Anti-Inflammatory Effect of Dietary Pentadecanoic Fatty Acid Supplementation on Inflammatory Bowel Disease in SAMP1/YitFc Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Measures to Improve Reproducibility
2.2. Spontaneous Ileitis and DSS Colitis in SAMP Mice
2.3. Diet
2.4. Experimental Design
2.5. DSS Induced Colitis and Colitis Severity Assessment
2.6. Analysis of Intestinal Inflammation and Gut Permeability In Vivo
2.7. Histopathological Analysis
2.8. nCounter Nanostring Gene Expression Analysis
2.9. Data Analysis
3. Results
3.1. Three-Week C:15 Supplementation Prevented the Occurrence of Severe DSS-Colitis in SAMP Mice
3.2. Six-Week C:15 Supplementation Reduced the Endoscopic Severity of DSS-Colitis in SAMP Mice
3.3. Six-Week Supplementation Reduces the Severity of Advanced Ileitis in SAMP Mice
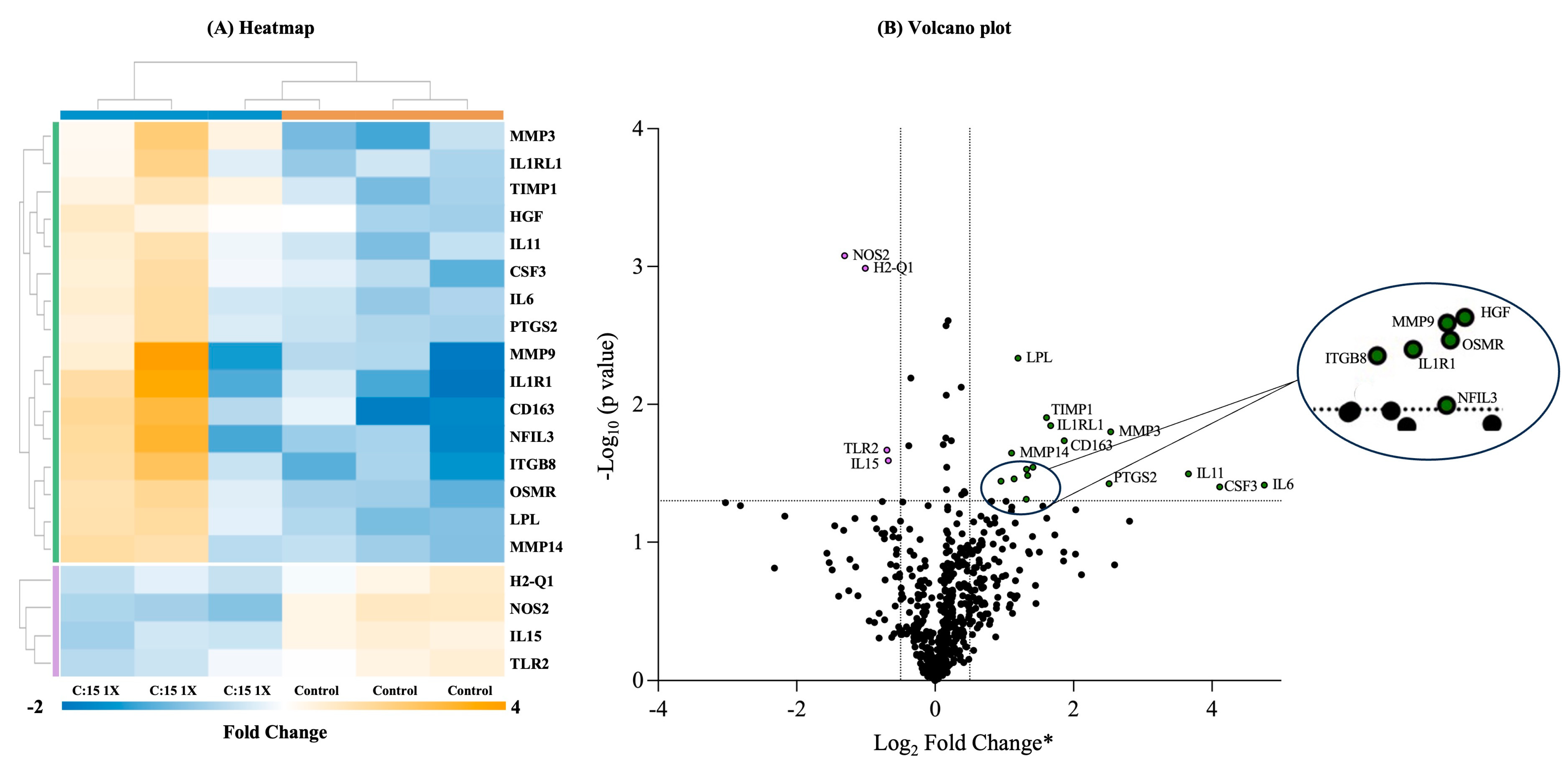
3.4. Three-Week C:15 Supplementation Altered the Expression of Various Genes Involved in Different Immunological Pathways in SAMP Mice
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn‘s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef]
- Bielefeldt, K.; Davis, B.; Binion, D.G. Pain and inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, R. Mechanisms of inflammatory bowel disease. Gastroenterol. Hepatol. 2013, 9, 529–532. [Google Scholar]
- Hong, S.M.; Baek, D.H. Diagnostic Procedures for Inflammatory Bowel Disease: Laboratory, Endoscopy, Pathology, Imaging, and Beyond. Diagnostics 2024, 14, 1384. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Ruemmele, F.M. Nutritional interventions for the treatment of IBD: Current evidence and controversies. Ther. Adv. Gastroenterol. 2019, 12, 1756284819890534. [Google Scholar] [CrossRef]
- Wark, G.; Samocha-Bonet, D.; Ghaly, S.; Danta, M. The Role of Diet in the Pathogenesis and Management of Inflammatory Bowel Disease: A Review. Nutrients 2020, 13, 135. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Piotrowska, M.; Binienda, A.; Fichna, J. The role of fatty acids in Crohn's disease pathophysiology—An overview. Mol. Cell Endocrinol. 2021, 538, 111448. [Google Scholar] [CrossRef]
- Yan, D.; Ye, S.; He, Y.; Wang, S.; Xiao, Y.; Xiang, X.; Deng, M.; Luo, W.; Chen, X.; Wang, X. Fatty acids and lipid mediators in inflammatory bowel disease: From mechanism to treatment. Front. Immunol. 2023, 14, 1286667. [Google Scholar] [CrossRef]
- Jenkins, B.; West, J.A.; Koulman, A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15:0) and heptadecanoic Acid (c17:0) in health and disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef] [PubMed]
