The Course of Minipuberty in Daughters of Women with Low Gestational Vitamin D Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Laboratory Assays
2.4. Statistical Analysis
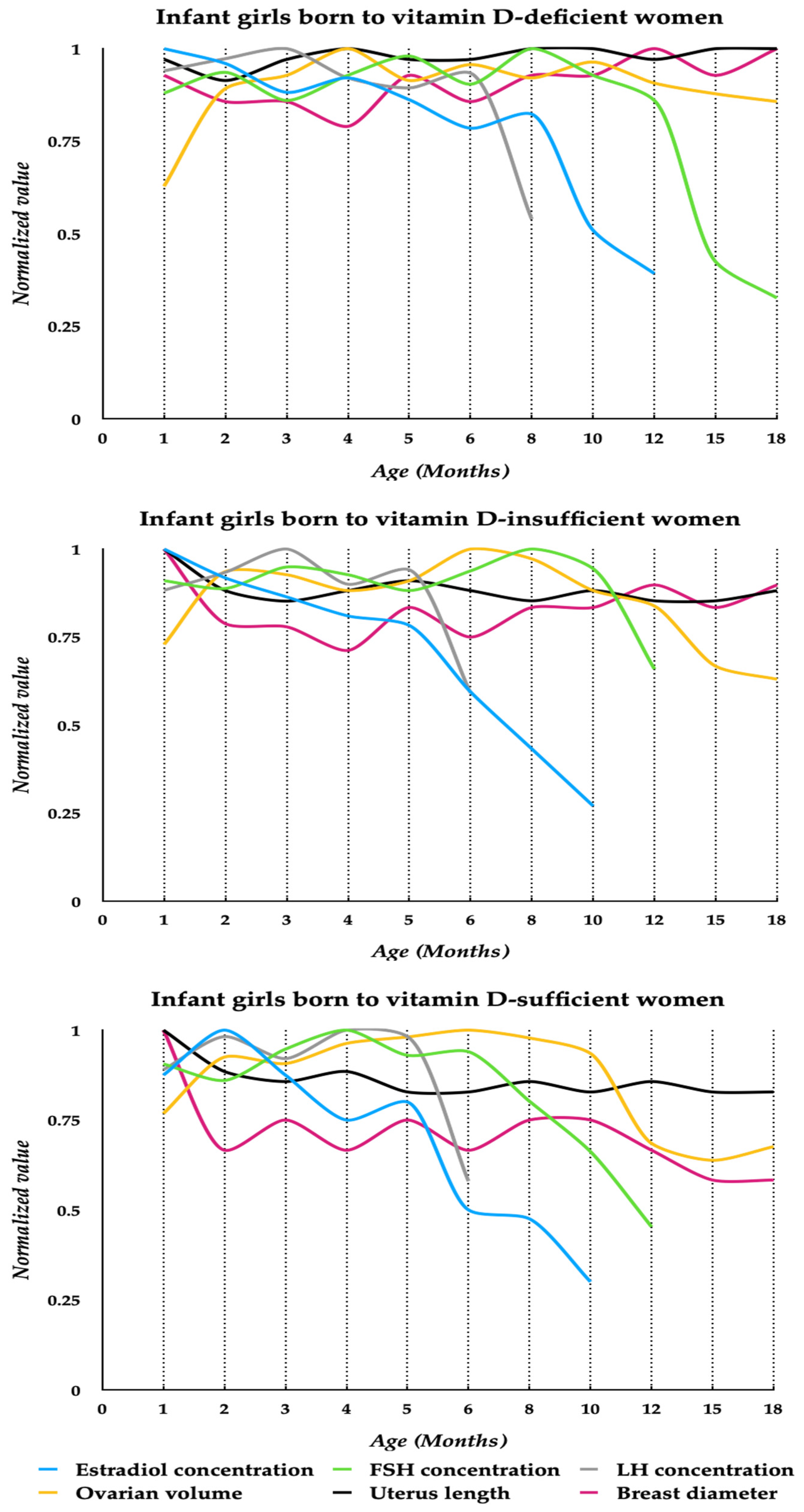
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lanciotti, L.; Cofini, M.; Leonardi, A.; Penta, L.; Esposito, S. Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Front. Endocrinol. 2018, 9, 410. [Google Scholar] [CrossRef]
- Becker, M.; Hesse, V. Minipuberty: Why does it happen? Horm. Res. Paediatr. 2020, 93, 76–84. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Sankilampi, U.; Dunkel, L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: Minipuberty. Horm. Res. Paediatr. 2014, 82, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, C.; Cappa, M. Ontogeny of hypothalamus-pituitary gonadal axis and minipuberty: An ongoing debate? Front. Endocrinol. 2020, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Lucaccioni, L.; Trevisani, V.; Boncompagni, A.; Marrozzini, L.; Berardi, A.; Iughetti, L. Minipuberty: Looking back to understand moving forward. Front. Pediatr. 2021, 8, 612235. [Google Scholar] [CrossRef]
- Ljubicic, M.L.; Busch, A.S.; Upners, E.N.; Fischer, M.B.; Petersen, J.H.; Raket, L.L.; Frederiksen, H.; Johannsen, T.H.; Juul, A.; Hagen, C.P. A biphasic pattern of reproductive hormones in healthy female infants: The Copenhagen minipuberty study. J. Clin. Endocrinol. Metab. 2022, 107, 2598–2605. [Google Scholar] [CrossRef]
- Chachlaki, K.; Le Duc, K.; Storme, L.; Prevot, V. Novel insights into minipuberty and GnRH: Implications on neurodevelopment, cognition, and COVID-19 therapeutics. J. Neuroendocrinol. 2024, e13387. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, X.; Wang, Y.; Yan, S.; Li, D.; Cui, W. The association between vitamin D levels and precocious puberty: A meta-analysis. J. Pediatr. Endocrinol. Metab. 2020, 33, 427–429. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, D.; Gao, H. An updated meta-analysis of the relationship between vitamin D levels and precocious puberty. Front. Endocrinol. 2023, 14, 1298374. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Yan, F.; Cui, Y.; Song, Y.; Yan, S.; Cui, W. Does vitamin D have a potential role in precocious puberty? A meta-analysis. Food Funct. 2023, 14, 5301–5310. [Google Scholar] [CrossRef]
