Correlation of Co-Morbidities with Symptom Severity of Children with Autism Spectrum Disorder: A Cross-Sectional Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
- (a)
- The ABC is used for behavioral examination, auxiliary diagnosis, and screening of ASD. A cut-off total score greater than 67 points indicates a high probability of ASD, while scores of 53–67 indicate potential ASD [18].
- (b)
- The CARS is used to evaluate the symptoms and duration of ASD. Scores of 30–36 points indicate moderate ASD and scores > 36 points indicate severe ASD [19].
- (c)
- The SRS is used to screen the social behavior of children and adolescents with ASD to determine the severity of social disorders [20].
2.3. Lab Measurements
2.4. Physical Examination
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Serum Nutrient Levels and Body Composition
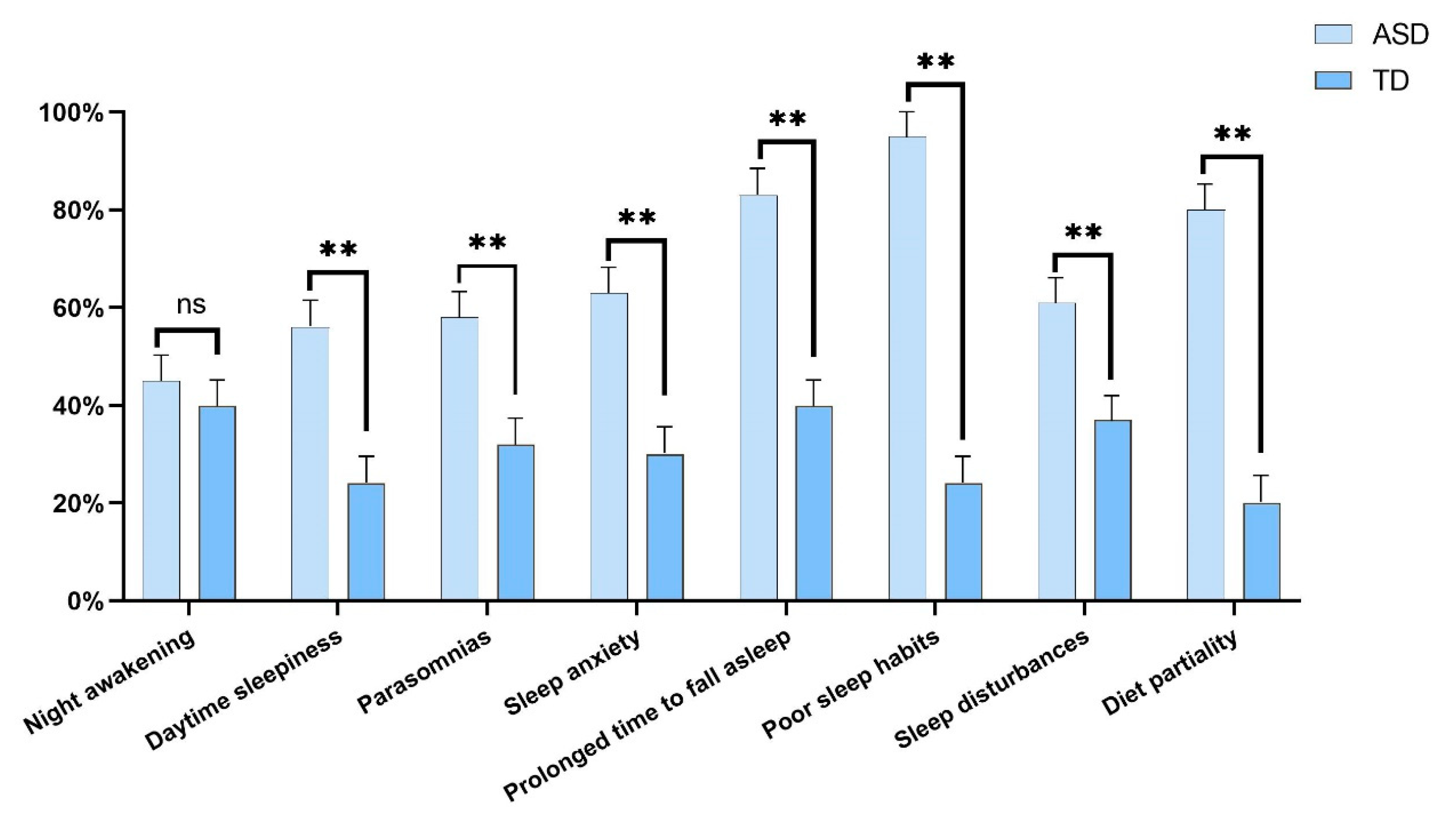
3.3. Sleep Disturbances and Diet Partiality
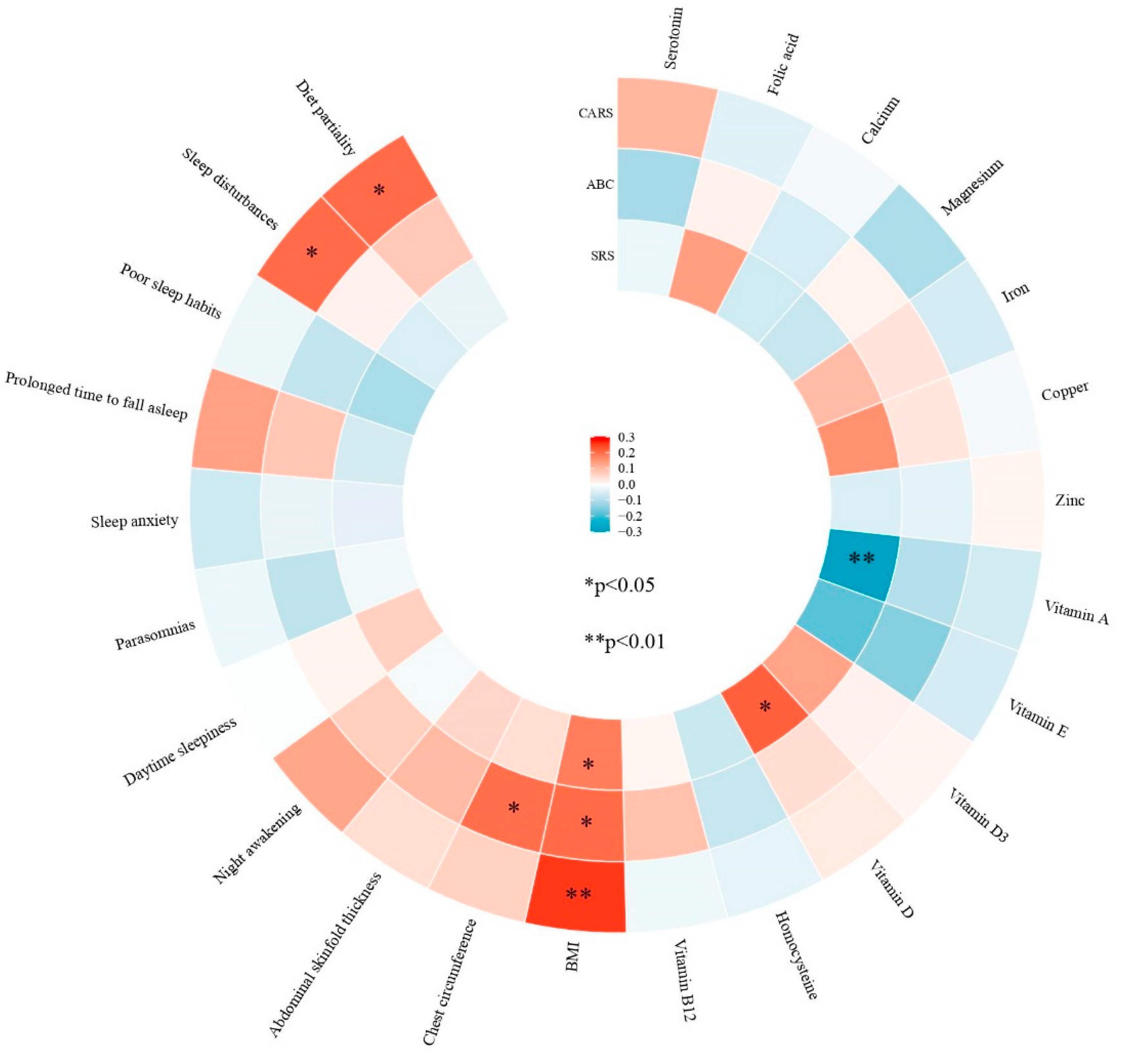
3.4. Correlation Analyses in Children with ASD
4. Discussion
Limitations and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Sleep Habits Questionnaire and Dietary Behavior Questionnaire
| Classification | Main Problems | Items | Option |
| Sleep disturbances | Night awakening | Whether your child wakes up at night? | 1. YES; 2. NO |
| Daytime sleepiness | Whether your child has one of the following conditions? ① Inability to wake up spontaneously in the morning requires someone else to wake up. ② Waking up in the morning in a bad state. ③ Fatigue or sleepiness during the day while watching TV. ④ Fatigue or sleepiness during the day when travelling in a car. | 1. YES; 2. NO | |
| Parasomnias | Whether your child has one of the following conditions? ① Grind teeth (during sleep). ② Talk in sleep. ③ Sleepwalking. ④ Have nightmares. ⑤ Restlessness (often with large body movements). ⑥ Snoring loudly. ⑦ Sleep apnea. | 1. YES; 2. NO | |
| Sleep anxiety | Whether your child has one of the following conditions? ① Afraid of the dark. ② Crying before bedtime. ③ Afraid to sleep alone. ④ Difficulty sleeping in unfamiliar surroundings. ⑤ Moving into someone else’s bed in the middle of the night. ⑥ Waking up in the middle of the night crying and being difficult to calm. ⑦ Dyspnoea | 1. YES; 2. NO | |
| Prolonged time to fall asleep | Is your child unable to fall asleep within 20 minutes of going to bed? | 1. YES; 2. NO | |
| Poor sleep habits | Whether your child has one of the following conditions? ① Unable to sleep alone. ② Needs to be put to sleep. | 1. YES; 2. NO | |
| Dietary behavior | Diet partiality | Does your child have a significant diet partiality? | 1. YES; 2. NO |
| Please list the types of food your child resists eating. | Unrestricted options |
