Vitamin D Deficiency after Anterior Cruciate Ligament Reconstruction Associates with Knee Osteoarthritis: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Cases Compared to Controls
3.2.1. Patient Demographics
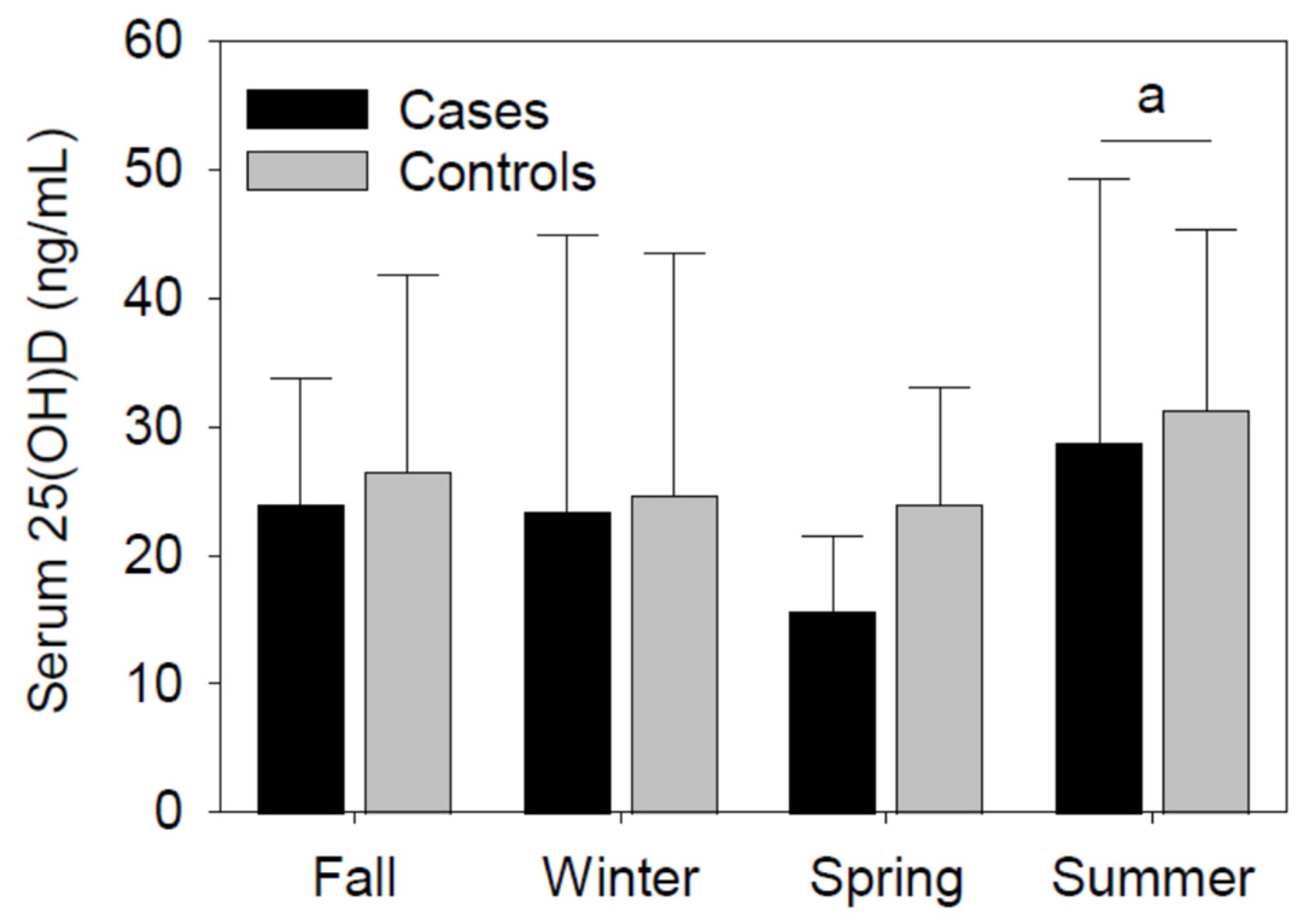
3.2.2. Serum 25(OH)D Concentrations
3.2.3. Timing of Knee OA Diagnosis for Cases and Window of Record Review for Controls
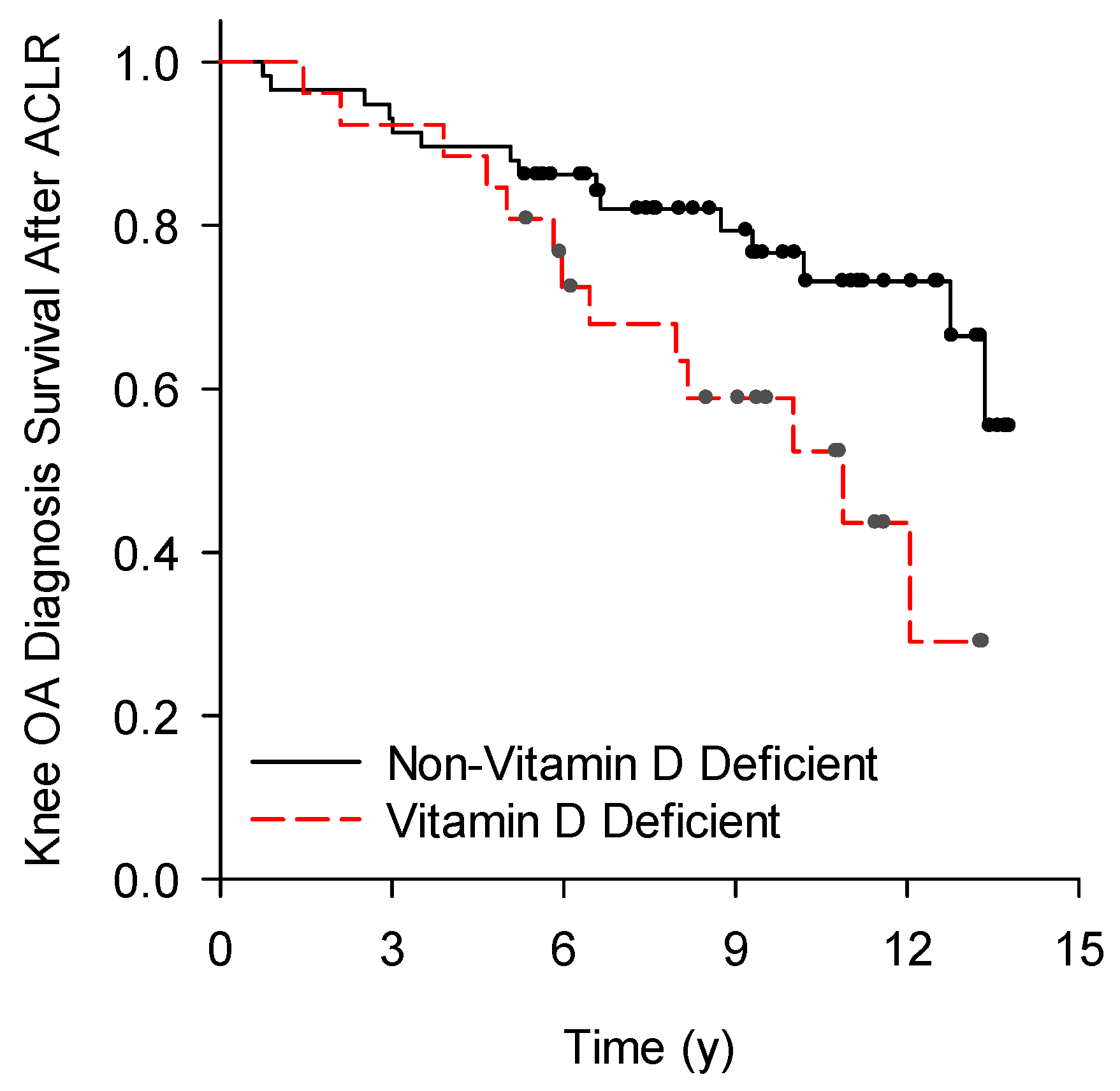
3.3. Vitamin D Deficient Compared to Non-Vitamin D Deficient Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bollen, S.R.; Scott, B.W. Rupture of the anterior cruciate ligament—A quiet epidemic? Injury 1996, 27, 407. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756. [Google Scholar] [CrossRef]
- Everhart, J.S.; Jones, M.H.; Yalcin, S.; Reinke, E.K.; Huston, L.J.; Andrish, J.T.; Cox, C.L.; Flanigan, D.C.; Kaeding, C.C.; Magnussen, R.A.; et al. The Clinical Radiographic Incidence of Posttraumatic Osteoarthritis 10 Years After Anterior Cruciate Ligament Reconstruction: Data From the MOON Nested Cohort. Am. J. Sports Med. 2021, 49, 1251. [Google Scholar] [CrossRef] [PubMed]
- Tourville, T.W.; Johnson, R.J.; Slauterbeck, J.R.; Naud, S.; Beynnon, B.D. Assessment of early tibiofemoral joint space width changes after anterior cruciate ligament injury and reconstruction: A matched case-control study. Am. J. Sports Med. 2013, 41, 769. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 2006, 20, 739. [Google Scholar] [CrossRef]
- Bank, N.C.; Sanghvi, P.; Hecht, C.J., 2nd; Mistovich, R.J. The Epidemiology of Posttraumatic Osteoarthritis of the Knee in the United States: An Analysis of 948,853 Patients From 2000 to 2022. J. Am. Acad. Orthop. Surg. 2024, 32, e313. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S. [Google Scholar] [CrossRef]
- McAlindon, T.; LaValley, M.; Schneider, E.; Nuite, M.; Lee, J.Y.; Price, L.L.; Lo, G.; Dawson-Hughes, B. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: A randomized controlled trial. JAMA 2013, 309, 155. [Google Scholar] [CrossRef]
- Ding, C.; Cicuttini, F.; Parameswaran, V.; Burgess, J.; Quinn, S.; Jones, G. Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: The Tasmanian older adult cohort study. Arthritis Rheum. 2009, 60, 1381. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Felson, D.T.; Zhang, Y.; Hannan, M.T.; Aliabadi, P.; Weissman, B.; Rush, D.; Wilson, P.W.; Jacques, P. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann. Intern. Med. 1996, 125, 353. [Google Scholar] [CrossRef]