- Gan, D.M.; Fang, J.; Zhang, P.P.; Zhao, Y.D.; Xu, Y.N. Serum 25-hydroxyvitamin D levels and the risk of idiopathic central precocious puberty in girls. Clinics 2023, 78, 100244. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, W.; Xiao, Y.; Cao, N.N.; Wang, Y.F.; Zhang, H.R.; Jiang, S.Q. Correlation between serum vitamin D level and uterine volume in girls with idiopathic central precocious puberty. J. Pediatr. Endocrinol. Metab. 2023, 37, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Marin, C.; Mora-Plazas, M.; Baylin, A. Vitamin D deficiency and age at menarche: A prospective study. Am. J. Clin. Nutr. 2011, 94, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Akın, O.; Bideci, A.; Döğer, E.; Demet Akbaş, E.; Kilinç Uğurlu, A.; Yavuz, S.T.; Elbeğ, Ş.; Çamurdan, O.; Cinaz, P. Vitamin D status and premature adrenarche. Pediatr. Int. 2018, 60, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, P.; Yuan, D. Confirming the association between low serum 25OHD levels in girls with central precocious puberty and its severity. BMC Pediatr. 2023, 23, 624. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.R.; Mascarenhas, L.P.; Satler, F.; Boguszewski, M.C.; Spritzer, P.M. Vitamin D receptor gene polymorphisms and sex steroid secretion in girls with precocious pubarche in Southern Brazil: A pilot study. J. Endocrinol. Investig. 2012, 35, 725–729. [Google Scholar]
- Gaml-Sørensen, A.; Brix, N.; Ernst, A.; Lunddorf, L.L.; Lindh, C.; Toft, G.; Henriksen, T.B.; Arah, O.A.; Ramlau-Hansen, C.H. The estimated effect of season and vitamin D in the first trimester on pubertal timing in girls and boys: A cohort study and an instrumental variable analysis. Int. J. Epidemiol. 2023, 52, 1328–1340. [Google Scholar] [CrossRef]
- Knuschke, P. Sun exposure and vitamin D. Curr. Probl. Dermatol. 2021, 55, 296–315. [Google Scholar] [PubMed]
- Kowalcze, K.; Krysiak, R.; Obuchowicz, A. Minipuberty in sons of women with low vitamin D status during pregnancy. Nutrients 2023, 15, 4729. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Cardiometabolic effects of cabergoline and combined oral contraceptive pills in young women with hyperprolactinemia: A pilot study. J. Clin. Med. 2023, 12, 3208. [Google Scholar] [CrossRef]
- Kowalcze, K.; Krysiak, R.; Obuchowicz, A. The impact of maternal hypothyroidism during pregnancy on minipuberty in boys. J. Clin. Med. 2023, 12, 7649. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Rios-Leyvraz, M.; Yao, Q. Calcium, zinc, and vitamin D in breast milk: A systematic review and meta-analysis. Int. Breastfeed. J. 2023, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, L.S.; Hagen, C.P.; Assens, M.; Busch, A.S.; Skakkebæk, N.E.; Almstrup, K.; Main, K.M. Genetic variations in FSH action affect sex hormone levels and breast tissue size in infant girls: A pilot study. J. Clin. Endocrinol. Metab. 