References
- Sato, M.; Nakai, N.; Fujima, S.; Choe, K.Y.; Takumi, T. Social circuits and their dysfunction in autism spectrum disorder. Mol. Psychiatry 2023, 28, 3194–3206. [Google Scholar] [CrossRef]
- Jasim, S.; Perry, A. Repetitive and restricted behaviors and interests in autism spectrum disorder: Relation to individual characteristics and mental health problems. BMC Psychiatry 2023, 23, 356. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Esposito, M.; Mirizzi, P.; Fadda, R.; Pirollo, C.; Ricciardi, O.; Mazza, M.; Valenti, M. Food Selectivity in Children with Autism: Guidelines for Assessment and Clinical Interventions. Int. J. Environ. Res. Public Health 2023, 20, 5092. [Google Scholar] [CrossRef] [PubMed]
- Arija, V.; Esteban-Figuerola, P.; Morales-Hidalgo, P.; Jardí, C.; Canals-Sans, J. Nutrient intake and adequacy in children with autism spectrum disorder: EPINED epidemiological study. Autism Int. J. Res. Pract. 2023, 27, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, L.; Zhang, Q.; Chen, L.; Dai, Y.; Liu, L.; Feng, J.; Cai, X.; Cheng, Q.; Chen, J.; et al. Vitamin and mineral status of children with autism spectrum disorder in Hainan Province of China: Associations with symptoms. Nutr. Neurosci. 2020, 23, 803–810. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Abushaikha, A.; Alnaser, K.; Obeidat, M.D.; Al-Shami, I. Nutritional Status of Pre-school Children and Determinant Factors of Autism: A Case-Control Study. Front. Nutr. 2021, 8, 627011. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.A.; Mittal, R.; Trivedi, M.; Castejon, A.M.; Deth, R.C.; Mittal, J.; Eshraghi, R.S.; Zukerman, R.; Karhu, E. Nutritional interventions for autism spectrum disorder. Nutr. Rev. 2020, 78, 515–531. [Google Scholar] [CrossRef]
- Feng, J.; Shan, L.; Miao, C.; Xue, Y.; Yue, X.; Jia, F. The association of vitamin A, zinc and copper levels with clinical symptoms in children with autism spectrum disorders in Jilin Province, China. BMC Pediatr. 2023, 23, 173. [Google Scholar] [CrossRef]
- van der Lubbe, A.; Swaab, H.; Vermeiren, R.; van den Akker, E.; Ester, W. Novel Insights into Obesity in Preschool Children with Autism Spectrum Disorder. Child Psychiatry Hum. Dev. 2024, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Hu, X.; Li, F.; Deng, J.; Shi, J.; Lin, Q. Eating Habits and Their Association with Weight Status in Chinese School-Age Children: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 3571. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D. Sleep, sleep disorders and inflammation in children. Sleep Med. 2009, 10, S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.; Mannion, A.; Madden, A.; Berger, F.; Costello, R.; Ghadiri, S.; Leader, G. Examining the Relationship Between Sleep Quality, Social Functioning, and Behavior Problems in Children with Autism Spectrum Disorder: A Systematic Review. Nat. Sci. Sleep 2022, 14, 675–695. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Cardinali, D.P.; Shakunthala, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Understanding the role of sleep and its disturbances in Autism spectrum disorder. Int. J. Neurosci. 2020, 130, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Le Couteur, A.; Haden, G.; Hammal, D.; McConachie, H. Diagnosing Autism Spectrum Disorders in Pre-school Children Using Two Standardised Assessment Instruments: The ADI-R and the ADOS. J. Autism Dev. Disord. 2007, 38, 362–372. [Google Scholar] [CrossRef]
- Marteleto, M.R.; Pedromônico, M.R. Validity of Autism Behavior Checklist (ABC): Preliminary study. Rev. Bras. Psiquiatr. 2005, 27, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Breidbord, J.; Croudace, T.J. Reliability Generalization for Childhood Autism Rating Scale. J. Autism Dev. Disord. 2013, 43, 2855–2865. [Google Scholar] [CrossRef]
- Cen, C.Q.; Liang, Y.Y.; Chen, Q.R.; Chen, K.Y.; Deng, H.Z.; Chen, B.Y.; Zou, X.B. Investigating the validation of the Chinese Mandarin version of the Social Responsiveness Scale in a Mainland China child population. BMC Psychiatry 2017, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Flieger, W.; Flieger, M.; Forma, A.; Sitarz, E.; Skórzyńska-Dziduszko, K.; Grochowski, C.; Maciejewski, R.; Karakuła-Juchnowicz, H. Autism spectrum disorder: Trace elements imbalances and the pathogenesis and severity of autistic symptoms. Neurosci. Biobehav. Rev. 2021, 129, 117–132. [Google Scholar] [CrossRef]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Notova, S.V.; Chernova, L.N.; Skalny, A.A.; Burtseva, T.I.; Tinkov, A.A. Hair trace element concentrations in autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD). J. Trace Elem. Med. Biol. 2020, 61, 126539. [Google Scholar] [CrossRef]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Zhao, G.; Wang, W.; Wang, C.; Zhang, L.; Zhang, H.; Lu, D.; Ruan, S.; Zhang, A.; Liu, Q.; et al. Metallomic profiling and natural copper isotopic signatures of childhood autism in serum and red blood cells. Chemosphere 2023, 330, 138700. [Google Scholar] [CrossRef]
- Wu, J.; Wang, D.; Yan, L.; Jia, M.; Zhang, J.; Han, S.; Han, J.; Wang, J.; Chen, X.; Zhang, R. Associations of essential element serum concentrations with autism spectrum disorder. Environ. Sci. Pollut. Res. Int. 2022, 29, 88962–88971. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, B.; Hoxha, M.; Domi, E.; Gervasoni, J.; Persichilli, S.; Malaj, V.; Zappacosta, B. Folic Acid and Autism: A Systematic Review of the Current State of Knowledge. Cells 2021, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, M.; Yang, T.; Lai, X.; Tang, T.; Chen, J.; Li, L.; Li, T. Nutritional Status and Symptoms in Preschool Children With Autism Spectrum Disorder: A Two-Center Comparative Study in Chongqing and Hainan Province, China. Front. Pediatr. 2020, 8, 469. [Google Scholar] [CrossRef]