- Policastro, V.; Righelli, D.; Rava, L.; Vernocchi, P.; Bianchi, M.; Vallone, C.; Signore, F.; Manco, M. Dietary Fatty Acids Contribute to Maintaining the Balance between Pro-Inflammatory and Anti-Inflammatory Responses during Pregnancy. Nutrients 2023, 15, 2432. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.K.; Butterworth, C.N. Broader and safer clinically-relevant activities of pentadecanoic acid compared to omega-3: Evaluation of an emerging essential fatty acid across twelve primary human cell-based disease systems. PLoS ONE 2022, 17, e0268778. [Google Scholar] [CrossRef]
- To, N.B.; Nguyen, Y.T.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic Acid, an Odd-Chain Fatty Acid, Suppresses the Stemness of MCF-7/SC Human Breast Cancer Stem-Like Cells through JAK2/STAT3 Signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Abdoul-Aziz, S.K.A.; Zhang, Y.; Wang, J. Milk Odd and Branched Chain Fatty Acids in Dairy Cows: A Review on Dietary Factors and Its Consequences on Human Health. Animals 2021, 11, 3210. [Google Scholar] [CrossRef]
- Albani, V.; Celis-Morales, C.; Marsaux, C.F.; Forster, H.; O'Donovan, C.B.; Woolhead, C.; Macready, A.L.; Fallaize, R.; Navas-Carretero, S.; San-Cristobal, R.; et al. Exploring the association of dairy product intake with the fatty acids C15:0 and C17:0 measured from dried blood spots in a multipopulation cohort: Findings from the Food4Me study. Mol. Nutr. Food Res. 2016, 60, 834–845. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Dornan, K.; Gunenc, A.; Oomah, B.D.; Hosseinian, F. Odd chain fatty acids and odd chain phenolic lipids (alkylresorcinols) are essential for diet. J. Am. Oil Chem. Soc. 2021, 98, 813–824. [Google Scholar] [CrossRef]
- Djousse, L.; Biggs, M.L.; Matthan, N.R.; Ix, J.H.; Fitzpatrick, A.L.; King, I.; Lemaitre, R.N.; McKnight, B.; Kizer, J.R.; Lichtenstein, A.H.; et al. Serum Individual Nonesterified Fatty Acids and Risk of Heart Failure in Older Adults. Cardiology 2021, 146, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Trieu, K.; Bhat, S.; Dai, Z.; Leander, K.; Gigante, B.; Qian, F.; Korat, A.V.A.; Sun, Q.; Pan, X.F.; Laguzzi, F.; et al. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: A cohort study, systematic review, and meta-analysis. PLoS Med. 2021, 18, e1003763. [Google Scholar] [CrossRef]