- Alabajos-Cea, A.; Herrero-Manley, L.; Suso-Martí, L.; Viosca-Herrero, E.; Cuenca-Martínez, F.; Varangot-Reille, C.; Blanco-Díaz, M.; Calatayud, J.; Casaña, J. The Role of Vitamin D in Early Knee Osteoarthritis and Its Relationship with Their Physical and Psychological Status. Nutrients 2021, 13, 4035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, S.; Abhilasha, A.; Singh, A.; Kumar, U. The Role of Matrix Metalloproteinase 13 and Vitamin D in Osteoarthritis: A Hospital-Based Observational Study. Cureus 2023, 15, e45437. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.K.; Gantaguru, A.; Nanda, S.N.; Velagada, S.; Srinivasan, A.; Mangaraj, M. Association of vitamin D and knee osteoarthritis in younger individuals. World J. Orthop. 2020, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Almalki, A.; Gharib, A.F.; Almehmadi, M.; Alharthi, A.; Alsalmi, O.; Alsulimani, A.H.; Alanazi, R.H.; AlWthenani, A.A.; Alotaibi, M.; AlZaidi, F.T. The Association of Vitamin D, Growth/Differentiation Factor 5 (GDF-5) Gene Polymorphism, and Serum GDF-5 Protein in Obese Patients With Knee Osteoarthritis. Cureus 2023, 15, e48350. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Speranza, T.C.; Mor, D.; Mason, R.S.; Bartlett, J.R.; Duque, G.; Levinger, I.; Levinger, P. Skeletal muscle vitamin D in patients with end stage osteoarthritis of the knee. J. Steroid Biochem. Mol. Biol. 2017, 173, 180. [Google Scholar] [CrossRef]
- Felson, D.T.; Niu, J.; Clancy, M.; Aliabadi, P.; Sack, B.; Guermazi, A.; Hunter, D.J.; Amin, S.; Rogers, G.; Booth, S.L. Low levels of vitamin D and worsening of knee osteoarthritis: Results of two longitudinal studies. Arthritis Rheum. 2007, 56, 129. [Google Scholar] [CrossRef]
- Zhang, F.F.; Driban, J.B.; Lo, G.H.; Price, L.L.; Booth, S.; Eaton, C.B.; Lu, B.; Nevitt, M.; Jackson, B.; Garganta, C.; et al. Vitamin D deficiency is associated with progression of knee osteoarthritis. J. Nutr. 2014, 144, 2002. [Google Scholar] [CrossRef]
- Konstari, S.; Paananen, M.; Heliovaara, M.; Knekt, P.; Marniemi, J.; Impivaara, O.; Arokoski, J.; Karppinen, J. Association of 25-hydroxyvitamin D with the incidence of knee and hip osteoarthritis: A 22-year follow-up study. Scand. J. Rheumatol. 2012, 21, 74. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Kjeldsberg, C.R.; Trawick, R.H.; Hill, H.R. Circulating interferon-gamma correlates with 1,25(OH)D and the 1,25(OH)D-to-25(OH)D ratio. Cytokine 2012, 60, 23. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Trawick, R.H.; Weaver, L.K.; Traber, M.G. Low vitamin D impairs strength recovery after anterior cruciate ligament surgery. J. Evid.-Based Compl. Altern. Med. 2011, 16, 201. [Google Scholar] [CrossRef]