2016, 101, 3191–3198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Brien, K.M.; Upson, K.; Cook, N.R.; Weinberg, C.R. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ. Health Perspect. 2016, 124, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Basiak, M.; Machnik, G.; Szkróbka, W.; Okopień, B. Vitamin D status determines cardiometabolic effects of cabergoline in women with elevated prolactin levels: A pilot study. Nutrients 2023, 15, 2303. [Google Scholar] [CrossRef] [PubMed]
- Kuiri-Hänninen, T.; Haanpää, M.; Turpeinen, U.; Hämäläinen, E.; Seuri, R.; Tyrväinen, E.; Sankilampi, U.; Dunkel, L. Postnatal ovarian activation has effects in estrogen target tissues in infant girls. J. Clin. Endocrinol. Metab. 2013, 98, 4709–4716. [Google Scholar] [CrossRef] [PubMed]
- Chin, H.B.; Baird, D.D.; Kaplan, S.L.; Darge, K.; Adgent, M.A.; Ford, E.G.; Rogan, W.J.; Stallings, V.A.; Umbach, D.M. Characterization of ovarian development in girls from born to 9 months. Paediatr. Paediatr. Perinat. Epidemiol. 2021, 35, 75–82. [Google Scholar] [CrossRef]
- Kuiri-Hanninen, T.; Kallio, S.; Seuri, R.; Tyrvainen, E.; Liakka, A.; Tapanainen, J.; Sankilampi, U.; Dunkel, L. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J. Clin. Endocrinol. Metab. 2011, 96, 3432–3439. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Dunkel, L.; Sankilampi, U. Sexual dimorphism in postnatal gonadotrophin levels in infancy reflects diverse maturation of the ovarian and testicular hormone synthesis. Clin. Endocrinol. 2018, 89, 85–92. [Google Scholar] [CrossRef]
- Kalaycı, F.M.; Gürsoy Doruk, Ö.; Erbaş, İ.M.; İnce, O.T.; Tan, M.N.; Aydın, A.; Abacı, A.; Böber, E.; Demir, K. Salivary sex steroid levels in infants and the relation with infantile colic. J. Clin. Res. Pediatr. Endocrinol. 2024, 16, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Bidlingmaier, F.; Strom, T.M.; Dörr, H.G.; Eisenmenger, W.; Knorr, D. Estrone and estradiol concentrations in human ovaries, testes, and adrenals during the first two years of life. J. Clin. Endocrinol. Metab. 1987, 65, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, U.; Mullis, P.E. The aromatase cytochrome P-450 and its clinical impact. Horm. Res. 2002, 57, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Villaggio, B.; Soldano, S.; Cutolo, M. 1,25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clin. Exp. Rheumatol. 