- Sawh, M.C.; Wallace, M.; Shapiro, E.; Goyal, N.P.; Newton, K.P.; Yu, E.L.; Bross, C.; Durelle, J.; Knott, C.; Gangoiti, J.A.; et al. Dairy Fat Intake, Plasma Pentadecanoic Acid, and Plasma Iso-heptadecanoic Acid Are Inversely Associated With Liver Fat in Children. J. Pediatr. Gastroenterol. Nutr. 2021, 72, e90–e96. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.R.; LaSalla, A.; Lam, G.; Kulpins, D.; Moen, E.L.; Sundrud, M.S.; Miyoshi, J.; Ilic, S.; Theriault, B.R.; Cominelli, F.; et al. Artificial microbiome heterogeneity spurs six practical action themes and examples to increase study power-driven reproducibility. Sci. Rep. 2020, 10, 5039. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palacios, A.; Aladyshkina, N.; Ezeji, J.C.; Erkkila, H.L.; Conger, M.; Ward, J.; Webster, J.; Cominelli, F. ‘Cyclical Bias’ in Microbiome Research Revealed by A Portable Germ-Free Housing System Using Nested Isolation. Sci. Rep. 2018, 8, 3801. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palacios, A.; Kodani, T.; Kaydo, L.; Pietropaoli, D.; Corridoni, D.; Howell, S.; Katz, J.; Xin, W.; Pizarro, T.T.; Cominelli, F. Stereomicroscopic 3D-pattern profiling of murine and human intestinal inflammation reveals unique structural phenotypes. Nat. Commun. 2015, 6, 7577. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Rodriguez-Palacios, A.; Aladyshkina, N.; Cominelli, F. Stereomicroscopy and 3D-target myeloperoxidase intestinal phenotyping following a fecal flora homogenization protocol. Protocol Exchange, 17 July 2015; Version 1. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Khoretonenko, M.V.; Ilic, S. Institutional protocols for the oral administration (gavage) of chemicals and microscopic microbial communities to mice: Analytical consensus. Exp. Biol. Med. 2019, 244, 459–470. [Google Scholar] [CrossRef]
- Kodani, T.; Rodriguez-Palacios, A.; Corridoni, D.; Lopetuso, L.; Di Martino, L.; Marks, B.; Pizarro, J.; Pizarro, T.; Chak, A.; Cominelli, F. Flexible colonoscopy in mice to evaluate the severity of colitis and colorectal tumors using a validated endoscopic scoring system. J. Vis. Exp. 2013, 80, e50843. [Google Scholar] [CrossRef]
- Menghini, P.; Corridoni, D.; Butto, L.F.; Osme, A.; Shivaswamy, S.; Lam, M.; Bamias, G.; Pizarro, T.T.; Rodriguez-Palacios, A.; Dinarello, C.A.; et al. Neutralization of IL-1alpha ameliorates Crohn's disease-like ileitis by functional alterations of the gut microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 26717–26726. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466 e454. [Google Scholar] [CrossRef]
- Burns, R.C.; Rivera-Nieves, J.; Moskaluk, C.A.; Matsumoto, S.; Cominelli, F.; Ley, K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology 2001, 121, 1428–1436. [Google Scholar] [CrossRef]
