Effects and Mechanisms of Polyunsaturated Fatty Acids on Age-Related Musculoskeletal Diseases: Sarcopenia, Osteoporosis, and Osteoarthritis—A Narrative Review
Abstract
1. Introduction
2. Methods
3. Sarcopenia
3.1. Prevalence of Sarcopenia
3.2. Pathogenesis of Sarcopenia
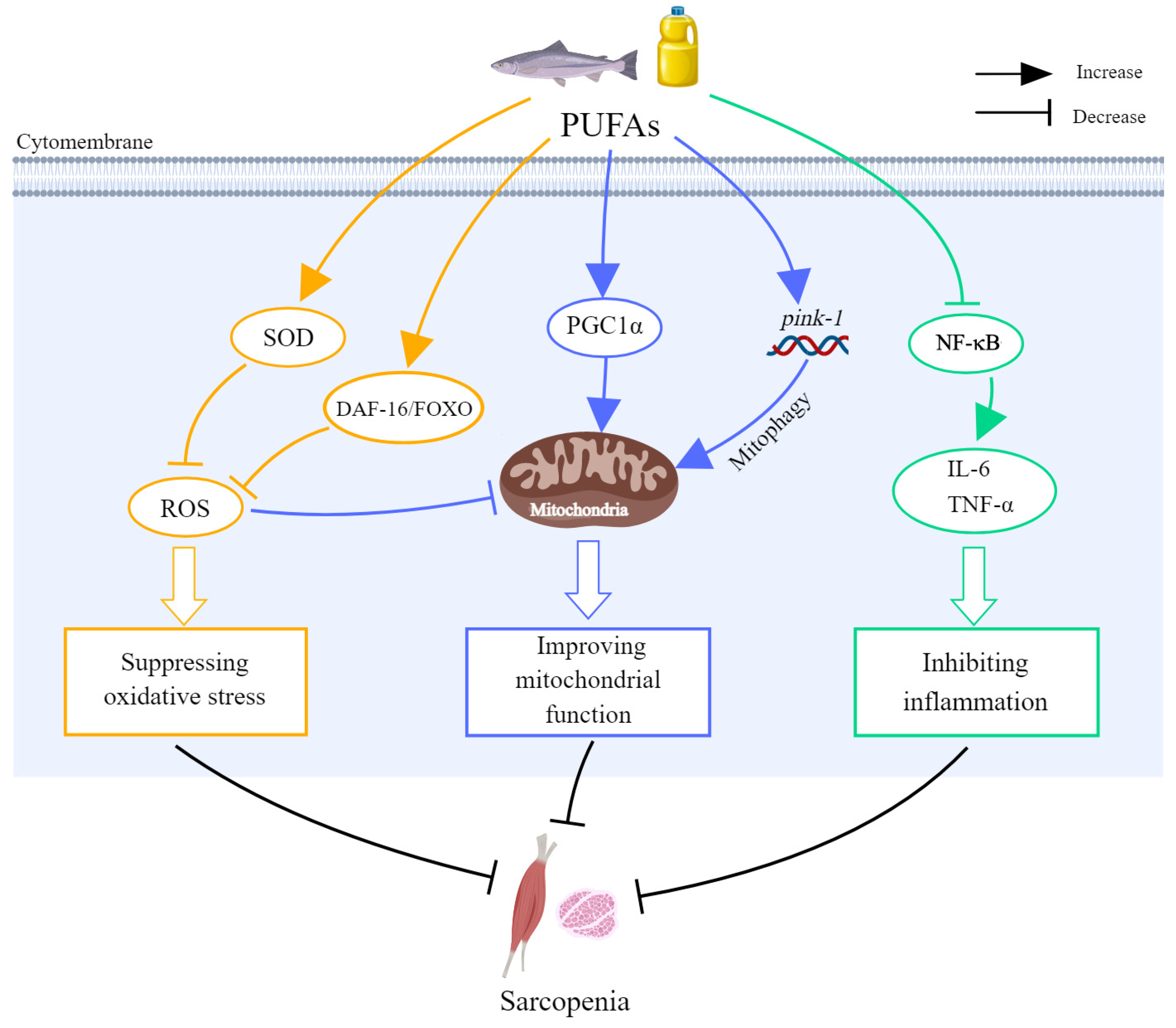
3.3. Effects of PUFAs on Sarcopenia
4. Osteoporosis
4.1. Prevalence of Osteoporosis
4.2. Pathogenesis of Osteoporosis
4.3. Effects of PUFAs on Osteoporosis
5. Osteoarthritis
5.1. Prevalence of Osteoarthritis
5.2. Pathogenesis of Osteoarthritis
5.3. Effects of PUFAs on Osteoarthritis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Decade of Healthy Ageing: Baseline Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular senescence: Aging, cancer, and injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Cai, H.-L.; Bao, J.-P.; Wu, L.-D. Dehydroepiandrosterone and age-related musculoskeletal diseases: Connections and therapeutic implications. Ageing Res. Rev. 2020, 62, 101132. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Gray-Gaillard, E.F.; Elisseeff, J.H. Cellular senescence in musculoskeletal homeostasis, diseases, and regeneration. Bone Res. 2021, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.J.; Wu, F.; Guo, Y.; Robledo, L.M.G.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L.; Pufah Group. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ-Br. Med. J. 2019, 366, l4697. [Google Scholar] [CrossRef]
- Bork, C.S.; Venø, S.K.; Lasota, A.N.; Lundbye-Christensen, S.; Schmidt, E.B. Marine and plant-based n-3 PUFA and atherosclerotic cardiovascular disease. Proc. Nutr. Soc. 2020, 79, 22–29. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Prevention of cardiovascular events with omega-3 polyunsaturated fattyacids and the mechanism involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef]
- Dierge, E.; Debock, E.; Guilbaud, C.; Corbet, C.; Mignolet, E.; Mignard, L.; Bastien, E.; Dessy, C.; Larondelle, Y.; Feron, O. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 2021, 33, 1701–1715 e1705. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; Campos, M.M. Protective effects of omega-3 fatty acids in cancer-related complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A. Role of mTORC1 in mechanically induced increases in translation and skeletal muscle mass. J. Appl. Physiol. 2019, 127, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPARgamma as a molecular target of EPA anti-inflammatory activity during TNF-alpha-impaired skeletal muscle cell differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.; Leslie, M.; Moghadasian, M.H.; Arendt, B.M.; Allard, J.P.; Ma, D.W.L. The role of n-6 and n-3 polyunsaturated fatty acids in the manifestation of the metabolic syndrome in cardiovascular disease and non-alcoholic fatty liver disease. Food Funct. 2014, 5, 426–435. [Google Scholar] [CrossRef]
- Mustonen, A.-M.; Nieminen, P. Dihomo-gamma-linolenic acid (20:3n-6)-metabolism, derivatives, and potential significance in chronic inflammation. Int. J. Mol. Sci. 2023, 24, 2116. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; Souza, F.d.C.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Burdge, G.C. Is essential fatty acid interconversion an important source of PUFA in humans? Br. J. Nutr. 2019, 121, 615–624. [Google Scholar] [CrossRef]
- Lins, M.; Puppin Zandonadi, R.; Raposo, A.; Ginani, V. Food waste on foodservice: An overview through the perspective of sustainable dimensions. Foods 2021, 10, 1175. [Google Scholar] [CrossRef]
- Capdeville, F. Narrative Review—An Overview; Produção e Consumo Sustentáveis; Ministério do Meio Ambiente—Departamento de Produção e Consumo Sustentáveis—DPCS: Brasilia, Brasil, 2012. [Google Scholar]
- Traub, J.; Bergheim, I.; Eibisberger, M.; Stadlbauer, V. Sarcopenia and liver cirrhosis-comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients 2020, 12, 547. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Bosaeus, I.; Rothenberg, E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc. Nutr. Soc. 2016, 75, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Hai, S.; Wang, H.; Cao, L.; Liu, P.; Zhou, J.; Yang, Y.; Dong, B. Association between sarcopenia with lifestyle and family function among community-dwelling Chinese aged 60 years and older. BMC Geriatr. 2017, 17, 187. [Google Scholar] [CrossRef]