2012, 30, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Swami, S.; Feldman, D. Vitamin D and breast cancer: Inhibition of estrogen synthesis and signaling. J. Steroid Biochem. Mol. Biol. 2010, 121, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Franssen, D.; Barroso, A.; Ruiz-Pino, F.; Vázquez, M.J.; García-Galiano, D.; Castellano, J.M.; Onieva, R.; Ruiz-Cruz, M.; Poutanen, M.; Gaytán, F.; et al. AMP-activated protein kinase (AMPK) signaling in GnRH neurons links energy status and reproduction. Metabolism 2021, 115, 154460. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.J.; Velasco, I.; Tena-Sempere, M. Novel mechanisms for the metabolic control of puberty: Implications for pubertal alterations in early-onset obesity and malnutrition. J. Endocrinol. 2019, 242, R51–R65. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Vitamin D status determines the impact of metformin on gonadotropin levels in postmenopausal women. J. Clin. Med. 2023, 12, 3715. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Guerrero, J.A.; León-Cabrera, S.; Escobedo, G. Molecular mechanisms involved in fetal programming and disease origin in adulthood. J. Pediatr. Endocrinol. Metab. 2023, 36, 615–627. [Google Scholar] [CrossRef]
- Burlo, F.; Lorenzon, B.; Tamaro, G.; Fabretto, A.; Buonomo, F.; Peinkhofer, M.; Vidonis, V.; Vittori, G.; Faleschini, E.; Barbi, E.; et al. Prevalence and characteristics of thelarche variant. Front. Endocrinol. 2023, 14, 1303989. [Google Scholar] [CrossRef]
- Renaul, C.H.; Aksglaede, L.; Wøjdemann, D.; Hansen, A.B.; Jensen, R.B.; Juul, A. Minipuberty of human infancy—A window of opportunity to evaluate hypogonadism and differences of sex development? Ann. Pediatr. Endocrinol. Metab. 2020, 25, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ljubicic, M.L.; Madsen, A.; Upners, E.N.; Fischer, M.B.; Busch, A.S.; Frederiksen, H.; Johannsen, T.H.; Juul, A.; Hagen, C.P. Longitudinal evaluation of breast tissue in healthy infants: Prevalence and relation to reproductive hormones and growth factors. Front. Endocrinol. 2022, 13, 1048660. [Google Scholar] [CrossRef] [PubMed]
- Kılınç, S.; Atay, E.; Ceran, Ö.; Atay, Z. Evaluation of vitamin D status and its correlation with gonadal function in children at mini-puberty. Clin. Endocrinol. 2019, 90, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Auer, M.K.; Nordenström, A.; Lajic, S.; Reisch, N. Congenital adrenal hyperplasia. Lancet 2023, 401, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Fairney, A.; Saphier, P.W. Studies on the measurement of 25-hydroxy vitamin D in human saliva. Br. J. Nutr. 1987, 57, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Heureux, N. Vitamin D testing-where are we and what is on the horizon? Adv. Clin. Chem. 2017, 78, 59–101. [Google Scholar] [PubMed]