- Shin, D.W.; Lim, B.O. Nutritional Interventions Using Functional Foods and Nutraceuticals to Improve Inflammatory Bowel Disease. J. Med. Food 2020, 23, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Charlet, R.; Le Danvic, C.; Sendid, B.; Nagnan-Le Meillour, P.; Jawhara, S. Oleic Acid and Palmitic Acid from Bacteroides thetaiotaomicron and Lactobacillus johnsonii Exhibit Anti-Inflammatory and Antifungal Properties. Microorganisms 2022, 10, 1803. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Altomare, A.; Cocca, S.; Solida, E.; Ribolsi, M.; Carotti, S.; Rainer, A.; Francesconi, M.; Morini, S.; Cicala, M.; et al. Palmitic Acid Affects Intestinal Epithelial Barrier Integrity and Permeability In Vitro. Antioxidants 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- de Silva, P.S.; Luben, R.; Shrestha, S.S.; Khaw, K.T.; Hart, A.R. Dietary arachidonic and oleic acid intake in ulcerative colitis etiology: A prospective cohort study using 7-day food diaries. Eur. J. Gastroenterol. Hepatol. 2014, 26, 11–18. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, Z.; Yang, H.; Zhao, F.; Liu, C.; Chen, J.; Lu, S.; Zou, Z.; Zhou, Y.; Zhang, X. Differential effects of EPA and DHA on DSS-induced colitis in mice and possible mechanisms involved. Food Funct. 2021, 12, 1803–1817. [Google Scholar] [CrossRef]
- Wang, L.; Choi, H.S.; Su, Y.; Lee, B.; Choi, J.H.; Jang, S.H.; Jang, Y.S.; Seo, J.W. Protective effect of 17S-epoxy-docosapentaenoic acid against dextran sulfate sodium induced ulcerative colitis in BALB/c mice. Mol. Med. Rep. 2022, 26, 12794. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Vasu, R.; Zhang, H. The Role of Long-Chain Fatty Acids in Inflammatory Bowel Disease. Mediat. Inflamm. 2019, 2019, 8495913. [Google Scholar] [CrossRef]
- Varnalidis, I.; Ioannidis, O.; Karamanavi, E.; Ampas, Z.; Poutahidis, T.; Taitzoglou, I.; Paraskevas, G.; Botsios, D. Omega 3 fatty acids supplementation has an ameliorative effect in experimental ulcerative colitis despite increased colonic neutrophil infiltration. Rev. Esp. Enferm. Dig. 2011, 103, 511–518. [Google Scholar] [CrossRef]
- Sharma, M.; Kaur, R.; Kaushik, K.; Kaushal, N. Redox modulatory protective effects of omega-3 fatty acids rich fish oil against experimental colitis. Toxicol. Mech. Methods 2019, 29, 244–254. [Google Scholar] [CrossRef]
- Brahmbhatt, V.; Oliveira, M.; Briand, M.; Perrisseau, G.; Bastic Schmid, V.; Destaillats, F.; Pace-Asciak, C.; Benyacoub, J.; Bosco, N. Protective effects of dietary EPA and DHA on ischemia-reperfusion-induced intestinal stress. J. Nutr. Biochem. 2013, 24, 104–111. [Google Scholar] [CrossRef]