- Liu, X.; Hou, L.; Xia, X.; Liu, Y.; Zuo, Z.; Zhang, Y.; Zhao, W.; Hao, Q.; Yue, J.; Dong, B. Prevalence of sarcopenia in multi ethnics adults and the association with cognitive impairment: Findings from West-China health and aging trend study. BMC Geriatr. 2020, 20, 63. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. Screening sarcopenia in community-dwelling older adults: SARC-F vs SARC-F combined with Calf circumference (SARC-CalF). J. Am. Med. Dir. Assoc. 2018, 19, 277 e271–277 e278. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metab.-Clin. Exp. 2023, 144, 155533. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R. Molecular mechanism and pathogenesis of sarcopenia: An overview. Int. J. Mol. Sci. 2021, 22, 3032. [Google Scholar] [CrossRef] [PubMed]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: A narrative review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of age-related mitochondrial dysfunction in sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Carosio, S.; Berardinelli, M.G.; Aucello, M.; Musarò, A. Impact of ageing on muscle cell regeneration. Ageing Res. Rev. 2011, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive oxygen and nitrogen species regulate key metabolic, anabolic, and catabolic pathways in skeletal muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef]
- Dumont, N.A.; Wang, Y.X.; Rudnicki, M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 2015, 142, 1572–1581. [Google Scholar] [CrossRef]
- Huo, F.; Liu, Q.; Liu, H. Contribution of muscle satellite cells to sarcopenia. Front. Physiol. 2022, 13, 892749. [Google Scholar] [CrossRef]
- Price, F.D.; von Maltzahn, J.; Bentzinger, C.F.; Dumont, N.A.; Yin, H.; Chang, N.C.; Wilson, D.H.; Frenette, J.; Rudnicki, M.A. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 2014, 20, 1174–1181. [Google Scholar] [CrossRef]
- Liu, L.; Charville, G.W.; Cheung, T.H.; Yoo, B.; Santos, P.J.; Schroeder, M.; Rando, T.A. Impaired notch signaling leads to a decrease in p53 activity and mitotic catastrophe in aged muscle stem cells. Cell Stem Cell 2018, 23, 544–556 e544. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia. Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Meng, S.-J.; Yu, L.-J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Lee, K.H.; Nam, S.W.; Kim, J.S.; Yang, J.W.; Lee, J.Y.; Smith, L.; et al. Sarcopenia in autoimmune and rheumatic diseases: A comprehensive review. Int. J. Mol. Sci. 2020, 21, 5678. [Google Scholar] [CrossRef] [PubMed]
- Standley, R.; Liu, S.; Jemiolo, B.; Trappe, S.; Trappe, T. Prostaglandin E2 induces transcription of skeletal muscle mass regulators interleukin-6 and muscle RING finger-1 in humans. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Wauters, E.; Dedeyne, L.; Vercauteren, L.; Amini, N.; Lapauw, L.; Matthys, C.; Verschueren, S.; Tournoy, J.; Koppo, K.; et al. Are dietary intake and nutritional status of specific polyunsaturated fatty acids correlated with sarcopenia outcomes in community-dwelling older adults with sarcopenia?—Exploratory results from ENHANce. BMC Geriatr. 2023, 23, 272. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, A.S.; Limirio, L.S.; Santos, H.O.; de Oliveira, E.P. Intake of polyunsaturated fatty acids and to ω-3 are protective factors for sarcopenia in kidney transplant patients. Nutrition 2021, 81, 110929. [Google Scholar] [CrossRef]
- Bae, Y.-J.; Cui, X.-S.; Shin, S.-H. Increased omega-3 fatty acid intake Is associated with low grip strength in ederly Korean females. Nutrients 2022, 14, 2374. [Google Scholar] [CrossRef]
- Belury, M.A.; Cole, R.M.; Andridge, R.; Keiter, A.; Raman, S.V.; Lustberg, M.B.; Kiecolt-Glaser, J.K. Erythrocyte long-chain ω-3 fatty acids are positively associated with lean mass and grip strength in women with recent diagnoses of breast cancer. J. Nutr. 2021, 151, 2125–2133. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Miki, A.; Kaji, A.; Sakai, R.; Iwai, K.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of KAMOGAWA-DM cohort study. J. Clin. Biochem. Nutr. 2020, 66, 233–237. [Google Scholar] [CrossRef]