- Wudy, S.A.; Schuler, G.; Sánchez-Guijo, A.; Hartmann, M.F. The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2018, 179, 88–103. [Google Scholar] [CrossRef]
- Streiner, D.L. Regression toward the mean: Its etiology, diagnosis, and treatment. Can. J. Psychiatry 2001, 46, 72–76. [Google Scholar] [CrossRef]

| Variable | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Sufficient | p-Values | ||
|---|---|---|---|---|---|---|
| Deficient vs. Insufficient | Deficient vs. Insufficient | Insufficient vs. Sufficient | ||||
| Number (n) | 28 | 27 | 28 | - | - | - |
| Gestational age at delivery (weeks) | 39 ± 2 | 39 ± 2 | 40 ± 1 | 1.0000 | 0.2430 | 0.2621 |
| Birth order: first/second/third and subsequent (%) | 50/43/7 | 44/44/11 | 46/43/11 | 0.7236 | 0.7545 | 0.8375 |
| Body length (cm) | 54.1 ± 1.8 | 53.8 ± 1.5 | 54.3 ± 1.9 | 0.5057 | 0.6875 | 0.2848 |
| Head circumference (cm) | 37.0 ± 0.8 | 37.2 ± 0.7 | 36.8 ± 0.8 | 0.3290 | 0.3537 | 0.0896 |
| Body weight (kg) | 4.28 ± 0.52 | 4.40 ± 0.53 | 4.43 ± 0.55 | 0.4006 | 0.2988 | 0.8380 |
| BMI (kg/m2) | 14.6 ± 1.4 | 15.2 ± 1.3 | 15.0 ± 1.1 | 0.1158 | 0.2398 | 0.5402 |
| Breastfeeding (%) | 79 | 85 | 82 | - | - | - |
| Total daily vitamin D intake (µg) | 13.1 ± 1.5 | 13.2 ± 1.7 | 13.4 ± 1.8 | 0.8178 | 0.5010 | 0.6738 |
| Variable | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Sufficient | p-Values | ||
|---|---|---|---|---|---|---|
| Deficient vs. Insufficient | Deficient vs. Insufficient | Insufficient vs. Sufficient | ||||
| Number (n) | 28 | 27 | 28 | - | - | - |
| Age (years) | 34 ± 9 | 34 ± 9 | 35 ± 8 | 1.0000 | 0.6792 | 0.6820 |
| University/secondary/primary or vocational education (%) | 39/43/18 | 44/41/15 | 43/43/14 | 0.6231 | 0.6084 | 0.7325 |
| Employment rate/white-collar/pink-collar/blue-collar workers (%) | 86/25/29/32 | 85/19/30/37 | 82/29/32/21 | 0.7568 | 0.8228 | 0.7611 |
| Smoking during pregnancy (%) | 32 | 30 | 29 | - | - | - |
| BMI (kg/m2) | 24.8 ± 4.1 | 24.1 ± 3.7 | 23.9 ± 3.8 | 0.5096 | 0.3980 | 0.8441 |
| Systolic blood pressure (mmHg) | 122 ± 20 | 118 ± 16 | 116 ± 18 | 0.4175 | 0.2432 | 0.6654 |
| Diastolic blood pressure (mmHg) | 81 ± 6 | 80 ± 6 | 79 ± 7 | 0.5394 | 0.2561 | 0.5725 |
| Mean 25OHD concentrations (nmol/L) | 34 ± 8 | 63 ± 7 | 114 ± 20 | <0.0001 | <0.0001 | <0.0001 |
| Mean daily vitamin D intake during pregnancy (µg) | 11.2 ± 3.7 | 18.1 ± 4.3 | 41.2 ± 11.8 | <0.0001 | <0.0001 | <0.0001 |
| Cumulative vitamin D intake during pregnancy (mg) | 3.9 ± 1.5 | 6.0 ± 1.8 | 13.1 ± 3.7 | <0.0001 | <0.0001 | <0.0001 |