- Albright, J.A.; Chang, K.; Byrne, R.A.; Quinn, M.S.; Meghani, O.; Daniels, A.H.; Owens, B.D. A Diagnosis of Vitamin D Deficiency Is Associated With Increased Rates of Anterior Cruciate Ligament Tears and Reconstruction Failure. Arthroscopy 2023, 39, 2477. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Latham, C.M.; Moore, A.N.; Thomas, N.T.; Lancaster, B.D.; Reeves, K.A.; Keeble, A.R.; Fry, C.S.; Johnson, D.L.; Thompson, K.L.; et al. Vitamin D status associates with skeletal muscle loss after anterior cruciate ligament reconstruction. JCI Insight 2023, 8, e170518. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Zhang, Y.; Kiel, D.P.; Felson, D.T. Positive association between serum 25-hydroxyvitamin D level and bone density in osteoarthritis. Arthritis Rheum. 2005, 53, 821. [Google Scholar] [CrossRef] [PubMed]
- Bodkin, S.G.; Werner, B.C.; Slater, L.V.; Hart, J.M. Post-traumatic osteoarthritis diagnosed within 5 years following ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 790. [Google Scholar] [CrossRef]
- van Driel, M.; Koedam, M.; Buurman, C.J.; Roelse, M.; Weyts, F.; Chiba, H.; Uitterlinden, A.G.; Pols, H.A.; van Leeuwen, J.P. Evidence that both 1alpha,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J. Cell Biochem. 2006, 99, 922. [Google Scholar] [CrossRef]
- Hilal, G.; Martel-Pelletier, J.; Pelletier, J.P.; Ranger, P.; Lajeunesse, D. Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: Possible role in subchondral bone sclerosis. Arthritis Rheum. 1998, 41, 891. [Google Scholar] [CrossRef]
- Corrado, A.; Neve, A.; Macchiarola, A.; Gaudio, A.; Marucci, A.; Cantatore, F.P. RANKL/OPG ratio and DKK-1 expression in primary osteoblastic cultures from osteoarthritic and osteoporotic subjects. J. Rheumatol. 2013, 40, 684. [Google Scholar] [CrossRef]
- Neve, A.; Cantatore, F.P.; Corrado, A.; Gaudio, A.; Ruggieri, S.; Ribatti, D. In vitro and in vivo angiogenic activity of osteoarthritic and osteoporotic osteoblasts is modulated by VEGF and vitamin D3 treatment. Regul. Pept. 2013, 184, 81. [Google Scholar] [CrossRef]
- Tetlow, L.C.; Smith, S.J.; Mawer, E.B.; Woolley, D.E. Vitamin D receptors in the rheumatoid lesion: Expression by chondrocytes, macrophages, and synoviocytes. Ann. Rheum. Dis. 1999, 58, 118. [Google Scholar] [CrossRef]
- Tetlow, L.C.; Woolley, D.E. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthr. Cartil. 2001, 9, 423. [Google Scholar] [CrossRef]