- Itoh, S.; Nagao, Y.; Morita, K.; Kurihara, T.; Tomino, T.; Kosai-Fujimoto, Y.; Harada, N.; Fujita, N.; Ushijima, Y.; Mori, M.; et al. Association between sarcopenia and omega-3 polyunsaturated fatty acid in patients with hepatocellular carcinoma. JMA J. 2022, 5, 169–176. [Google Scholar] [CrossRef]
- Ghzaiel, I.; Zarrouk, A.; Nury, T.; Libergoli, M.; Florio, F.; Hammouda, S.; Ménétrier, F.; Avoscan, L.; Yammine, A.; Samadi, M.; et al. Antioxidant properties and cytoprotective effect of Pistacia lentiscus L. seed oil against 7β-hydroxycholesterol-Induced toxicity in C2C12 myoblasts: Reduction in oxidative stress, mitochondrial and peroxisomal dysfunctions and attenuation of cell death. Antioxidants 2021, 10, 1772. [Google Scholar] [CrossRef]
- Nisr, R.B.; Shah, D.S.; Hundal, H.S. Mono- and polyunsaturated fatty acids counter palmitate-induced mitochondrial dysfunction in rat skeletal muscle cells. Cell. Physiol. Biochem. 2020, 54, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Zhang, T.; Guo, Y.; Pei, W.; Liu, R.; Chang, M.; Wang, X. Linolenic acid ameliorates sarcopenia in C. elegans by promoting mitophagy and fighting oxidative stress. Food Funct. 2023, 14, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Nishimura, M.; Uehara, M.; Kuribara-Souta, A.; Yamamoto, M.; Yoshikawa, N.; Morohashi, K.-I.; Tanaka, H. Eicosapentaenoic acid changes muscle transcriptome and intervenes in aging-related fiber type transition in male mice. Am. J. Physiol.-Endocrinol. Metab. 2021, 320, E346–E358. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Yang, L.; Yang, X.; Xu, D.; Wang, S.; Gao, H.; Liu, H.; Xia, H.; Yang, C.; Lu, Y.; et al. Potential nutritional strategies to prevent and reverse sarcopenia in aging process: Role of fish oil-derived omega-3 polyunsaturated fatty acids, wheat oligopeptide and their combined intervention. J. Adv. Res. 2023, 57, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, Y.; Yang, X.; Pan, D.; Wang, Y.; Yin, S.; Wang, S.; Sun, G. Effects of fish oil-derived n-3 polyunsaturated fatty acid on body composition, muscle strength and physical performance in older people: A secondary analysis of a randomised, double-blind, placebo-controlled trial. Age Ageing 2022, 51, afac274. [Google Scholar] [CrossRef]
- Alkhedhairi, S.A.; Alkhayl, F.F.A.; Ismail, A.D.; Rozendaal, A.; German, M.; MacLean, B.; Johnston, L.; Miller, A.; Hunter, A.; Macgregor, L.; et al. The effect of krill oil supplementation on skeletal muscle function and size in older adults: A randomised controlled trial. Clin. Nutr. 2022, 41, 1228–1235. [Google Scholar] [CrossRef]
- Kunz, H.E.; Michie, K.L.; Gries, K.J.; Zhang, X.; Ryan, Z.C.; Lanza, I.R. A randomized trial of the effects of dietary n-3 PUFAs on skeletal muscle function and acute exercise response in healthy older adults. Nutrients 2022, 14, 3537. [Google Scholar] [CrossRef]
- Strandberg, E.; Ponsot, E.; Piehl-Aulin, K.; Falk, G.; Kadi, F. Resistance training alone or combined with n-3 PUFA-rich diet in older women: Effects on muscle fiber hypertrophy. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2019, 74, 489–493. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhu, Y.; Liu, X.; Xia, H.; Yang, X.; Sun, G. Treatment for 6 months with fish oil-derived n-3 polyunsaturated fatty acids has neutral effects on glycemic control but improves dyslipidemia in type 2 diabetic patients with abdominal obesity: A randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2017, 56, 2415–2422. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: The DO-HEALTH randomized clinical trial. JAMA-J. Am. Med. Assoc. 2020, 324, 1855–1868. [Google Scholar] [CrossRef]