| Study Month | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Sufficient | p-Values | ||
|---|---|---|---|---|---|---|
| Deficient vs. Insufficient | Deficient vs. Insufficient | Insufficient vs. Sufficient | ||||
| 1 | 51 ± 20 | 37 ± 18 | 35 ± 16 | 0.0087 | 0.0017 | 0.6647 |
| 2 | 49 ± 16 | 34 ± 14 | 40 ± 12 | 0.0005 | 0.0208 | 0.0934 |
| 3 | 45 ± 17 | 32 ± 14 | 35 ± 14 | 0.0032 | 0.0197 | 0.3968 |
| 4 | 47 ± 19 | 30 ± 15 | 30 ± 18 | 0.0006 | 0.0011 | 1.0000 |
| 5 | 44 ± 20 | 29 ± 16 | 32 ± 19 | 0.0034 | 0.0253 | 0.6385 |
| 6 | 40 ± 23 | 22 ± 14 | 20 ± 12 | 0.0010 | 0.0001 | 0.5714 |
| 8 | 42 ± 18 | 16 ± 8 | 19 ± 14 | <0.0001 | <0.0001 | 0.3460 |
| 10 | 26 ± 10 | 10 ± 7 | 12 ± 7 | <0.0001 | <0.0001 | 0.2943 |
| 12 | 20 ± 12 | Below LOD | Below LOD | <0.0001 | <0.0001 | - |
| Study Month | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Sufficient | p-Values | ||
|---|---|---|---|---|---|---|
| Deficient vs. Insufficient | Deficient vs. Insufficient | Insufficient vs. Sufficient | ||||
| 1 | 2.20 ± 0.81 | 1.63 ± 0.71 | 1.56 ± 0.80 | 0.0077 | 0.0044 | 0.7329 |
| 2 | 2.34 ± 0.75 | 1.59 ± 0.80 | 1.48 ± 0.75 | 0.0007 | 0.0001 | 0.6009 |
| 3 | 2.15 ± 0.68 | 1.70 ± 0.91 | 1.63 ± 0.59 | 0.0421 | 0.0035 | 0.7341 |
| 4 | 2.32 ± 0.80 | 1.66 ± 0.67 | 1.72 ± 0.64 | 0.0017 | 0.0031 | 0.7365 |
| 5 | 2.45 ± 0.90 | 1.58 ± 0.82 | 1.60 ± 0.73 | 0.0008 | 0.0002 | 0.9290 |
| 6 | 2.26 ± 0.83 | 1.68 ± 0.93 | 1.62 ± 0.80 | 0.0180 | 0.0049 | 0.7980 |
| 8 | 2.50 ± 1.01 | 1.79 ± 0.85 | 1.38 ± 0.60 | 0.0068 | <0.0001 | 0.0431 |
| 10 | 2.32 ± 0.78 | 1.69 ± 0.64 | 1.14 ± 0.63 | 0.0019 | <0.0001 | 0.002 |
| 12 | 2.15 ± 0.84 | 1.18 ± 0.55 | 0.78 ± 0.52 | <0.0001 | <0.0001 | 0.0077 |
| 15 | 1.06 ± 0.62 | Below LOD | Below LOD | <0.0001 | <0.0001 | - |
| 18 | 0.82 ± 0.55 | Below LOD | Below LOD | <0.0001 | <0.0001 | - |
| Study Month | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Sufficient | p-Values | ||
|---|---|---|---|---|---|---|
| Deficient vs. Insufficient | Deficient vs. Insufficient | Insufficient vs. Sufficient | ||||
| 1 | 1.43 ± 0.60 | 1.06 ± 0.40 | 0.98 ± 0.44 | 0.0098 | 0.0023 | 0.4840 |
| 2 | 1.48 ± 0.53 | 1.12 ± 0.52 | 1.12 ± 0.55 | 0.0140 | 0.0157 | 1.0000 |
| 3 | 1.52 ± 0.55 | 1.20 ± 0.60 | 1.05 ± 0.60 | 0.0440 | 0.0035 | 0.3582 |
| 4 | 1.40 ± 0.48 | 1.08 ± 0.56 | 1.14 ± 0.62 | 0.0268 | 0.0450 | 0.7083 |
| 5 | 1.36 ± 0.51 | 1.13 ± 0.46 | 1.12 ± 0.49 | 0.0452 | 0.0443 | 0.9381 |
| 6 | 1.42 ± 0.50 | 0.71 ± 0.35 | 0.65 ± 0.32 | <0.0001 | <0.0001 | 0.5096 |
| 8 | 0.82 ± 0.46 | Below LOD | Below LOD | <0.0001 | <0.0001 | - |
| Study Month | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Sufficient | p-Values | ||