- Cao, Q.; Lin, Y.; Yue, C.; Wang, Y.; Quan, F.; Cui, X.; Bi, R.; Tang, X.; Yang, Y.; Wang, C.; et al. IL-6 deficiency promotes colitis by recruiting Ly6C(hi) monocytes into inflamed colon tissues in a CCL2-CCR2-dependent manner. Eur. J. Pharmacol. 2021, 904, 174165. [Google Scholar] [CrossRef]
- Nishina, T.; Deguchi, Y.; Kawauchi, M.; Xiyu, C.; Yamazaki, S.; Mikami, T.; Nakano, H. Interleukin 11 confers resistance to dextran sulfate sodium-induced colitis in mice. iScience 2023, 26, 105934. [Google Scholar] [CrossRef]
- Cox, C.B.; Storm, E.E.; Kapoor, V.N.; Chavarria-Smith, J.; Lin, D.L.; Wang, L.; Li, Y.; Kljavin, N.; Ota, N.; Bainbridge, T.W.; et al. IL-1R1-dependent signaling coordinates epithelial regeneration in response to intestinal damage. Sci. Immunol. 2021, 6, abe8856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Geboes, K.; Colpaert, S.; D'Haens, G.R.; Rutgeerts, P.; Ceuppens, J.L. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J. Immunol. 2000, 164, 3608–3615. [Google Scholar] [CrossRef] [PubMed]
- Meisel, M.; Mayassi, T.; Fehlner-Peach, H.; Koval, J.C.; O'Brien, S.L.; Hinterleitner, R.; Lesko, K.; Kim, S.; Bouziat, R.; Chen, L.; et al. Interleukin-15 promotes intestinal dysbiosis with butyrate deficiency associated with increased susceptibility to colitis. ISME J. 2017, 11, 15–30. [Google Scholar] [CrossRef]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Meshkibaf, S.; Martins, A.J.; Henry, G.T.; Kim, S.O. Protective role of G-CSF in dextran sulfate sodium-induced acute colitis through generating gut-homing macrophages. Cytokine 2016, 78, 69–78. [Google Scholar] [CrossRef]
- Kuroda, N.; Masuya, M.; Tawara, I.; Tsuboi, J.; Yoneda, M.; Nishikawa, K.; Kageyama, Y.; Hachiya, K.; Ohishi, K.; Miwa, H.; et al. Infiltrating CCR2(+) monocytes and their progenies, fibrocytes, contribute to colon fibrosis by inhibiting collagen degradation through the production of TIMP-1. Sci. Rep. 2019, 9, 8568. [Google Scholar] [CrossRef]
- Eba, H.; Murasawa, Y.; Iohara, K.; Isogai, Z.; Nakamura, H.; Nakamura, H.; Nakashima, M. The anti-inflammatory effects of matrix metalloproteinase-3 on irreversible pulpitis of mature erupted teeth. PLoS ONE 2012, 7, e52523. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Jiang, C.; Pan, K.; Deng, J.; Wan, C. MMP9 protects against LPS-induced inflammation in osteoblasts. Innate Immun. 