Anti-Neuroinflammatory Potential of Areca Nut Extract and Its Bioactive Compounds in Anthracene-Induced BV-2 Microglial Cell Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of AC Extracts
2.3. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
2.4. Molecular Docking
2.5. Lipinski’s Rule and Pharmacokinetic Property Analysis
2.6. Cell Culture and Treatment
2.7. Cell Viability Assay
2.8. RNA Extraction and qPCR Analysis
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Intracellular ROS Analysis
2.11. Immunofluorescence Analysis
2.12. Preparation of Cytoplasmic and Nuclear Proteins
2.13. Whole Protein Extraction and Immunoblotting Analysis
2.14. Statistical Analysis
3. Results
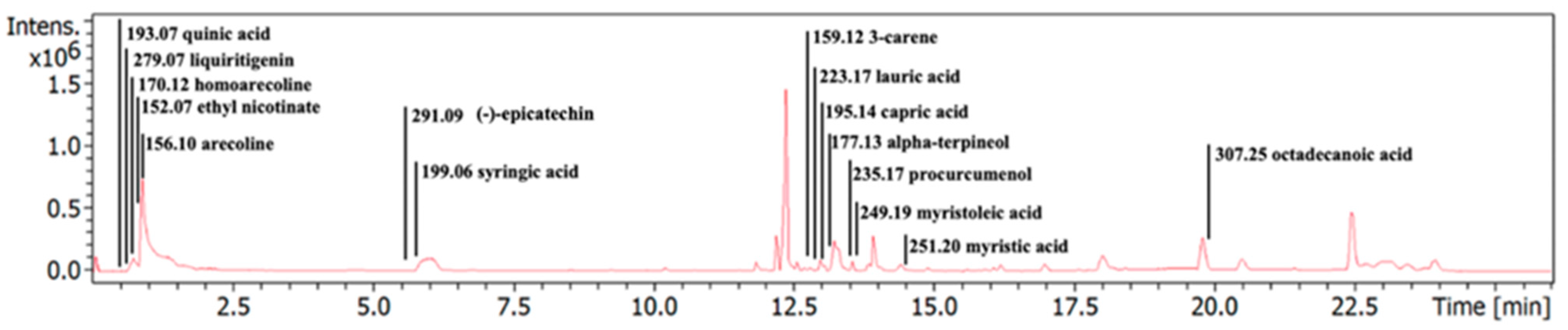