- Guo, B.Q.; Li, H.B.; Ding, S.B. Blood homocysteine levels in children with autism spectrum disorder: An updated systematic review and meta-analysis. Psychiatry Res. 2020, 291, 113283. [Google Scholar] [CrossRef]
- Wołoszynowska-Fraser, M.U.; Kouchmeshky, A.; McCaffery, P. Vitamin A and Retinoic Acid in Cognition and Cognitive Disease. Annu. Rev. Nutr. 2020, 40, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Eyles, D.W. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients 2022, 14, 4353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, Y.; Lu, Z.; Song, M.; Huang, X.; Mi, L.; Yang, J.; Cui, X. Effects of Vitamin D Supplementation on Children with Autism Spectrum Disorder: A Systematic Review and Meta-analysis. Clin. Psychopharmacol. Neurosci. 2023, 21, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zhang, Q.; Zhu, J.; Yang, T.; Guo, M.; Li, Q.; Liu, H.; Wu, Q.H.; Chen, J.; Li, T.Y. A weekly vitamin A supplementary program alleviates social impairment in Chinese children with autism spectrum disorders and vitamin A deficiency. Eur. J. Clin. Nutr. 2021, 75, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Eyles, D.W.; Magnusson, C.; Newschaffer, C.J.; McGrath, J.J.; Kvaskoff, D.; Ko, P.; Dalman, C.; Karlsson, H.; Gardner, R.M. Developmental vitamin D and autism spectrum disorders: Findings from the Stockholm Youth Cohort. Mol. Psychiatry 2021, 26, 1578–1588. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism-related traits: The Generation R Study. Mol. Psychiatry 2018, 23, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; White, T.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism spectrum disorder. BJPsych Open 2017, 3, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Wen, X.; Han, X.R.; Wang, S.; Wang, Y.J.; Shen, M.; Fan, S.H.; Zhuang, J.; Li, M.Q.; Hu, B.; et al. Relationship Between Neonatal Vitamin D at Birth and Risk of Autism Spectrum Disorders: The NBSIB Study. J. Bone Miner. Res. 2018, 33, 458–466. [Google Scholar] [CrossRef]