- Dong, H.; Hutchins-Wiese, H.; Kleppinger, A.; Annis, K.; Liva, E.; Lammi-Keefe, C.; Durham, H.; Feinn, R.; Kenny, A.M. Effects of omega-3 polyunsaturated fatty acid supplementation on bone turnover in older women. Int. J. Vitam. Nutr. Res. 2014, 84, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Hossain, S.; Wakatsuki, H.; Tanabe, Y.; Ohno, M.; Kato, S.; Shido, O.; Hashimoto, M. Perilla seed oil improves bone health by inhibiting bone resorption in healthy Japanese adults: A 12-month, randomized, double-blind, placebo-controlled trial. Phytother. Res. 2023, 37, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Hutchins-Wiese, H.L.; Picho, K.; Watkins, B.A.; Li, Y.; Tannenbaum, S.; Claffey, K.; Kenny, A.M. High-dose eicosapentaenoic acid and docosahexaenoic acid supplementation reduces bone resorption in postmenopausal breast cancer survivors on aromatase inhibitors: A pilot study. Nutr. Cancer 2014, 66, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.S.; Eide, I.A.; Jenssen, T.; Åsberg, A.; Bollerslev, J.; Godang, K.; Hartmann, A.; Schmidt, E.B.; Svensson, M. Marine n-3 polyunsaturated fatty acids and bone mineral density in kidney transplant recipients: A randomized, placebo-controlled trial. Nutrients 2021, 13, 2361. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alcaraz, A.; Tiraboschi, J.; Gómez, C.; Candas-Estébanez, B.; Saumoy, M.; Imaz, A.; Podzamczer, D. Lack of benefit with omega-3 fatty acid supplementation in HIV patients: A randomized pilot study. HIV Res. Clin. Pract. 2019, 20, 99–105. [Google Scholar] [CrossRef]
- Suzuki, Y.; Fukushima, M.; Sakuraba, K.; Sawaki, K.; Sekigawa, K. Krill oil improves mild knee joint pain: A randomized control trial. PLoS ONE 2016, 11, e0162769. [Google Scholar] [CrossRef]
- Stonehouse, W.; Benassi-Evans, B.; Bednarz, J.; Vincent, A.D.; Hall, S.; Hill, C.L. Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: A 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2022, 116, 672–685. [Google Scholar] [CrossRef]
- MacFarlane, L.A.; Cook, N.R.; Kim, E.; Lee, I.; Iversen, M.D.; Gordon, D.; Buring, J.E.; Katz, J.N.; Manson, J.E.; Costenbader, K.H. The effects of vitamin D and marine omega-3 fatty acid supplementation on chronic knee pain in older US adults: Results from a randomized trial. Arthritis Rheumatol. 2020, 72, 1836–1844. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef]
- Zhang, J.; Dennison, E.; Prieto-Alhambra, D. Osteoporosis epidemiology using international cohorts. Curr. Opin. Rheumatol. 2020, 32, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, W.; Yin, X.; Cui, L.; Tang, S.; Jiang, N.; Cui, L.; Zhao, N.; Lin, Q.; Chen, L.; et al. Prevalence of osteoporosis and fracture in China: The China Osteoporosis Prevalence Study. JAMA Netw. Open 2021, 4, e2121106. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Xia, W. The epidemiology of osteoporosis, associated fragility fractures, and management gap in China. Arch. Osteoporos. 2019, 14, 32. [Google Scholar] [CrossRef]
- Odén, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporos. Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef]
- Noh, J.-Y.; Yang, Y.; Jung, H. Molecular mechanisms and emerging therapeutics for osteoporosis. Int. J. Mol. Sci. 2020, 21, 7623. [Google Scholar] [CrossRef]
- Lerner, U.H.; Kindstedt, E.; Lundberg, P. The critical interplay between bone resorbing and bone forming cells. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 33–51. [Google Scholar] [CrossRef]
- Liang, B.; Burley, G.; Lin, S.; Shi, Y.-C. Osteoporosis pathogenesis and treatment: Existing and emerging avenues. Cell. Mol. Biol. Lett. 2022, 27, 72. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG pathway: A mechanism Involved in exercise-induced bone remodeling. Biomed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.-R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-alpha directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef]
- Yokota, K.; Sato, K.; Miyazaki, T.; Kitaura, H.; Kayama, H.; Miyoshi, F.; Araki, Y.; Akiyama, Y.; Takeda, K.; Mimura, T. Combination of tumor necrosis factor alpha and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014, 66, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, X.; Wang, S.; Su, Y.; Chen, X.; Cao, L.; Zhi, X.; Qiu, Z.; Wang, Y.; Jiang, H.; et al. Triptolide prevents bone loss via suppressing osteoclastogenesis through inhibiting PI3K-AKT-NFATc1 pathway. J. Cell. Mol. Med. 2020, 24, 6149–6161. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B-Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lin, S.; Wu, X.; Yang, S.; Cao, L.; Zheng, A.; Wu, J.; Jiang, X. The Function of Wnt ligands on osteocyte and bone remodeling. J. Dent. Res. 2019, 98, 930–938. [Google Scholar] [CrossRef]