3.1. Characterization of Phytochemical Components in the ACEE
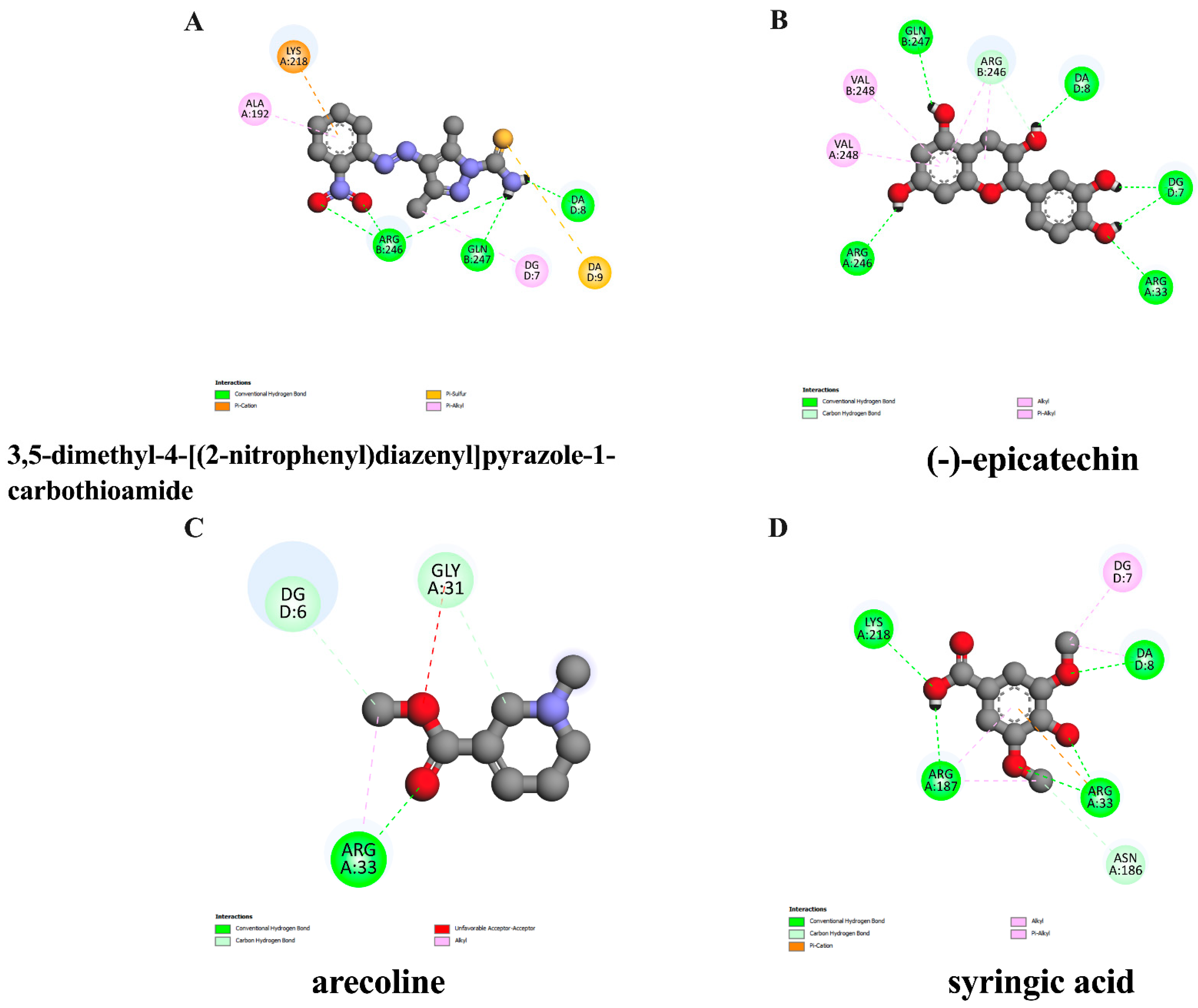
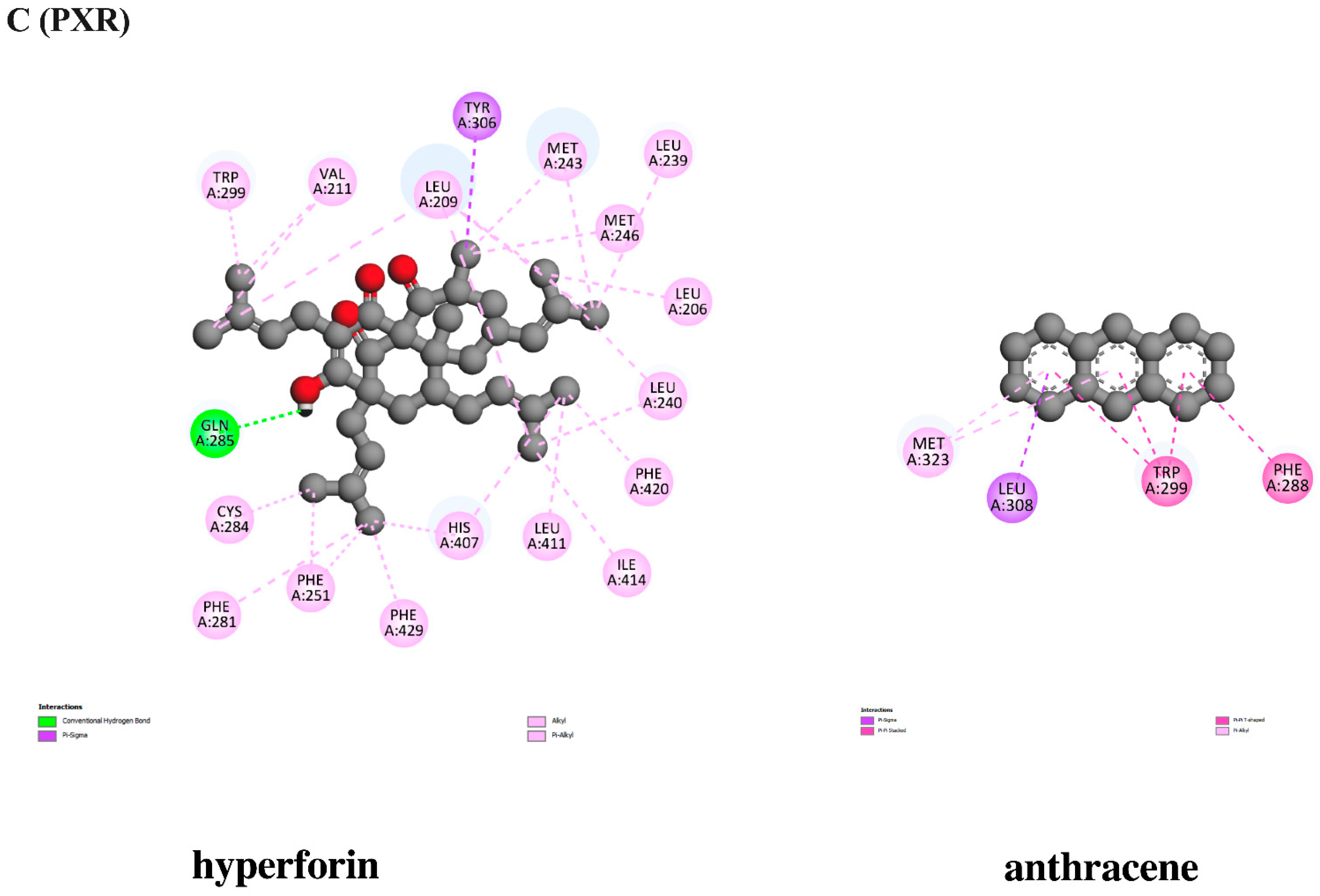
3.2. In Silico Evaluation of Identified Compounds in ACEE and NF-κB Protein
3.3. Lipinski’s Rule of Five Parameters and ADMET Properties of Pure Compounds in ACEE