- Orfanidou, T.; Malizos, K.N.; Varitimidis, S.; Tsezou, A. 1,25-Dihydroxyvitamin D(3) and extracellular inorganic phosphate activate mitogen-activated protein kinase pathway through fibroblast growth factor 23 contributing to hypertrophy and mineralization in osteoarthritic chondrocytes. Exp. Biol. Med. 2012, 237, 241. [Google Scholar] [CrossRef] [PubMed]
- Mikesky, A.E.; Mazzuca, S.A.; Brandt, K.D.; Perkins, S.M.; Damush, T.; Lane, K.A. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Rheum. 2006, 55, 690. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Baker, K.; Niu, J.; Clancy, M.; Goggins, J.; Guermazi, A.; Grigoryan, M.; Hunter, D.J.; Felson, D.T. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009, 60, 189. [Google Scholar] [CrossRef] [PubMed]
- Tourville, T.W.; Jarrell, K.M.; Naud, S.; Slauterbeck, J.R.; Johnson, R.J.; Beynnon, B.D. Relationship between isokinetic strength and tibiofemoral joint space width changes after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2014, 42, 302. [Google Scholar] [CrossRef]
- Hipsley, A.; Hall, M.; Saxby, D.J.; Bennell, K.L.; Wang, X.; Bryant, A.L. Quadriceps muscle strength at 2 years following anterior cruciate ligament reconstruction is associated with tibiofemoral joint cartilage volume. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 1949. [Google Scholar] [CrossRef]
- Slemenda, C.; Brandt, K.D.; Heilman, D.K.; Mazzuca, S.; Braunstein, E.M.; Katz, B.P.; Wolinsky, F.D. Quadriceps weakness and osteoarthritis of the knee. Ann. Intern. Med. 1997, 127, 97. [Google Scholar] [CrossRef]
- Qiu, J.; Choi, C.Y.; Man, G.C.; He, X.; Yu, M.; Cao, M.; Wang, Q.; Ng, J.P.; Yung, P.S.; Ong, M.T. Serum vitamin D insufficiency is correlated with quadriceps neuromuscular functions in patients with anterior cruciate ligament injury: A preliminary study. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2024, 35, 76. [Google Scholar] [CrossRef]
- Snoeker, B.; Turkiewicz, A.; Magnusson, K.; Frobell, R.; Yu, D.; Peat, G.; Englund, M. Risk of knee osteoarthritis after different types of knee injuries in young adults: A population-based cohort study. Br. J. Sports Med. 2020, 54, 725. [Google Scholar] [CrossRef]
- Poulsen, E.; Goncalves, G.H.; Bricca, A.; Roos, E.M.; Thorlund, J.B.; Juhl, C.B. Knee osteoarthritis risk is increased 4-6 fold after knee injury—A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 1454. [Google Scholar] [CrossRef]