2020, 26, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; Blazquez-Prieto, J.; Amado-Rodriguez, L.; Lopez-Alonso, I.; Batalla-Solis, E.; Gonzalez-Lopez, A.; Sanchez-Perez, M.; Mayoral-Garcia, C.; Gutierrez-Fernandez, A.; Albaiceta, G.M. Matrix metalloproteinase-14 triggers an anti-inflammatory proteolytic cascade in endotoxemia. J. Mol. Med. 2017, 95, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Frolova, L.; Drastich, P.; Rossmann, P.; Klimesova, K.; Tlaskalova-Hogenova, H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: Upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J. Histochem. Cytochem. 2008, 56, 267–274. [Google Scholar] [CrossRef]
- Gobert, A.P.; Cheng, Y.; Akhtar, M.; Mersey, B.D.; Blumberg, D.R.; Cross, R.K.; Chaturvedi, R.; Drachenberg, C.B.; Boucher, J.L.; Hacker, A.; et al. Protective role of arginase in a mouse model of colitis. J. Immunol. 2004, 173, 2109–2117. [Google Scholar] [CrossRef]
- Scheeren, F.A.; Kuo, A.H.; van Weele, L.J.; Cai, S.; Glykofridis, I.; Sikandar, S.S.; Zabala, M.; Qian, D.; Lam, J.S.; Johnston, D.; et al. A cell-intrinsic role for TLR2-MYD88 in intestinal and breast epithelia and oncogenesis. Nat. Cell Biol. 2014, 16, 1238–1248. [Google Scholar] [CrossRef]
- Alarfaj, S.J.; Mostafa, S.A.; Negm, W.A.; El-Masry, T.A.; Kamal, M.; Elsaeed, M.; El Nakib, A.M. Mucosal Genes Expression in Inflammatory Bowel Disease Patients: New Insights. Pharmaceuticals 2023, 16, 324. [Google Scholar] [CrossRef]
- West, N.R.; Hegazy, A.N.; Owens, B.M.J.; Bullers, S.J.; Linggi, B.; Buonocore, S.; Coccia, M.; Gortz, D.; This, S.; Stockenhuber, K.; et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017, 23, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mizuno, S.; Nakamura, T. Antinecrotic and antiapoptotic effects of hepatocyte growth factor on cholestatic hepatitis in a mouse model of bile-obstructive diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G639–G646. [Google Scholar] [CrossRef][Green Version]
- Pena-Silva, R.A.; Chalouhi, N.; Wegman-Points, L.; Ali, M.; Mitchell, I.; Pierce, G.L.; Chu, Y.; Ballas, Z.K.; Heistad, D.; Hasan, D. Novel role for endogenous hepatocyte growth factor in the pathogenesis of intracranial aneurysms. Hypertension 2015, 65, 587–593. [Google Scholar] [CrossRef]
- Bengtsson, E.; Hultman, K.; Edsfeldt, A.; Persson, A.; Nitulescu, M.; Nilsson, J.; Goncalves, I.; Bjorkbacka, H. CD163+ macrophages are associated with a vulnerable plaque phenotype in human carotid plaques. Sci. Rep. 2020, 10, 14362. [Google Scholar] [CrossRef]