3.4. Effect of ACEE and Pure Compounds on the Viability of BV-2 Cells
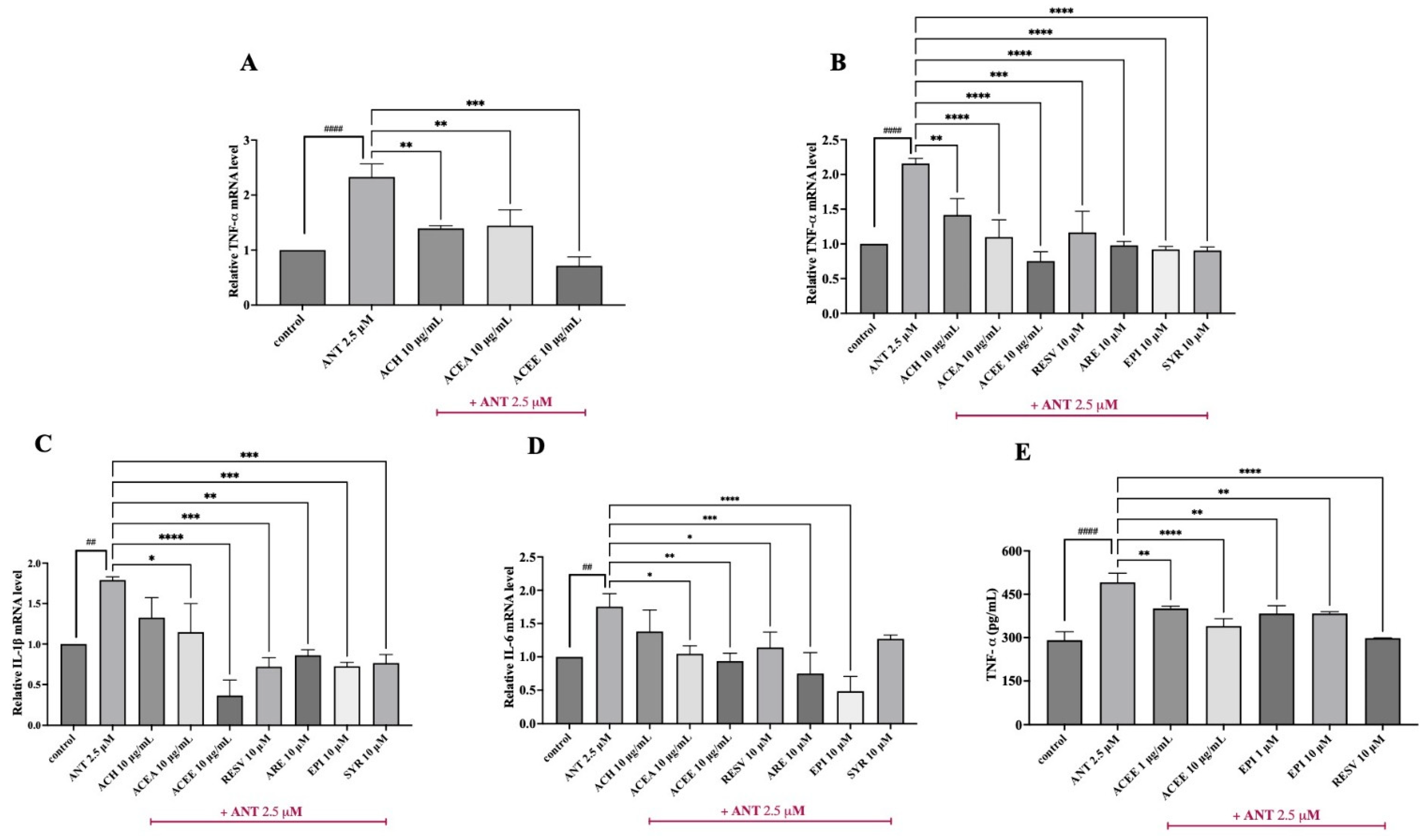
3.5. Inhibitory Effect of ACEE and Pure Compounds on the Levels of Pro-Inflammatory Cytokines