- Barker, T.; May, H.T.; Doty, J.R.; Lappe, D.L.; Knowlton, K.U.; Carlquist, J.; Konery, K.; Inglet, S.; Chisum, B.; Galenko, O.; et al. Vitamin D supplementation protects against reductions in plasma 25-hydroxyvitamin D induced by open-heart surgery: Assess-d trial. Physiol. Rep. 2021, 9, e14747. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Brown, K.B.; Rogers, V.E. Soluble TNF receptors are modulated by vitamin D status but not by acute perturbations in 25-hydroxyvitamin D following a bolus of supplemental vitamin D. J. Cytokine Biol. 2017, 2, 1. [Google Scholar] [CrossRef]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Levy, M.; Schneider, E.D.; Templeton, J.; Goldfine, H.; Dixon, B.M.; Rasmussen, G.L.; Trawick, R.H.; et al. Circulating cytokine concentrations are not altered by supplemental vitamin D in knee osteoarthritis: A pilot study. J. Nutr. Intermed. Metab. 2019, 18, 1. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266. [Google Scholar] [CrossRef] [PubMed]
- Barker, T. Vitamin D and inflammation. In Nutrition & Physical Activity in Inflammatory Diseases, 1st ed.; Garg, M.L., Wood, L.G., Eds.; CABI: Boston, UK, 2013; p. 75. [Google Scholar]
- Edfeldt, K.; Liu, P.T.; Chun, R.; Fabri, M.; Schenk, M.; Wheelwright, M.; Keegan, C.; Krutzik, S.R.; Adams, J.S.; Hewison, M.; et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 22593. [Google Scholar] [CrossRef] [PubMed]
- Hummel, D.M.; Fetahu, I.S.; Groschel, C.; Manhardt, T.; Kallay, E. Role of proinflammatory cytokines on expression of vitamin D metabolism and target genes in colon cancer cells. J. Steroid Biochem. Mol. Biol. 2013, 144, 91–95. [Google Scholar] [CrossRef]
- Pryke, A.M.; Duggan, C.; White, C.P.; Posen, S.; Mason, R.S. Tumor necrosis factor-alpha induces vitamin D-1-hydroxylase activity in normal human alveolar macrophages. J. Cell Physiol. 1990, 142, 652. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37. [Google Scholar] [CrossRef]
- Smolders, J.; van den Ouweland, J.; Geven, C.; Pickkers, P.; Kox, M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism 2020, 115, 154434. [Google Scholar] [CrossRef]

| ACLR to Serum 25(OH)D | Serum 25(OH)D | Age at ACLR | ||||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| Serum 25(OH)D | 0.11 | 0.33 | ||||
| Age at ACLR | −0.13 | 0.24 | 0.16 | 0.16 | ||
| BMI | −0.14 | 0.19 | −0.19 | 0.08 | −0.05 | 0.69 |
| Cases | Controls | p-Value | |
|---|---|---|---|
| n (m/f) | 28 (13/15) | 56 (26/30) | 1.00 |
| Age at ACLR, y | 34.8 (20.0) | 34.3 (17.8) | 0.73 |
| Height, m | 1.68 (0.13) | 1.73 (0.14) | 0.48 |
| Body mass, kg | 89.9 (28.7) | 85.3 (25.2) | 0.36 |
| BMI, kg/m2 | 30.0 (9.4) | 27.9 (7.1) | 0.24 |
| Additional procedures at ACLR, n | 0.87 | ||
| No | 9 | 19 | |