- Bal, Z.; Kushioka, J.; Kodama, J.; Kaito, T.; Yoshikawa, H.; Korkusuz, P.; Korkusuz, F. BMP and TGFbeta use and release in bone regeneration. Turk. J. Med. Sci. 2020, 50, 1707–1722. [Google Scholar] [CrossRef]
- Xu, Y.; Shu, B.; Tian, Y.; Chelly, M.; Morandi, M.M.; Barton, S.; Shang, X.; Dong, Y. Notch activation promotes osteoblast mineralization by inhibition of apoptosis. J. Cell. Physiol. 2018, 233, 6921–6928. [Google Scholar] [CrossRef]
- Meyer, M.B.; Benkusky, N.A.; Pike, J.W. The RUNX2 cistrome in osteoblasts: Characterization, down-regulation following differentiation, and relationship to gene expression. J. Biol. Chem. 2014, 289, 16016–16031. [Google Scholar] [CrossRef]
- Komori, T. Molecular mechanism of Runx2-dependent bone development. Mol. Cells 2020, 43, 168–175. [Google Scholar] [CrossRef]
- Roncero-Martín, R.; Aliaga, I.; Moran, J.M.; Puerto-Parejo, L.M.; Rey-Sánchez, P.; Canal-Macías, M.d.l.L.; Sánchez-Fernández, A.; Pedrera-Zamorano, J.D.; López-Espuela, F.; Vera, V.; et al. Plasma fatty acids and quantitative ultrasound, DXA and pQCT derived parameters in postmenopausal Spanish women. Nutrients 2021, 13, 1454. [Google Scholar] [CrossRef]
- Feehan, O.; Magee, P.J.; Pourshahidi, L.K.; Armstrong, D.J.; Slevin, M.M.; Allsopp, P.J.; Conway, M.C.; Strain, J.J.; McSorley, E.M. Associations of long chain polyunsaturated fatty acids with bone mineral density and bone turnover in postmenopausal women. Eur. J. Nutr. 2023, 62, 95–104. [Google Scholar] [CrossRef]
- Liang, H.; Xiong, C.; Luo, Y.; Zhang, J.; Huang, Y.; Zhao, R.; Zhou, N.; Zhao, Z.; Luo, X. Association between serum polyunsaturated fatty acids and bone mineral density in US adults: NHANES 2011–2014. Front. Endocrinol. 2023, 14, 1266329. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Yoo, H.J.; Park, S.J.; Kwak, M.K.; Lee, S.H.; Kim, S.J.; Hamrick, M.W.; Isales, C.M.; Ahn, S.H.; Koh, J.-M. Association of blood n-3 fatty acid with bone mass and bone marrow TRAP-5b in the elderly with and without hip fracture. Osteoporos. Int. 2019, 30, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi-Oyelere, B.L.; Brough, L.; Coad, J.; Roy, N.; Kruger, M.C. The Relationship between nutrient patterns and bone mineral density in postmenopausal women. Nutrients 2019, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Liang, H.; Zhou, N.; Huang, T.; Zhao, Z.; Luo, X. Polyunsaturated fatty acids level and bone mineral density: A two-sample Mendelian randomization study. Front. Endocrinol. 2022, 13, 858851. [Google Scholar] [CrossRef]
- Mei, Z.; Chen, G.-C.; Hu, J.; Lin, C.; Sun, Z.; Liu, C.; Geng, X.; Yuan, C.; Qi, Q.; Zheng, Y. Habitual use of fish oil supplements, genetic predisposition, and risk of fractures: A large population-based study. Am. J. Clin. Nutr. 2021, 114, 945–954. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, M.; Chen, X.; Zhou, Y.; Chen, Z. Metabolomic analysis to elucidate the change of the n-3 polyunsaturated fatty acids in senescent osteoblasts. Biosci. Biotechnol. Biochem. 2021, 85, 611–620. [Google Scholar] [CrossRef]
- Siriarchavatana, P.; Kruger, M.C.; Miller, M.R.; Tian, H.; Wolber, F.M. Non-polar lipid from greenshell mussel (Perna canaliculus) inhibits osteoclast differentiation. Bone Rep. 2021, 15, 101132. [Google Scholar] [CrossRef]
- Abou-Saleh, H.; Ouhtit, A.; Halade, G.V.; Rahman, M. Bone bnefits of fish oil supplementation depend on its EPA and DHA content. Nutrients 2019, 11, 2701. [Google Scholar] [CrossRef]
- Farahnak, Z.; Freundorfer, M.T.; Lavery, P.; Weiler, H.A. Dietary docosahexaenoic acid contributes to increased bone mineral accretion and strength in young female Sprague-Dawley rats. Prostaglandins Leukot. Essent. Fat. Acids 2019, 144, 32–39. [Google Scholar] [CrossRef]
- Maíra, D.C.d.A.; Letícia, R.P.; da Costa, L.R.; Boueri, B.F.d.C.; Carolina, R.P.; Pereira, A.D.; Ribeiro, D.C.; da Silva, E.M.; da Costa, C.A.S.; Gilson, T.B. Flaxseed (linum usitatissimum) flour contributes to bone health in adult male rats. Nutrition 2018, 49, 48–50. [Google Scholar] [CrossRef]