- Kowal, K.; Silver, R.; Slawinska, E.; Bielecki, M.; Chyczewski, L.; Kowal-Bielecka, O. CD163 and its role in inflammation. Folia Histochem. Cytobiol. 2011, 49, 365–374. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsuoka, K.; Sheikh, S.Z.; Elloumi, H.Z.; Kamada, N.; Hisamatsu, T.; Hansen, J.J.; Doty, K.R.; Pope, S.D.; Smale, S.T.; et al. NFIL3 is a regulator of IL-12 p40 in macrophages and mucosal immunity. J. Immunol. 2011, 186, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Travis, M.A.; Reizis, B.; Melton, A.C.; Masteller, E.; Tang, Q.; Proctor, J.M.; Wang, Y.; Bernstein, X.; Huang, X.; Reichardt, L.F.; et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 2007, 449, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Ziouzenkova, O.; Perrey, S.; Asatryan, L.; Hwang, J.; MacNaul, K.L.; Moller, D.E.; Rader, D.J.; Sevanian, A.; Zechner, R.; Hoefler, G.; et al. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: Evidence for an antiinflammatory role for lipoprotein lipase. Proc. Natl. Acad. Sci. USA 2003, 100, 2730–2735. [Google Scholar] [CrossRef]
- Hamada, Y.; Murakami, I.; Kato, E.; Yamane, S.; Fujino, H.; Matsumoto, K.; Tashima, K.; Horie, S.; Murayama, T. Neurogenic contraction of mouse rectum via the cyclooxygenase pathway: Changes of PGE2-induced contraction with dextran sulfate sodium-induced colitis. Pharmacol. Res. 2010, 61, 48–57. [Google Scholar] [CrossRef]
- Prusakiewicz, J.J.; Duggan, K.C.; Rouzer, C.A.; Marnett, L.J. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry 2009, 48, 7353–7355. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.; McLean, M.H.; Durum, S.K. Cytokine Tuning of Intestinal Epithelial Function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Meyer, F.; Wendling, D.; Demougeot, C.; Prati, C.; Verhoeven, F. Cytokines and intestinal epithelial permeability: A systematic review. Autoimmun. Rev. 2023, 22, 103331. [Google Scholar] [CrossRef]
- Biel, C.; Faber, K.N.; Bank, R.A.; Olinga, P. Matrix metalloproteinases in intestinal fibrosis. J. Crohns Colitis 2024, 18, 462–478. [Google Scholar] [CrossRef]
- Vilardi, A.; Przyborski, S.; Mobbs, C.; Rufini, A.; Tufarelli, C. Current understanding of the interplay between extracellular matrix remodelling and gut permeability in health and disease. Cell Death Discov. 2024, 10, 258. [Google Scholar] [CrossRef]
- Derkacz, A.; Olczyk, P.; Olczyk, K.; Komosinska-Vassev, K. The Role of Extracellular Matrix Components in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Neurath, M.F. Cytokines in inflammatory bowel diseases—Update 2020. Pharmacol Res 2020, 158, 104835. [Google Scholar] [CrossRef] [PubMed]
- Maronek, M.; Marafini, I.; Gardlik, R.; Link, R.; Troncone, E.; Monteleone, G. Metalloproteinases in Inflammatory Bowel Diseases. J. Inflamm. Res. 2021, 14, 1029–1041. [Google Scholar] [CrossRef] [PubMed]

