 2017, 96, e5761. [Google Scholar] [CrossRef]
- Kreft, D.; Doblhammer, G.; Guthoff, R.F.; Frech, S. Prevalence, incidence, and risk factors of primary open-angle glaucoma—A cohort study based on longitudinal data from a German public health insurance. BMC Public Health 2019, 19, 851. [Google Scholar] [CrossRef]
- Yadav, K.S.; Rajpurohit, R.; Sharma, S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci. 2019, 221, 362–376. [Google Scholar] [CrossRef]
- Vergroesen, J.E.; de Crom, T.O.E.; van Duijn, C.M.; Voortman, T.; Klaver, C.C.W.; Ramdas, W.D. MIND diet lowers risk of open-angle glaucoma: The Rotterdam Study. Eur. J. Nutr. 2023, 62, 477–487. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, Y.; Yuan, Y.; Xiong, R.; Hu, Y.; Ning, K.; Ha, J.; Wang, W.; Han, X.; He, M. The Mediterranean Diet and Age-Related Eye Diseases: A Systematic Review. Nutrients 2023, 15, 2043. [Google Scholar] [CrossRef]
- Moreno-Montañés, J.; Gándara, E.; Gutierrez-Ruiz, I.; Moreno-Galarraga, L.; Ruiz-Canela, M.; Bes-Rastrollo, M.; Martinez-Gonzalez, M.A.; Fernandez-Montero, A. Healthy Lifestyle Score and Incidence of Glaucoma: The Sun Project. Nutrients 2022, 14, 779. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Buys, E.S.; Sappington, R.M. The nitric oxide-guanylate cyclase pathway and glaucoma. Nitric Oxide Biol. Chem. 2018, 77, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Stephan, B.C.M.; Minihane, A.M.; Mathers, J.C.; Siervo, M. Nitric Oxide Boosting Effects of the Mediterranean Diet: A Potential Mechanism of Action. J. Gerontol. Ser. Biol. Sci. Med. Sci. 2018, 73, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Reina-Torres, E.; De Ieso, M.L.; Pasquale, L.R.; Madekurozwa, M.; van Batenburg-Sherwood, J.; Overby, D.R.; Stamer, W.D. The vital role for nitric oxide in intraocular pressure homeostasis. Prog. Retin. Eye Res. 2021, 83, 100922. [Google Scholar] [CrossRef]
- Kang, J.H.; Willett, W.C.; Rosner, B.A.; Buys, E.; Wiggs, J.L.; Pasquale, L.R. Association of dietary nitrate intake with primary open-angle glaucoma: A prospective analysis from the nurses’ health study and health professionals follow-up study. JAMA Ophthalmol. 2016, 134, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Reyes, J.A.; Álvarez-Luis, D.; Arteaga-Hernández, V.; Sanchez-Mendez, M.; Abreu-Gonzalez, R. Mediterranean diet adherence by patients with primary open angle glaucoma Adherencia a la dieta mediterránea en pacientes afectos de glaucoma primario de ángulo abierto. Arch. Soc. Esp. Oftalmol. 2017, 92, 353–358. [Google Scholar] [CrossRef]
- Mvitu, M.; Longo-Mbenza, B.; Tulomba, M.D.; Nge, A. Reduced risk of metabolic syndrome due to regular intake of vegetables rich in antioxidants among African type 2 diabetics. Diabetes Metab. Syndr. Clin. Res. Rev. 2010, 4, 132–136. [Google Scholar] [CrossRef]
- Rusciano, D.; Pezzino, S.; Mutolo, M.G.; Giannotti, R.; Librando, A.; Pescosolido, N. Neuroprotection in Glaucoma: Old and New Promising Treatments. Adv. Pharmacol. Sci. 2017, 2017, 4320408. [Google Scholar] [CrossRef]