- Zhan, Q.; Tian, Y.; Han, L.; Wang, K.; Wang, J.; Xue, C. The opposite effects of Antarctic krill oil and arachidonic acid-rich oil on bone resorption in ovariectomized mice. Food Funct. 2020, 11, 7048–7060. [Google Scholar] [CrossRef] [PubMed]
- Degenerative joint disease (oasteoarthritis). JAMA-J. Am. Med. Assoc. 1973, 224, 740–744. [CrossRef]
- Safiri, S.; Kolahi, A.-A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.; Thoma, L.; Golightly, Y. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Swain, S.; Sarmanova, A.; Mallen, C.; Kuo, C.; Coupland, C.; Doherty, M.; Zhang, W. Trends in incidence and prevalence of osteoarthritis in the United Kingdom: Findings from the Clinical Practice Research Datalink (CPRD). Osteoarthr. Cartil. 2020, 28, 792–801. [Google Scholar] [CrossRef]
- Jordan, J.M.; Helmick, C.G.; Renner, J.B.; Luta, G.; Dragomir, A.D.; Woodard, J.; Fang, F.; Schwartz, T.A.; Abbate, L.M.; Callahan, L.F.; et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: The Johnston County Osteoarthritis Project. J. Rheumatol. 2007, 34, 172–180. [Google Scholar]
- Li, D.; Li, S.; Chen, Q.; Xie, X. The prevalence of symptomatic knee osteoarthritis in relation to age, sex, area, region, and body mass index in china: A systematic review and meta-analysis. Front. Med. 2020, 7, 304. [Google Scholar] [CrossRef]
- Yahaya, I.; Wright, T.; Babatunde, O.O.; Corp, N.; Helliwell, T.; Dikomitis, L.; Mallen, C.D. Prevalence of osteoarthritis in lower middle- and low-income countries: A systematic review and meta-analysis. Rheumatol. Int. 2021, 41, 1221–1231. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst century: Risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee osteoarthritis: Epidemiology, pathogenesis, and mesenchymal stem cells: What else is new? An update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Kim, H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Y.; Yang, G.; Yang, T.; He, L.; Wang, Y. Cathelicidin antimicrobial peptide (CAMP) gene promoter methylation induces chondrocyte apoptosis. Hum. Genom. 2021, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, X.; Chi, R.; Qi, J.; Xu, T. Moderate mechanical stress suppresses the IL-1beta-induced chondrocyte apoptosis by regulating mitochondrial dynamics. J. Cell. Physiol. 2021, 236, 7504–7515. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Zhang, N.; Ding, R.; Wang, Q.; Wang, W. Alterations of the subchondral bone in osteoarthritis: Complying with Wolff’s law. Curr. Rheumatol. Rev. 2022, 18, 178–185. [Google Scholar] [CrossRef]
- Zhang, L.; Kirkwood, C.L.; Sohn, J.; Lau, A.; Bayers-Thering, M.; Bali, S.K.; Rachala, S.; Marzo, J.M.; Anders, M.J.; Beier, F.; et al. Expansion of myeloid-derived suppressor cells contributes to metabolic osteoarthritis through subchondral bone remodeling. Arthritis Res. Ther. 2021, 23, 287. [Google Scholar] [CrossRef]
- Han, X.; Cui, J.; Chu, L.; Zhang, W.; Xie, K.; Jiang, X.; He, Z.; Du, J.; Ai, S.; Sun, Q.; et al. Abnormal subchondral trabecular bone remodeling in knee osteoarthritis under the influence of knee alignment. Osteoarthr. Cartil. 2022, 30, 100–109. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Matthan, N.; Lichtenstein, A.; Niu, J.; Guermazi, A.; Roemer, F.; Grainger, A.; Nevitt, M.; Clancy, M.; Lewis, C.; et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: The MOST study. Osteoarthr. Cartil. 2012, 20, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Alzughaibi, L.S.; Marzouki, Z.M. Dietary intake of fatty acids and antioxidants in relation to radiographic knee osteoarthritis: Results from a case-control study. J. Hum. Nutr. Diet. 2020, 33, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Driban, J.B.; Xu, C.; Lapane, K.L.; McAlindon, T.E.; Eaton, C.B. Dietary fat intake and radiographic progression of knee osteoarthritis: Data from the Osteoarthritis Initiative. Arthritis Care Res. 2017, 69, 368–375. [Google Scholar] [CrossRef]
- Felson, D.T.; Misra, D.; LaValley, M.; Clancy, M.; Rabasa, G.; Lichtenstein, A.; Matthan, N.; Torner, J.; Lewis, C.E.; Nevitt, M.C.; et al. Essential fatty acids and osteoarthritis. Arthritis Care Res. 2024, 76, 796–801. [Google Scholar] [CrossRef]