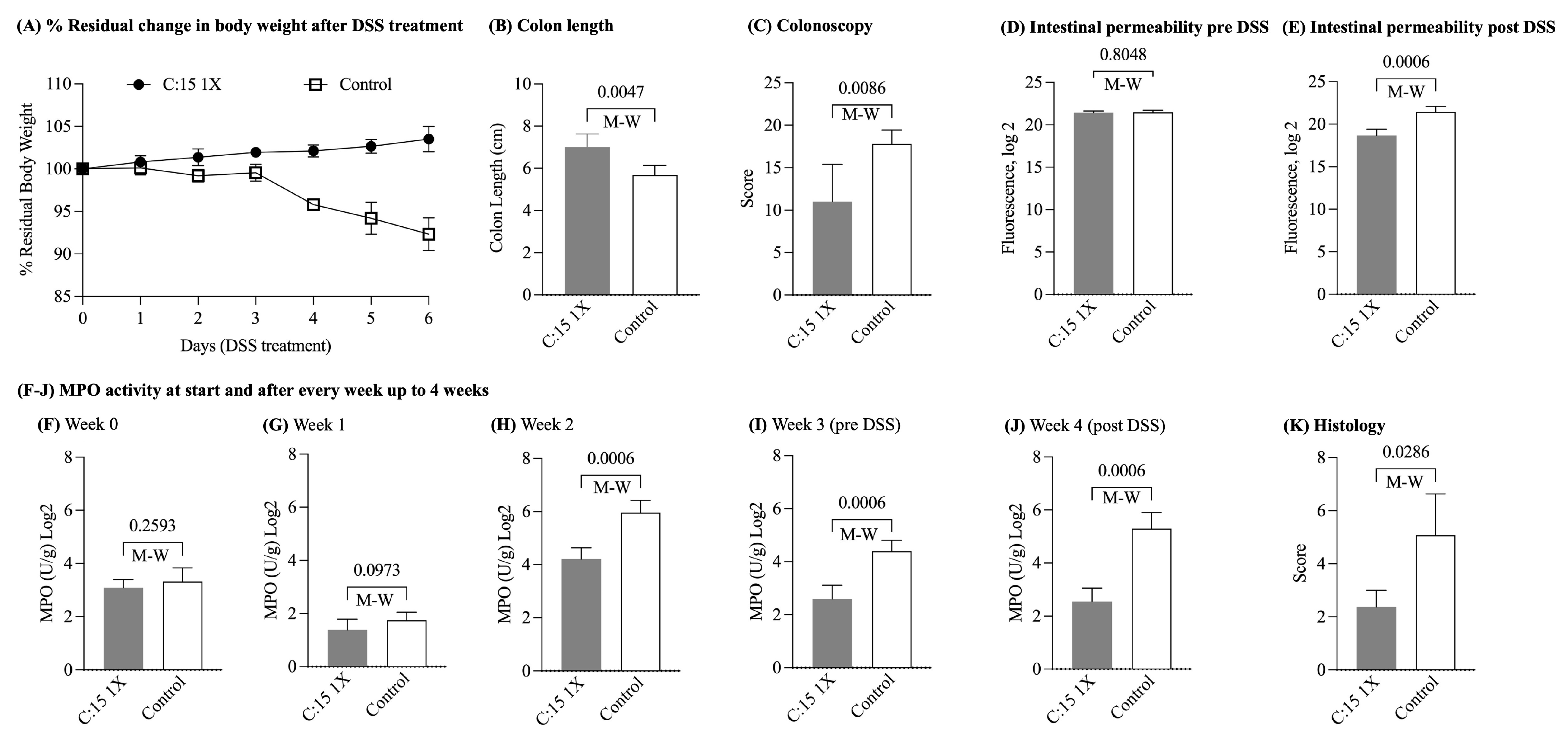
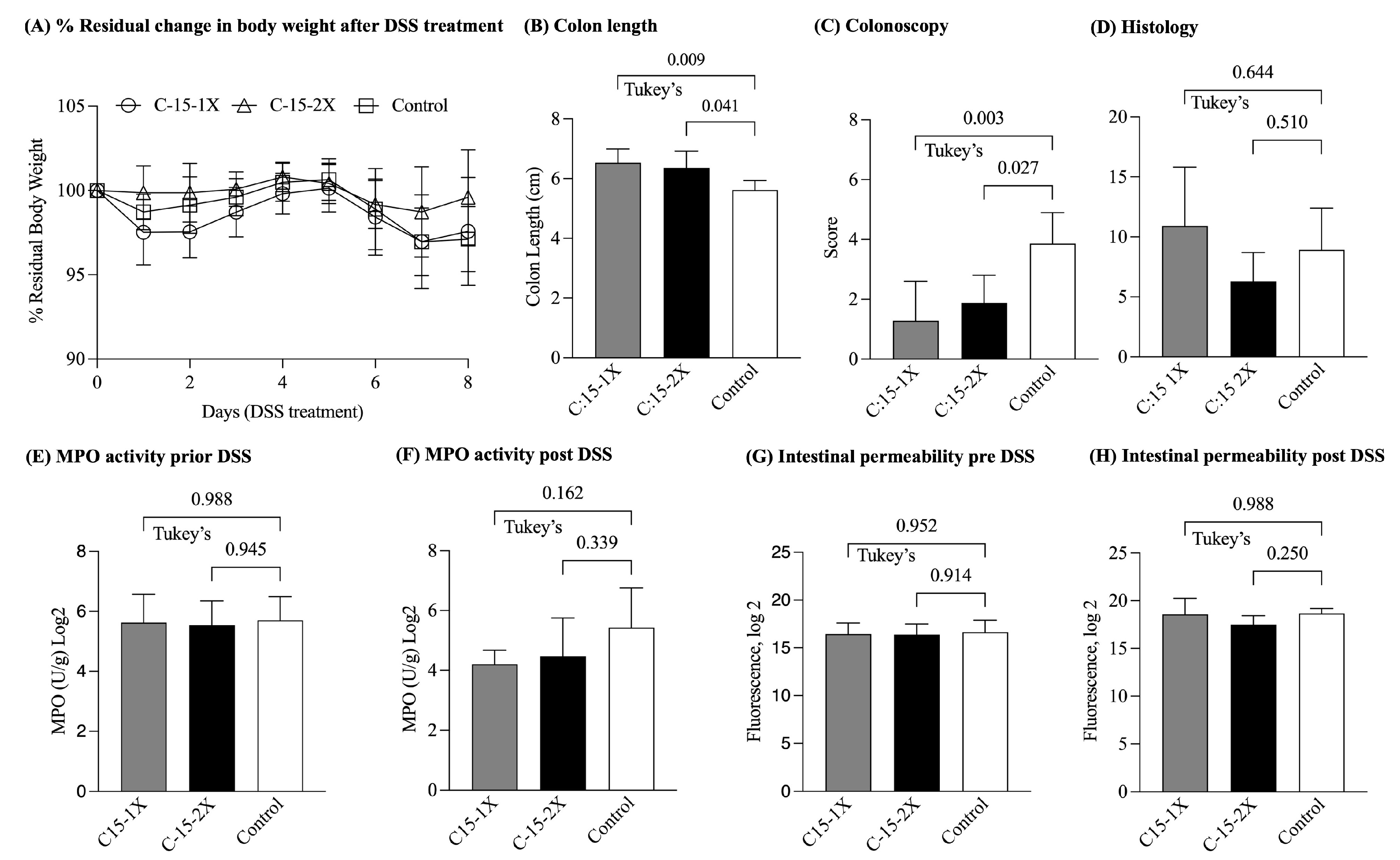

| Experiment | DSS Induced Colitis | DSS Induced Colitis | Chronic Ileitis |
|---|---|---|---|
| C:15 administration | Short term (3 weeks) | Long term (6 weeks) | 6 weeks |
| Mouse (age) | SAMP/YitFc (14 weeks) | SAMP/YitFc (14 weeks) | SAMP/YitFc (24 weeks) |
| Comparison | C:15(1X) vs. Control | C:15(1X) vs. (2X) vs. Control | C:15(1X) vs. Control |
| % Residual Body weight * | C:15: 103.1 ± 2.0 Control: 92.0 ± 2.8 | C:15(1X): 97.5 ± 2.9 C:15(2X): 99.5 ± 2.5 Control: 97.1 ± 1.7 | C:15: 105.2 ± 1.4 Control: 96.3 ± 1.3 |
| MPO U/mg Log2 * | C:15: 2.5 ± 0.5 Control: 5.2 ± 0.6 | C:15(1X): 3.1 ± 0.2 C:15(2X): 3.2 ± 0.9 Control: 7.1 ± 0.1 | C:15: 4.2 ± 0.5 Control: 6.2 ± 0.9 |
| Fluorescence Log2 * | C:15 18.6 ± 0.7 Control: 21.4 ± 0.6 | C:15(1X): 19.0 ± 2.1 C:15(2X): 17.6 ± 0.5 Control: 24.0 ± 0.5 | C:15: 15.4 ± 0.2 Control: 15.8 ± 0.2 |
| Colon length * | C:15: 7.0 ± 0.6 Control: 5.6 ± 0.4 | C:15(1X): 6.5 ± 0.4 C:15(2X): 6.3 ± 0.5 Control: 5.6 ± 0.3 | NA |
| Colonoscopy * | C:15: 3.1 ± 1.0 Control: 4.4 ± 1.5 | C:15(1X): 1.6 ± 0.8 C:15(2X): 1.6 ± 0.8 Control: 4.7 ± 0.9 | NA |
| Colon Histology * | C:15: 11.0 ± 4.3 Control: 17.8 ± 1.6 | C:15(1X): 10.9 ± 4.8 C:15(2X): 6.3 ± 2.3 Control: 8.9 ± 3.4 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.; Mehghini, P.; Rodriguez-Palacios, A.; Di Martino, L.; Cominelli, F.; Basson, A.R. Anti-Inflammatory Effect of Dietary Pentadecanoic Fatty Acid Supplementation on Inflammatory Bowel Disease in SAMP1/YitFc Mice. Nutrients 2024, 16, 3031. https://doi.org/10.3390/nu16173031
Singh D, Mehghini P, Rodriguez-Palacios A, Di Martino L, Cominelli F, Basson AR. Anti-Inflammatory Effect of Dietary Pentadecanoic Fatty Acid Supplementation on Inflammatory Bowel Disease in SAMP1/YitFc Mice. Nutrients. 2024; 16(17):3031. https://doi.org/10.3390/nu16173031
Chicago/Turabian StyleSingh, Drishtant, Paola Mehghini, Alexander Rodriguez-Palacios, Luca Di Martino, Fabio Cominelli, and Abigail Raffner Basson. 2024. "Anti-Inflammatory Effect of Dietary Pentadecanoic Fatty Acid Supplementation on Inflammatory Bowel Disease in SAMP1/YitFc Mice" Nutrients 16, no. 17: 3031. https://doi.org/10.3390/nu16173031
APA StyleSingh, D., Mehghini, P., Rodriguez-Palacios, A., Di Martino, L., Cominelli, F., & Basson, A. R. (2024). Anti-Inflammatory Effect of Dietary Pentadecanoic Fatty Acid Supplementation on Inflammatory Bowel Disease in SAMP1/YitFc Mice. Nutrients, 16(17), 3031. https://doi.org/10.3390/nu16173031