- Feng, Y.-R.; Zhang, Q.; Miao, J.-K.; Yang, T.; Chen, J.; Chen, H.-Y.; Mou, Q.-H.; Xiang, X.-L.; Long, D.; Wei, Q.-H.; et al. Association of the retinol to all-trans retinoic acid pathway with autism spectrum disorder. World J. Pediatr. 2024, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lucock, M.; Jones, P.; Martin, C.; Yates, Z.; Veysey, M.; Furst, J.; Beckett, E. Photobiology of vitamins. Nutr. Rev. 2018, 76, 512–525. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Role of Vitamin D in the Hygiene Hypothesis: The Interplay between Vitamin D, Vitamin D Receptors, Gut Microbiota, and Immune Response. Front. Immunol. 2016, 7, 627. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Molina-López, J.; Leiva-García, B.; Planells, E.; Planells, P. Food selectivity, nutritional inadequacies, and mealtime behavioral problems in children with autism spectrum disorder compared to neurotypical children. Int. J. Eat. Disord. 2021, 54, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- van De Sande, M.M.; van Buul, V.J.; Brouns, F.J. Autism and nutrition: The role of the gut-brain axis. Nutr. Res. Rev. 2014, 27, 199–214. [Google Scholar] [CrossRef]
- Sammels, O.; Karjalainen, L.; Dahlgren, J.; Wentz, E. Autism Spectrum Disorder and Obesity in Children: A Systematic Review and Meta-Analysis. Obes. Facts 2022, 15, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Kamal Nor, N.; Ghozali, A.H.; Ismail, J. Prevalence of Overweight and Obesity Among Children and Adolescents With Autism Spectrum Disorder and Associated Risk Factors. Front. Pediatr. 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Flores-Dorantes, M.T.; Díaz-López, Y.E.; Gutiérrez-Aguilar, R. Environment and Gene Association With Obesity and Their Impact on Neurodegenerative and Neurodevelopmental Diseases. Front. Neurosci. 2020, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Watanabe, T.; Sasaki, Y. Are sleep disturbances a cause or consequence of autism spectrum disorder? Psychiatry Clin. Neurosci. 2023, 77, 377–385. [Google Scholar] [CrossRef]
- Maurer, J.J.; Choi, A.; An, I.; Sathi, N.; Chung, S. Sleep disturbances in autism spectrum disorder: Animal models, neural mechanisms, and therapeutics. Neurobiol. Sleep Circadian Rhythm. 2023, 14, 100095. [Google Scholar] [CrossRef] [PubMed]
- Miike, T.; Toyoura, M.; Tonooka, S.; Konishi, Y.; Oniki, K.; Saruwatari, J.; Tajima, S.; Kinoshita, J.; Nakai, A.; Kikuchi, K. Neonatal irritable sleep-wake rhythm as a predictor of autism spectrum disorders. Neurobiol. Sleep Circadian Rhythm. 2020, 9, 100053. [Google Scholar] [CrossRef]
- MacDuffie, K.E.; Shen, M.D.; Dager, S.R.; Styner, M.A.; Kim, S.H.; Paterson, S.; Pandey, J.; St John, T.; Elison, J.T.; Wolff, J.J.; et al. Sleep Onset Problems and Subcortical Development in Infants Later Diagnosed With Autism Spectrum Disorder. Am. J. Psychiatry 2020, 177, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Richdale, A.L.; Baker, E.K.; Peiró, A.M. Sleep in autism: A biomolecular approach to aetiology and treatment. Sleep Med. Rev. 2020, 54, 101357. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Calderoni, S.; Apicella, F.; Cosenza, A.; Igliozzi, R.; Palermo, G.; Tancredi, R.; Tritto, G.; Craig, F.; Muratori, F.; et al. Impact of sleep disorders on behavioral issues in preschoolers with autism spectrum disorder. Front. Psychiatry 2023, 14, 1181466. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Smith, T.; DeMand, A.; Lecavalier, L.; Evans, V.; Gurka, M.; Swiezy, N.; Bearss, K.; Scahill, L. Exploring sleep quality of young children with autism spectrum disorder and disruptive behaviors. Sleep Med. 2018, 44, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.H.; Yi, J.H.; Kim, J.Y.; Solmi, M.; Cortese, S.; Smith, L.; Koyanagi, A.; Shin, J.I.; Cheon, K.A.; et al. Correlations between sleep problems, core symptoms, and behavioral problems in children and adolescents with autism spectrum disorder: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2023, 33, 1539–1549. [Google Scholar] [CrossRef]
- Miike, T.; Oniki, K.; Toyoura, M.; Tonooka, S.; Tajima, S.; Kinoshita, J.; Saruwatari, J.; Konishi, Y. Disruption of Circadian Sleep/Wake Rhythms in Infants May Herald Future Development of Autism Spectrum Disorder. Clocks Sleep 2024, 6, 170–182. [Google Scholar] [CrossRef] [PubMed]
| General Characteristics | ASD | TD | |
|---|---|---|---|
| Age | 4.06 ± 0.98 | 4.34 ± 0.88 | 0.024 |
| Sex (male) [n (%)] | 95 (79.2) | 81 (73.6) | 0.323 |
| Ethnicity (Han) [n (%)] | 111 (92.5) | 101 (91.8) | 0.847 |
| Resident (Urban) [n (%)] | 92 (66.7) | 110 (100) | <0.001 |
| Maternal abnormalities during pregnancy [n (%)] | 55 (45.8) | 38 (34.6) | 0.081 |
| Mother’s education [n (%)] | |||
| Illiterate/elementary/middle school | 37 (30.8) | 3 (2.7) | <0.001 |
| High school | 14 (11.7) | 6 (5.5) | |
| College or above | 69 (57.5) | 101 (91.8) | |
| Father’s education [n (%)] | |||
| Illiterate/elementary/middle school | 34 (28.3) | 2 (1.8) | <0.001 |
| High school | 13 (10.8) | 7 (6.4) | |
| College or above | 73 (60.8) | 101 (91.8) | |
| Family structure (Nuclear family) [n (%)] | 58 (48.33) | 53 (48.18) | 0.982 |
| Types | ASD | TD | ||
|---|---|---|---|---|
| Serotonin | 127.5 (81.75–172.75) | 133 (92.5–167) | −0.18 | 0.858 |
| Folic acid | 11.33 ± 3.29 | 12.48 ± 3.10 | −2.711 | 0.007 |
| Calcium | 92.9 (88.25–95.98) | 92.8 (87.2–98.4) | −0.816 | 0.414 |
| Magnesium | 19.86 ± 1.81 | 18.92 ± 1.54 | 4.266 | <0.001 |
| Iron | 1133 (873–1445.25) | 1087 (844.5–1346.5) | −0.442 | 0.658 |
| Copper | 1054.5 (929.75–1170.5) | 1123 (998.5–1301) | −3.303 | 0.001 |
| Zinc | 815.5 (728.5–887.75) | 810 (759–876) | −0.465 | 0.642 |
| Vitamin A | 0.36 ± 0.08 | 0.35 ± 0.07 | 0.565 | 0.572 |
| Vitamin E | 8.9 (7.6–10) | 8.4 (7.3–9.95) | −1.162 | 0.245 |
| Vitamin D3 | 18.55 (12.9–27.75) | 20.5 (15.25–28.05) | −0.785 | 0.433 |