- Yu, H.; Gong, Z.; Wang, G.; Cao, R.; Yin, H.; Ma, L.; Guo, A. DHA attenuates cartilage degeneration by mediating apoptosis and autophagy in human chondrocytes and rat models of osteoarthritis. Vitr. Cell. Dev. Biol.-Anim. 2023, 59, 455–466. [Google Scholar] [CrossRef]
- Xiong, T.; Huang, S.; Wang, X.; Shi, Y.; He, J.; Yuan, Y.; Wang, R.; Gu, H.; Liu, L. N-3 polyunsaturated fatty acids alleviate the progression of obesity-related osteoarthritis and protect cartilage through inhibiting the HMGB1-RAGE/TLR4 signaling pathway. Int. Immunopharmacol. 2024, 128, 111498. [Google Scholar] [CrossRef]
- Jin, X.; Dong, X.; Sun, Y.; Liu, Z.; Liu, L.; Gu, H. Dietary fatty acid regulation of the NLRP3 Inflammasome via the TLR4/NF-kappaB signaling pathway affects chondrocyte pyroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 3711371. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, W.; Zhong, Z.; Yu, H.; Zhang, P.; Shen, H. Docosahexaenoic acid inhibits bone remodeling and vessel formation in the osteochondral unit in a rat model. Biomed. Pharmacother. 2019, 114, 108811. [Google Scholar] [CrossRef]



| Study Type | PUFAs Type | Subject | Dose | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| In vitro | PLSO | 7β-OHC-induced murine C2C12 myoblasts | 100 μg/mL for 24 h | Prevented myoblast dysfunction and death Reduced oxidative stress | ↑ SOD, GPx ↓ ROS, MDA, and ΔΨm | [52] |
| In vitro | LO | SFA-induced rat skeletal (L6) myotubes | 100 mM for 16 h | Reduced inflammation and oxidation levels Improved mitochondrial function | ↑ PGC1α ↓ ROS, IL-6, and NF-κB | [53] |
| In vivo | LA | Caenorhabditis elegans | 50 μg/mL for 10 days | Improved skeletal muscle loss | ↑ DAF-16/FOXO and pink-1 ↓ ROS | [54] |
| In vivo | EPA | 75-week-old C57BL/6J mice | 1 wt% for 12 weeks, supplemented in diet | Suppressed aging-associated muscle dysfunction and muscle fiber type changes | Fast-to-slow fiber type transition; Muscle transcriptome alteration | [55] |
| In vivo | Fish oil | 25-month-old SD rats | 200, 400, 800 mg/kg for 10 weeks, oral gavage | Improved muscle atrophy, oxidative stress, and inflammatory levels and cell infiltration | Promoted protein synthesis and muscle regeneration | [56] |
| Study Type | Intervention | Subject | Dose | Results | Ref. |
|---|---|---|---|---|---|
| Sarcopenia | |||||
| RCT | EPA + DHA | Older Chinese people | 1.34 g EPA + 1.07 g DHA/d for 6 months | Increased mass, strength, and physical performance of muscle | [57] |
| RCT | Krill oil | Healthy elderly people | 4 g/d for 6 months | Increased muscle thickness, grip strength, and knee extensor maximal torque | [58] |
| RCT | EPA + DHA | Healthy older adults | 4 g/d for 6 months | Increased muscle strength Attenuated the acute response to exercise | [59] |
| RCT | n-3 PUFA-rich healthy diet + training | Older women | Fish and seafood intake ≥ 500 g/week for 24 weeks | Lowered the local level of inflammation Triggered growth responses in skeletal muscle | [60] |
| RCT | Fish oil | Type 2 diabetic patients with abdominal obesity | 4 g/d for 6 months | Increased serum EPA and DHA levels but no significant change in muscle mass | [61] |
| RCT | Omega-3 PUFA | Adults aged 70 years or older | 1 g/d for 3 years | Showed no significant increase in the scores of SPPB | [62] |
| Osteoporosis | |||||
| RCT | EPA/DHA | Older postmenopausal women | 1.2 g/d for 6 months | Reduced bone turnover Improved RBC DHA levels in short-term supplementation | [63] |
| RCT | PO | Japanese adults | 7.0 mL/d for 12 months | Had a positive effect on age-related BMD decline | [64] |
| RCT | Fish oil (EPA + DHA) | Postmenopausal breast cancer survivors | 4 g/d for 3 months | Changed serum fatty acid levels Inhibited bone resorption | [65] |
| RCT | Marine n-3 PUFA | Adult kidney transplant recipients | 2.6 g/d for 44 weeks | Showed no significant effect on promoting BMD | [66] |
| RCT | n-3 PUFA | HIV-infected patients | 2 g/d for 24 months | Had no beneficial effect on BMD | [67] |
| Osteoarthritis | |||||
| RCT | Krill oil | Japanese adults | 2 g/d for 30 days | Mitigated the pain and stiffness in knees | [68] |