| Yes | 19 | 37 | |
| Time from ACLR to serum 25(OH)D, y | 2.37 (3.54) | 2.56 (3.29) | 0.75 |
| Serum 25(OH)D, ng/mL | 21.6 (13.8) | 25.6 (13.3) | 0.14 |
| Season of serum 25(OH) assessment, n | 0.32 | ||
| Fall | 4 | 18 | |
| Winter | 9 | 17 | |
| Spring | 10 | 13 | |
| Summer | 5 | 8 | |
| Time from ACLR to OA diagnosis, y | 5.89 (5.77) | NA | NA |
| Time from ACLR to last records review, y | NA | 9.52 (4.48) | NA |
| Deficient | Non-Deficient | p-Value | |
|---|---|---|---|
| n (m/f) | 26 (11/15) | 58 (28/30) | 0.64 |
| Age at ACLR, y | 34.3 (19.6) | 34.5 (15.5) | 0.97 |
| Height, m | 1.70 (0.10) | 1.72 (0.15) | 0.47 |
| Body mass, kg | 97.5 (30.4) | 83.5 (22.6) | 0.04 |
| BMI, kg/m2 | 32.8 (10.3) | 27.5 (6.5) | 0.01 |
| Additional procedures at ACLR, n | 0.07 | ||
| No | 5 | 23 | |
| Yes | 21 | 35 | |
| Time from ACLR to serum 25(OH)D, y | 2.28 (2.76) | 2.66 (3.49) | 0.50 |
| Serum 25(OH)D, ng/mL | 15.1 (6.1) | 28.9 (13.8) | <0.01 |
| Season of serum 25(OH)D assessment, n | 0.03 | ||
| Fall | 5 | 17 | |
| Winter | 8 | 18 | |
| Spring | 12 | 11 | |
| Summer | 1 | 12 | |
| Post-ACLR knee OA, n | 0.03 | ||
| No | 13 | 43 | |
| Yes | 13 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.; Schmitt, L.C.; Burnett, Z.; Milliron, E.M.; Cavendish, P.A.; Magnussen, R.A.; Kaeding, C.C.; Flanigan, D.C.; Barker, T. Vitamin D Deficiency after Anterior Cruciate Ligament Reconstruction Associates with Knee Osteoarthritis: A Retrospective Study. Nutrients 2024, 16, 3029. https://doi.org/10.3390/nu16173029
Bae S, Schmitt LC, Burnett Z, Milliron EM, Cavendish PA, Magnussen RA, Kaeding CC, Flanigan DC, Barker T. Vitamin D Deficiency after Anterior Cruciate Ligament Reconstruction Associates with Knee Osteoarthritis: A Retrospective Study. Nutrients. 2024; 16(17):3029. https://doi.org/10.3390/nu16173029
Chicago/Turabian StyleBae, Sonu, Laura C. Schmitt, Zachary Burnett, Eric M. Milliron, Parker A. Cavendish, Robert A. Magnussen, Christopher C. Kaeding, David C. Flanigan, and Tyler Barker. 2024. "Vitamin D Deficiency after Anterior Cruciate Ligament Reconstruction Associates with Knee Osteoarthritis: A Retrospective Study" Nutrients 16, no. 17: 3029. https://doi.org/10.3390/nu16173029
APA StyleBae, S., Schmitt, L. C., Burnett, Z., Milliron, E. M., Cavendish, P. A., Magnussen, R. A., Kaeding, C. C., Flanigan, D. C., & Barker, T. (2024). Vitamin D Deficiency after Anterior Cruciate Ligament Reconstruction Associates with Knee Osteoarthritis: A Retrospective Study. Nutrients, 16(17), 3029. https://doi.org/10.3390/nu16173029