- D’Angelo, A.; Vitiello, L.; Lixi, F.; Abbinante, G.; Coppola, A.; Gagliardi, V.; Pellegrino, A.; Giannaccare, G. Optic Nerve Neuroprotection in Glaucoma: A Narrative Review. J. Clin. Med. 2024, 13, 2214. [Google Scholar] [CrossRef]
- Ramdas, W.D. The relation between dietary intake and glaucoma: A systematic review. Acta Ophthalmol. 2018, 96, 550–556. [Google Scholar] [CrossRef]
- Ohguro, H.; Ohguro, I.; Katai, M.T.; Tanaka, S. Two-Year Randomized, Placebo-Controlled Study of Black Currant Anthocyanins on Visual Field in Glaucoma. Ophthalmologica 2012, 228, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sohn, S.W.; Kee, C. Effect of Ginkgo Biloba Extract on Visual Field Progression in Normal Tension Glaucoma. J. Glaucoma 2013, 22, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Garcia-Medina, M.; Garrido-Fernandez, P.; Galvan-Espinosa, J.; Garcia-Maturana, C.; Zanon-Moreno, V.; Pinazo-Duran, M.D. A Two-Year Follow-up of Oral Antioxidant Supplementation in Primary Open-Angle Glaucoma: An Open-Label, Randomized, Controlled Trial. Acta Ophthalmol. 2015, 93, 546–554. [Google Scholar] [CrossRef]
- Keppel Hesselink, J.M.; Costagliola, C.; Fakhry, J.; Kopsky, D.J. Palmitoylethanolamide, a natural retinoprotectant: Its putative relevance for the treatment of glaucoma and diabetic retinopathy. J. Ophthalmol. 2015, 2015, 430596. [Google Scholar] [CrossRef] [PubMed]
- Hecht, I.; Achiron, A.; Bartov, E.; Maharshak, I.; Mendel, L.; Peer, L.; Bar, A.; Burgansky-Eliash, Z. Effects of dietary and lifestyle recommendations on patients with glaucoma: A randomized controlled pilot trial. Eur. J. Integr. Med. 2019, 25, 60–66. [Google Scholar] [CrossRef]
- Jabbarpoor Bonyadi, M.H.; Yazdani, S.; Saadat, S. The ocular hypotensive effect of saffron extract in primary open angle glaucoma: A pilot study. BMC Complement. Altern. Med. 2014, 14, 399. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; Lopez-Villarin, N.; Salobrar-Garcia, E.; Lopez-Cuenca, I.; Licastro, E.; Inarejos-Garcia, A.M.; Almodovar, P.; Pinazo-Duran, M.; et al. Neuroprotective and anti-inflammatory effects of a hydrophilic saffron extract in a model of glaucoma. Int. J. Mol. Sci. 2019, 20, 4110. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Aggul, A.G.; Taslimi, P.; Kuzu, M.; Uzun, N.; Bilginer, S.; Gulcin, I. Gulcin, Oleuropein and verbascoside—Their inhibition effects on carbonic anhydrase and molecular docking studies. J. Oleo Sci. 2021, 70, ess21106. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell. Biochem. 2014, 75, 349–359. [Google Scholar]
- Patel, S.; Mathan, J.J.; Vaghefi, E.; Braakhuis, A.J. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, V.; van Wijngaarden, P.; Steinberg, G.R.; Crowston, J.G. A short term high-fat high-sucrose diet in mice impairs optic nerve recovery after injury and this is not reversed by exercise. Exp. Eye Res. 2017, 162, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.X.; van Bergen, N.; Bui, B.V.; Chrysostomou, V.; Vingrys, A.J.; Trounce, I.A.; Crowston, J.G. Impact of aging and diet restriction on retinal function during and after acute intraocular pressure injury. Neurobiol. Aging 2012, 33, e15–e25. [Google Scholar] [CrossRef]