| Vitamin D | 19.75 (13.4–28.05) | 21.2 (15.9–28.2) | −0.769 | 0.442 |
| Homocysteine | 5.66 (4.953–6.523) | 5.39 (4.63–6.125) | −1.984 | 0.047 |
| Vitamin B12 | 736.52 (550.4–1039.4) | 919 (710.4–1131.4) | −3.49 | <0.001 |
| Body Dimension | ASD | TD | ||
|---|---|---|---|---|
| Head circumference | 50.5 (49.5–51.5) | 50.5 (49.35–51.55) | −0.259 | 0.796 |
| Chest circumference | 53.5 (51.8–56.8) | 52.6 (50.55–54.65) | −3.377 | 0.001 |
| Waistline | 49 (47.5–52.43) | 49.5 (47.25–51.7) | −0.016 | 0.987 |
| Hip circumference | 55 (52.05–59) | 55.7 (52.2–58.3) | −0.176 | 0.861 |
| Height | 104.2 ± 8.66 | 104.78 ± 7.8 | −0.535 | 0.593 |
| Weight | 18.50 ± 4.11 | 17.69 ± 3.25 | 1.646 | 0.101 |
| Triceps skinfold thickness | 9 (8–11) | 10 (8.5–11.5) | −0.756 | 0.45 |
| Subscapular skinfold thickness | 6 (5–7) | 6 (5.5–7) | −0.174 | 0.862 |
| Abdominal skinfold thickness | 6 (5–8.5) | 5.5 (4.5–7) | −3.028 | 0.002 |
| Total Body Water | 10.35 (8.75–11.79) | 9.8 (8.75–11.2) | −1.557 | 0.12 |
| Protein | 2.7 (2.3–3.1) | 2.6 (2.3–2.9) | −1.145 | 0.135 |
| Minerals | 0.87 (0.74–1.06) | 0.86 (0.74–0.98) | −0.75 | 0.454 |
| Body Fat Mass | 3.95 (3.1–5.3) | 4.1 (3.2–5.1) | −0.21 | 0.833 |
| Soft Lean Mass | 13.2 (11.25–15.18) | 12.6 (11.2–14.3) | −1.576 | 0.115 |
| Fat Free Mass | 14.05 (11.83–16.04) | 13.3 (11.8–15.15) | −1.486 | 0.137 |
| Skeletal Muscle Mass | 6.25 (5.017–7.388) | 5.9 (5–6.95) | −1.48 | 0.139 |
| Body Mass Index | 17.03 ± 1.94 | 16.02 ± 1.54 | 4.338 | <0.001 |
| Percent Body Fat | 22.75 (19.6–27.78) | 23.5 (19.4–26.8) | −0.003 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, M.; Zhang, Y.; Li, D.; Li, S.; Hu, J.; Gao, Y.; Cheng, Z.; Liu, S.; Wu, L.; Sun, C. Correlation of Co-Morbidities with Symptom Severity of Children with Autism Spectrum Disorder: A Cross-Sectional Survey. Nutrients 2024, 16, 2960. https://doi.org/10.3390/nu16172960
Zou M, Zhang Y, Li D, Li S, Hu J, Gao Y, Cheng Z, Liu S, Wu L, Sun C. Correlation of Co-Morbidities with Symptom Severity of Children with Autism Spectrum Disorder: A Cross-Sectional Survey. Nutrients. 2024; 16(17):2960. https://doi.org/10.3390/nu16172960
Chicago/Turabian StyleZou, Mingyang, Yilin Zhang, Dexin Li, Shengqi Li, Jingyi Hu, Ya Gao, Zeyu Cheng, Shidan Liu, Lijie Wu, and Caihong Sun. 2024. "Correlation of Co-Morbidities with Symptom Severity of Children with Autism Spectrum Disorder: A Cross-Sectional Survey" Nutrients 16, no. 17: 2960. https://doi.org/10.3390/nu16172960
APA StyleZou, M., Zhang, Y., Li, D., Li, S., Hu, J., Gao, Y., Cheng, Z., Liu, S., Wu, L., & Sun, C. (2024). Correlation of Co-Morbidities with Symptom Severity of Children with Autism Spectrum Disorder: A Cross-Sectional Survey. Nutrients, 16(17), 2960. https://doi.org/10.3390/nu16172960