| RCT | Krill oil | Adults with clinically diagnosed knee osteoarthritis or regular knee pain | 4 g/d for 6 months | Improved keen pain, stiffness, and physical function | [69] |
| RCT | Marine omega-3 fatty acids | US older adults | 1 g/d Omacor® + 840 mg EPA + DHA for 3.8–6.1 years | Did not alleviate knee pain, stiffness, or enhance function | [70] |
| Study Type | PUFAs Type | Subject | Dose | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| In vitro | n-3 PUFAs | Osteoblasts | NA | Increased bone metabolism gene expression Decreased aging-related genes expression, oxidative stress damage | ↑ RANKL/OPG, IGF-1 ↓ MDA, FOXO1 | [98] |
| In vitro | GSM oil | RAW 264.7 osteoclasts | 10–20 μg/mL for 48 h | Inhibited osteoclastogenic activity | ↓ TRAP and NFATc1 | [99] |
| In vivo | Fish oil | 12-month-old C57BL/6 mice | 1%, 4% for 12 months, supplemented in diet | Maintained higher BMD during aging | ↑ BMD, IL-12, and IFN-γ ↓ TRAP5b, RANKL, and NF-κB | [100] |
| In vivo | DHA | SD rats | 0.1, 0.4, 0.8, 1.2% w/w for 10 weeks, supplemented in diet | Increased bone mass, bone strength Improved trabecular microarchitecture | ↑ BMC, BMD | [101] |
| In vivo | Flaxseed flour | Adult Wistar rats | 25 g/100 g diet for 6 months | Produced greater BMD and femur resistance | ↑ BMD, BMC, and osteocalcin | [102] |
| In vivo | AA | Ovariectomized mice | 220 mg/kg for 3 months, oral administration | Impaired trabecular microstructure repair and BMD | ↑ PGE2, RANKL, and NF-κB ↓ BMD | [103] |
| Study Type | PUFAs Type | Subject | Dose | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| In vitro | DHA | Human osteoarthritis chondrocyte | 50 μg/mL for 1 h | Promoted chondrocyte proliferation Suppressed apoptosis and elevated autophagy | ↑ Beclin-1 and Bcl-2 ↓ p-JNK, p-p38, p-mTOR, and LC3-I/II ratio | [129] |
| In vivo | DHA | SD rats | 5 g/kg for 6 weeks, supplemented in diet | ↑ Collagen II–positive cell rate ↓ Mankin score | [129] | |
| In vitro | DHA | SW1353 cells | 10 μM for 24 h | Alleviated osteoarthritis progression | ↑ SIRT1 ↓ HMGB1, RAGE, TLR4, and Caspase-8 | [130] |
| In vivo | Fish oil | Obesity-related post-traumatic osteoarthritis mice | 8.4% w/w for 14 weeks, supplemented in diet | [130] | ||
| In vitro | n-3/n-6 PUFAs | SW1353 cells | NA | n-6 PUFAs exacerbated obesity-related osteoarthritis n-3 PUFAs were protective | n-6: ↑ TLR4, NF-κB, and NLRP3 n-3: ↓ TLR4, NF-κB, and NLRP3 | [131] |
| In vivo | n-3/n-6 PUFAs | Obesity-related post-traumatic osteoarthritis mice | [131] | |||
| In vitro | DHA | RAW264.7 cells | NA | Protected cartilage by inhibiting the ability of bone remodeling and angiogenesis | ↓ CTSK, TRAP, NFATc1, MITF, VEGF-C, VEGF-A, and VEGFR2 | [132] |
| In vivo | DHA | ACLT-induced rats | 1 mg/kg every other day for 2 months, injected in tail | ↓ RANKL, CD31, and endomucin | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Xiong, R.; Cheng, J.; Ye, J.; Qiu, Y.; Huang, S.; Li, M.; Liu, Z.; Pang, J.; Zhang, X.; et al. Effects and Mechanisms of Polyunsaturated Fatty Acids on Age-Related Musculoskeletal Diseases: Sarcopenia, Osteoporosis, and Osteoarthritis—A Narrative Review. Nutrients 2024, 16, 3130. https://doi.org/10.3390/nu16183130
Chen H, Xiong R, Cheng J, Ye J, Qiu Y, Huang S, Li M, Liu Z, Pang J, Zhang X, et al. Effects and Mechanisms of Polyunsaturated Fatty Acids on Age-Related Musculoskeletal Diseases: Sarcopenia, Osteoporosis, and Osteoarthritis—A Narrative Review. Nutrients. 2024; 16(18):3130. https://doi.org/10.3390/nu16183130
Chicago/Turabian StyleChen, Haoqi, Ruogu Xiong, Jin Cheng, Jialu Ye, Yingzhen Qiu, Siyu Huang, Mengchu Li, Zhaoyan Liu, Jinzhu Pang, Xuguang Zhang, and et al. 2024. "Effects and Mechanisms of Polyunsaturated Fatty Acids on Age-Related Musculoskeletal Diseases: Sarcopenia, Osteoporosis, and Osteoarthritis—A Narrative Review" Nutrients 16, no. 18: 3130. https://doi.org/10.3390/nu16183130
APA StyleChen, H., Xiong, R., Cheng, J., Ye, J., Qiu, Y., Huang, S., Li, M., Liu, Z., Pang, J., Zhang, X., Guo, S., Li, H., & Zhu, H. (2024). Effects and Mechanisms of Polyunsaturated Fatty Acids on Age-Related Musculoskeletal Diseases: Sarcopenia, Osteoporosis, and Osteoarthritis—A Narrative Review. Nutrients, 16(18), 3130. https://doi.org/10.3390/nu16183130