- Guo, X.; Kimura, A.; Azuchi, Y.; Akiyama, G.; Noro, T.; Harada, C.; Namekata, K.; Harada, T. Caloric restriction promotes cell survival in a mouse model of normal tension glaucoma. Sci. Rep. 2016, 6, 33950. [Google Scholar] [CrossRef]
- Li, M.; Gao, Z.L.; Zhang, Q.P.; Luo, A.-X.; Xu, W.-Y.; Duan, T.-Q.; Wen, X.-P.; Zhang, R.-Q.; Zeng, R.; Huang, J.-F. Autophagy in glaucoma pathogenesis: Therapeutic potential and future perspectives. Front. Cell Dev. Biol. 2022, 10, 1068213. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nazio, F.; Tettamanti, G.; Girardello, R.; Cianfanelli, V.; Cavaliere, F.; Morrone, L.A.; Corasaniti, M.T.; et al. Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival. Cell Death Dis. 2018, 9, 981. [Google Scholar] [CrossRef]
- Alkozi, H.; Sánchez-Naves, J.; de Lara, M.J.; Carracedo, G.; Fonseca, B.; Martinez-Auguila, A.; Pintor, J. Elevated intraocular pressure increases melatonin levels in the aqueous humour. Acta Ophthalmol. 2017, 95, e185–e189. [Google Scholar] [CrossRef]
- Hu, C.; Feng, Y.; Huang, G.; Cui, K.; Fan, M.; Xiang, W.; Shi, Y.; Ye, D.; Ye, H.; Bai, X.; et al. Melatonin prevents EAAC1 deletion-induced retinal ganglion cell degeneration by inhibiting apoptosis and senescence. J. Pineal Res. 2024, 76, e12916. [Google Scholar] [CrossRef]
- Agoraatos, A.; Huber, C.G. The role of melatonin in glaucoma: Implications concerning pathophysiological relevance and therapeutic potential. J. Pineal Res. 2011, 50, 1–7. [Google Scholar] [CrossRef]
- Romeo, A.; Kazsoki, A.; Musumeci, T.; Zelkó, R. A Clinical, Pharmacological, and Formulation Evaluation of Melatonin in the Treatment of Ocular Disorders-A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3999. [Google Scholar] [CrossRef]
- Aydin, E.; Sahin, S. Increased melatonin levels in aqueous humor of patients with proliferative retinopathy in type 2 diabetes mellitus. Int. J. Ophthalmol. 2016, 9, 721–724. [Google Scholar] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Lopez-Bernal, M.D.; Cobo-Martinez, A.; Zanon-Moreno, V.; Pinazo-Duran, M.D.; Del-Rio-Vellosillo, M. Glaucoma and Antioxidants: Review and Update. Antioxidants 2020, 9, 1031. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbai, O.; Torrisi, F.; Fabrizio, F.P.; Rabbeni, G.; Perrone, L. Effect of the Mediterranean Diet (MeDi) on the Progression of Retinal Disease: A Narrative Review. Nutrients 2024, 16, 3169. https://doi.org/10.3390/nu16183169
Sbai O, Torrisi F, Fabrizio FP, Rabbeni G, Perrone L. Effect of the Mediterranean Diet (MeDi) on the Progression of Retinal Disease: A Narrative Review. Nutrients. 2024; 16(18):3169. https://doi.org/10.3390/nu16183169
Chicago/Turabian StyleSbai, Oualid, Filippo Torrisi, Federico Pio Fabrizio, Graziella Rabbeni, and Lorena Perrone. 2024. "Effect of the Mediterranean Diet (MeDi) on the Progression of Retinal Disease: A Narrative Review" Nutrients 16, no. 18: 3169. https://doi.org/10.3390/nu16183169
APA StyleSbai, O., Torrisi, F., Fabrizio, F. P., Rabbeni, G., & Perrone, L. (2024). Effect of the Mediterranean Diet (MeDi) on the Progression of Retinal Disease: A Narrative Review. Nutrients, 16(18), 3169. https://doi.org/10.3390/nu16183169