|---|---|---|---|---|---|---|
| Deficient vs. Insufficient | Deficient vs. Insufficient | Insufficient vs. Sufficient | ||||
| 1 | 0.88 ± 0.30 | 0.81 ± 0.30 | 0.83 ± 0.28 | 0.3908 | 0.5218 | 0.7992 |
| 2 | 1.25 ± 0.35 | 1.04 ± 0.28 | 1.00 ± 0.24 | 0.0176 | 0.0029 | 0.5714 |
| 3 | 1.30 ± 0.46 | 1.03 ± 0.40 | 0.98 ± 0.25 | 0.0305 | 0.0024 | 0.6083 |
| 4 | 1.40 ± 0.52 | 0.98 ± 0.39 | 1.04 ± 0.36 | 0.0076 | 0.0018 | 0.5035 |
| 5 | 1.28 ± 0.38 | 1.01 ± 0.40 | 1.06 ± 0.40 | 0.0131 | 0.0395 | 0.6449 |
| 6 | 1.34 ± 0.43 | 1.11 ± 0.37 | 1.08 ± 0.42 | 0.0385 | 0.0260 | 0.7800 |
| 8 | 1.29 ± 0.35 | 1.08 ± 0.42 | 1.05 ± 0.40 | 0.0487 | 0.0204 | 0.7872 |
| 10 | 1.35 ± 0.48 | 0.98 ± 0.31 | 1.01 ± 0.30 | 0.0014 | 0.0025 | 0.7168 |
| 12 | 1.27 ± 0.41 | 0.93 ± 0.38 | 0.74 ± 0.32 | 0.0024 | <0.0001 | 0.0688 |
| 15 | 1.23 ± 0.49 | 0.74 ± 0.28 | 0.69 ± 0.30 | <0.0001 | <0.0001 | 0.5259 |
| 18 | 1.20 ± 0.46 | 0.70 ± 0.28 | 0.73 ± 0.28 | <0.0001 | <0.0001 | 0.6928 |
| Study Month | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Sufficient | p-Values | ||
|---|---|---|---|---|---|---|
| Deficient vs. Insufficient | Deficient vs. Insufficient | Insufficient vs. Sufficient | ||||
| 1 | 34 ± 5 | 34 ± 6 | 35 ± 6 | 1.0000 | 0.5010 | 0.5393 |
| 2 | 32 ± 6 | 30 ± 5 | 31 ± 5 | 0.1858 | 0.4617 | 0.4617 |
| 3 | 34 ± 5 | 29 ± 6 | 30 ± 5 | 0.0014 | 0.0042 | 0.5042 |
| 4 | 35 ± 5 | 30 ± 5 | 31 ± 6 | 0.0005 | 0.0090 | 0.5057 |
| 5 | 34 ± 4 | 31 ± 5 | 29 ± 5 | 0.0171 | 0.0001 | 0.1440 |
| 6 | 34 ± 6 | 30 ± 5 | 29 ± 5 | 0.0018 | 0.0013 | 0.4617 |
| 8 | 35 ± 5 | 29 ± 4 | 30 ± 4 | <0.0001 | 0.0001 | 0.3582 |
| 10 | 35 ± 4 | 30 ± 4 | 29 ± 4 | 0.0001 | <0.0001 | 0.3582 |
| 12 | 34 ± 4 | 29 ± 5 | 30 ± 5 | 0.0001 | 0.0017 | 0.4617 |
| 15 | 35 ± 5 | 29 ± 4 | 29 ± 5 | <0.0001 | <0.0001 | 1.0000 |
| 18 | 35 ± 4 | 30 ± 5 | 29 ± 4 | 0.0001 | <0.0001 | 0.4203 |
| Study Month | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Sufficient | p-Values | ||
|---|---|---|---|---|---|---|
| Deficient vs. Insufficient | Deficient vs. Insufficient | Insufficient vs. Sufficient | ||||
| 1 | 13 ± 5 | 12 ± 5 | 12 ± 5 | 0.4617 | 0.4575 | 1.0000 |
| 2 | 12 ± 5 | 9 ± 5 | 8 ± 4 | 0.0304 | 0.0017 | 0.4156 |
| 3 | 12 ± 5 | 9 ± 4 | 9 ± 3 | 0.0175 | 0.0087 | 1.0000 |
| 4 | 11 ± 5 | 8 ± 5 | 8 ± 4 | 0.0304 | 0.0163 | 1.0000 |
| 5 | 13 ± 5 | 10 ± 3 | 9 ± 5 | 0.0096 | 0.0046 | 0.3682 |
| 6 | 12 ± 4 | 9 ± 4 | 8 ± 4 | 0.0075 | 0.0004 | 0.3582 |
| 8 | 13 ± 3 | 10 ± 4 | 9 ± 3 | 0.0027 | <0.0001 | 0.2978 |
| 10 | 13 ± 4 | 10 ± 4 | 9 ± 5 | 0.0075 | 0.0017 | 0.4175 |
| 12 | 14 ± 3 | 11 ± 4 | 8 ± 3 | 0.0027 | 0.0028 | 0.0027 |
| 15 | 13 ± 4 | 10 ± 4 | 7 ± 3 | 0.0075 | <0.0001 | 0.0027 |
| 18 | 14 ± 4 | 11 ± 4 | 7 ± 3 | 0.0028 | <0.0001 | 0.0001 |
| Correlated Variables | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Sufficient | |
|---|---|---|---|---|
| Maternal 25OHD | Cumulative vitamin D intake | 0.65 [p < 0.0001] | 0.71 [p < 0.0001] | 0.76 [p < 0.0001] |
| Estradiol | LH | 0.43 [p = 0.0004] – 0.65 [p < 0.0001] | 0.46 [p = 0.0002] – 0.64 [p < 0.0001] | 0.47 [p = 0.0001] – 0.64 [p < 0.0001] |
| Ovarian volume | LH | 0.28 [p = 0.0485] – 0.44 [p = 0.0006] | 0.31 [p = 0.0376] – 0.42 [p = 0.0009 | 0.32 [p = 0.0246] – 0.43 [p = 0.0008] |
| Ovarian volume | FSH | 0.25 [p = 0.0487] – 0.38 [p = 0.0019] | 0.28 [p = 0.0464] – 0.41 [p = 0.0011] | 0.27 [p = 0.0481] – 0.44 [p = 0.0006] |
| Uterine length | Estradiol | 0.28 [p = 0.0395] – 0.42 [p = 0.0008] | 0.29 [p = 0.0385] – 0.44 [p = 0.0006] | 0.31 [p = 0.0285] – 0.47 [p = 0.0002] |
| Breast diameter | Estradiol | 0.34 [p = 0.0236] – 0.47 [p = 0.0002] | 0.36 [p = 0.0184] – 0.48 [p = 0.0001] | 0.35 [p = 0.0204] – 0.48 [p = 0.0001] |
| Breast diameter | FSH | 0.32 [p = 0.0248] – 0.41 [p = 0.0012] | 0.31 [p = 0.0305] – 0.46 [p = 0.0002] | 0.31 [p = 0.0294] – 0.44 [p = 0.0005] |
| Maternal 25OHD | Estradiol | -0.34 [p = 0.0214] – -0.46 [p = 0.0002] | -0.10 [p = 0.4785] – -0.21 [p = 0.0841] | -0.12 [p = 0.4032] – 0.14 [p = 0.2876] |
| Maternal 25OHD | LH | -0.30 [p = 0.0311] – -0.42 [p = 0.0006] | -0.08 [p = 0.5312] -0.19 [p = 0.1121] | -0.14 [p = 0.2912] – 0.13 [p = 0.3512] |
| Maternal 25OHD | FSH | -0.43 [p = 0.0004] – -0.50 [p < 0.0001] | -0.41 [p = 0.0010] – -0.48 [p = 0.0001] | -0.22 [p = 0.0610] – 0.04 [p = 0.6425] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalcze, K.; Krysiak, R.; Gullo, G.; Ott, J. The Course of Minipuberty in Daughters of Women with Low Gestational Vitamin D Status. Nutrients 2024, 16, 2362. https://doi.org/10.3390/nu16142362
Kowalcze K, Krysiak R, Gullo G, Ott J. The Course of Minipuberty in Daughters of Women with Low Gestational Vitamin D Status. Nutrients. 2024; 16(14):2362. https://doi.org/10.3390/nu16142362
Chicago/Turabian StyleKowalcze, Karolina, Robert Krysiak, Giuseppe Gullo, and Johannes Ott. 2024. "The Course of Minipuberty in Daughters of Women with Low Gestational Vitamin D Status" Nutrients 16, no. 14: 2362. https://doi.org/10.3390/nu16142362
APA StyleKowalcze, K., Krysiak, R., Gullo, G., & Ott, J. (2024). The Course of Minipuberty in Daughters of Women with Low Gestational Vitamin D Status. Nutrients, 16(14), 2362. https://doi.org/10.3390/nu16142362