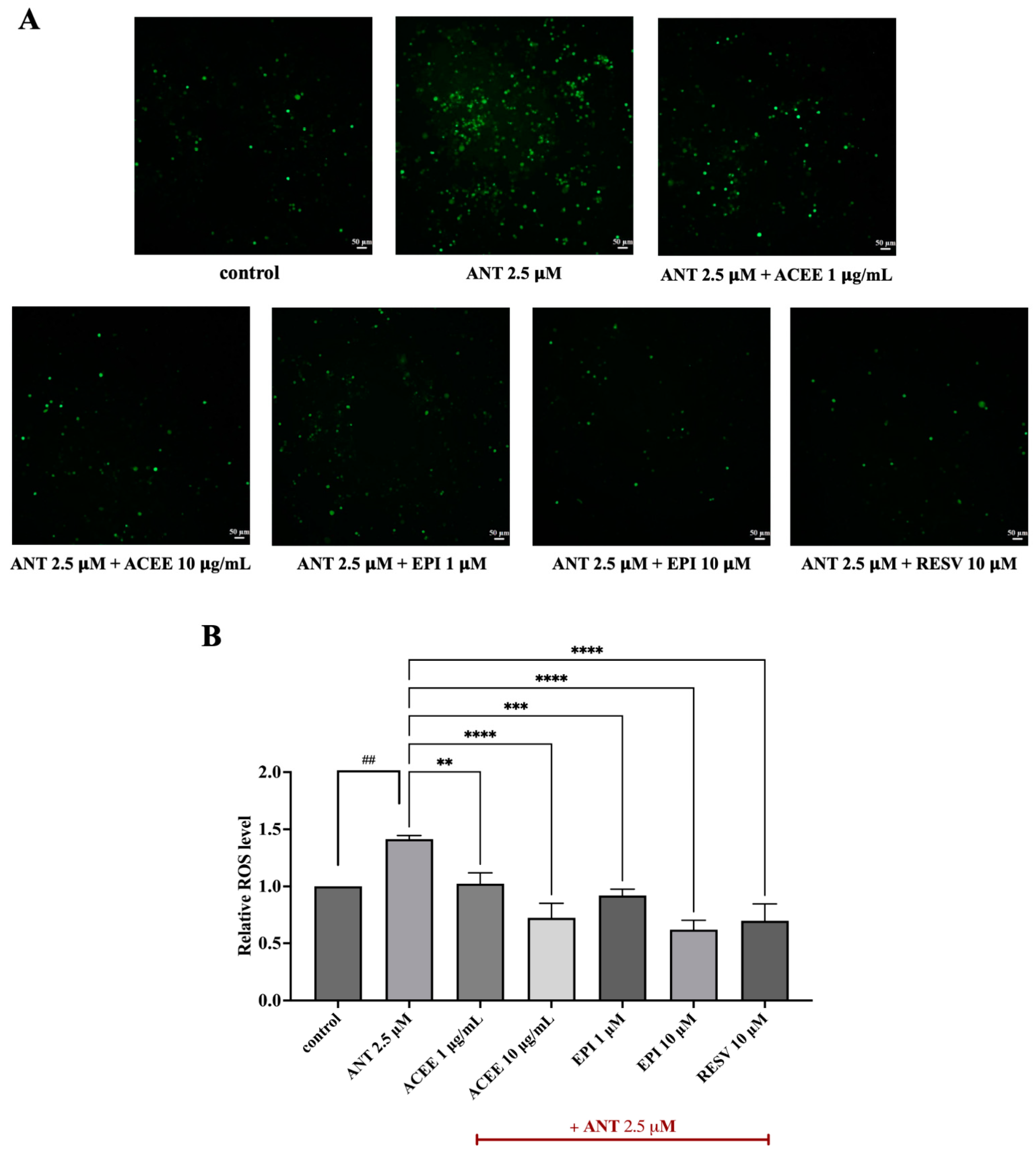
3.6. Inhibitory Effect of ACEE and (−)-Epicatechin on ROS Production
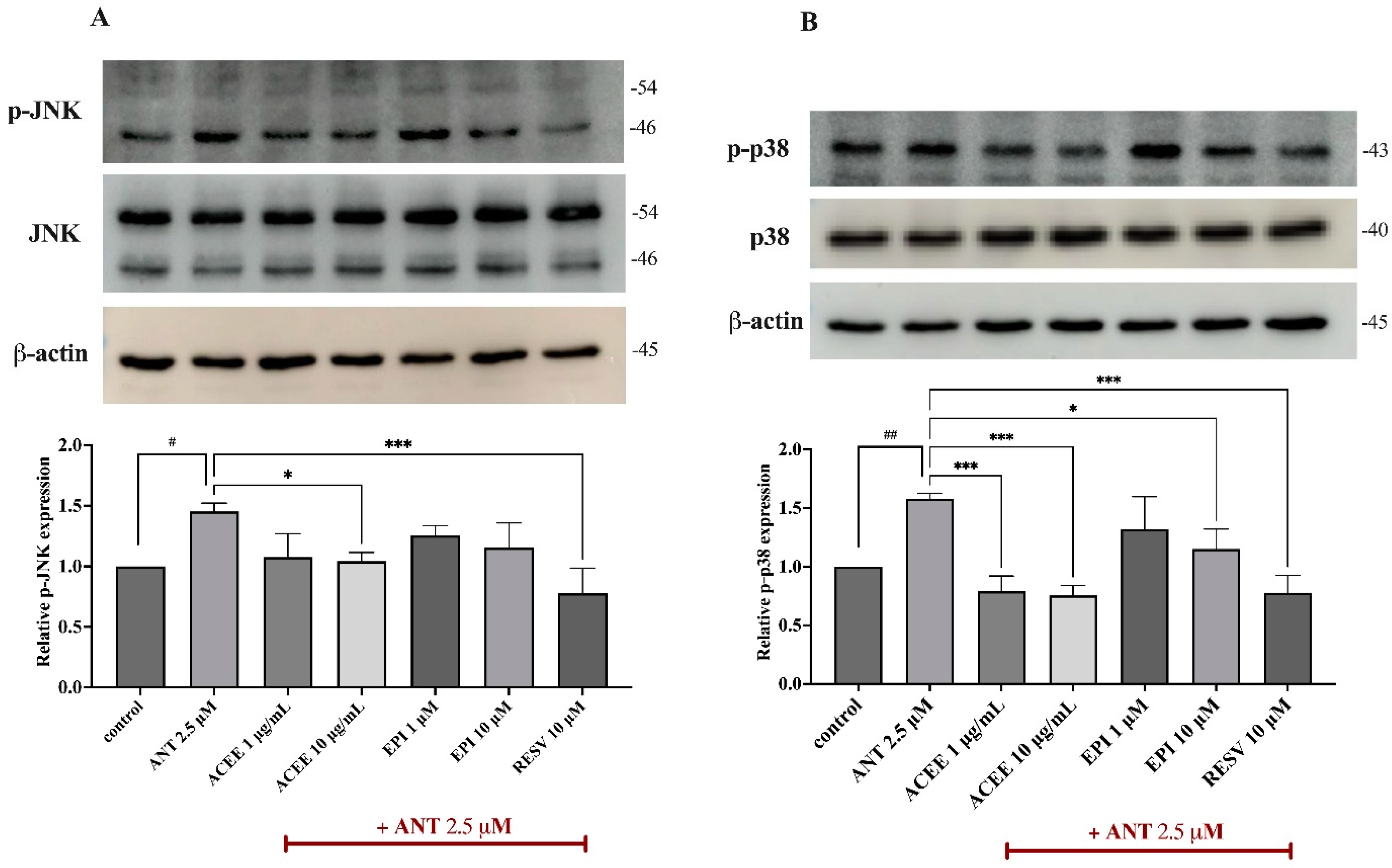
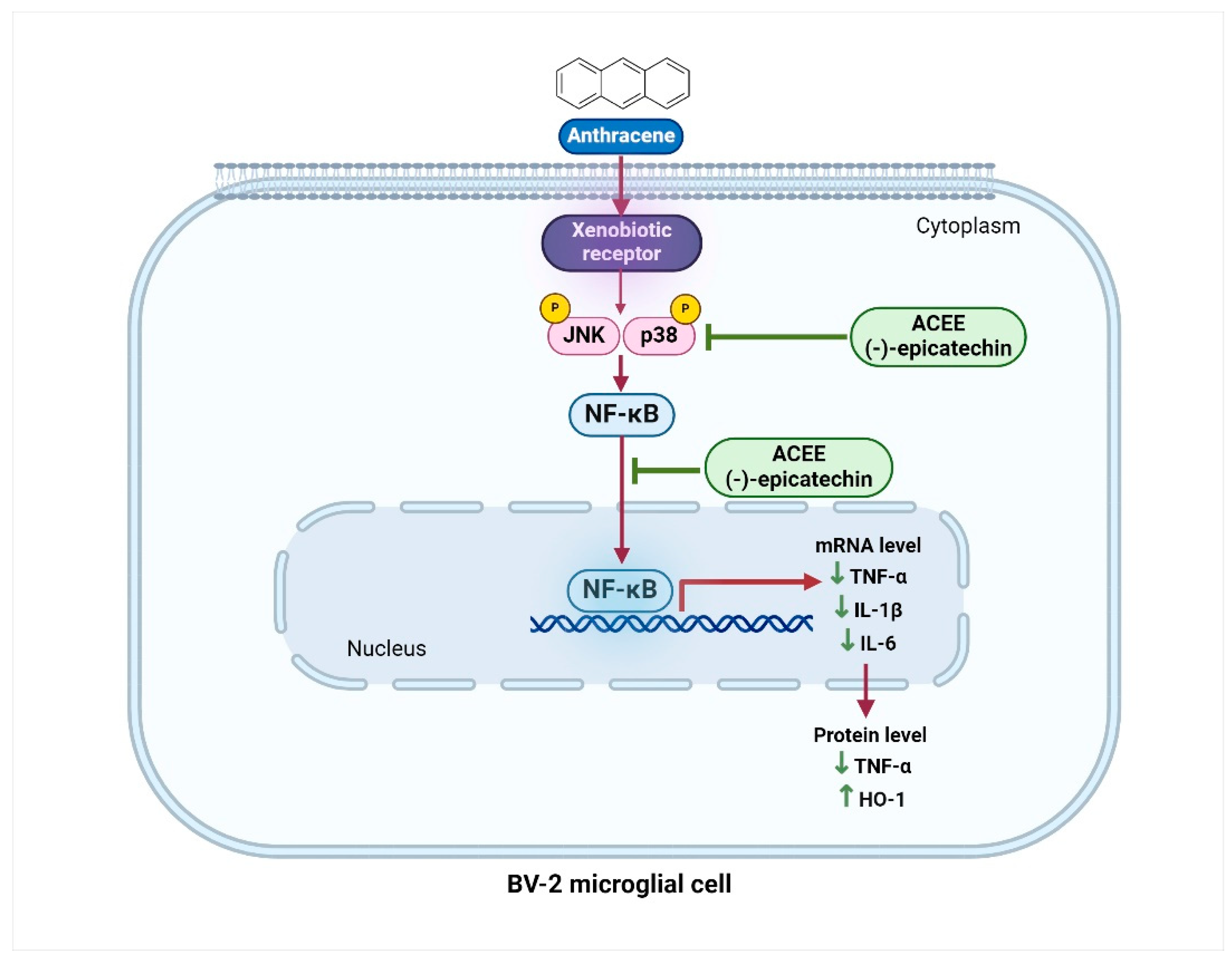
3.7. Effect of ACEE and (−)-Epicatechin on on MAPKs Signaling Activation
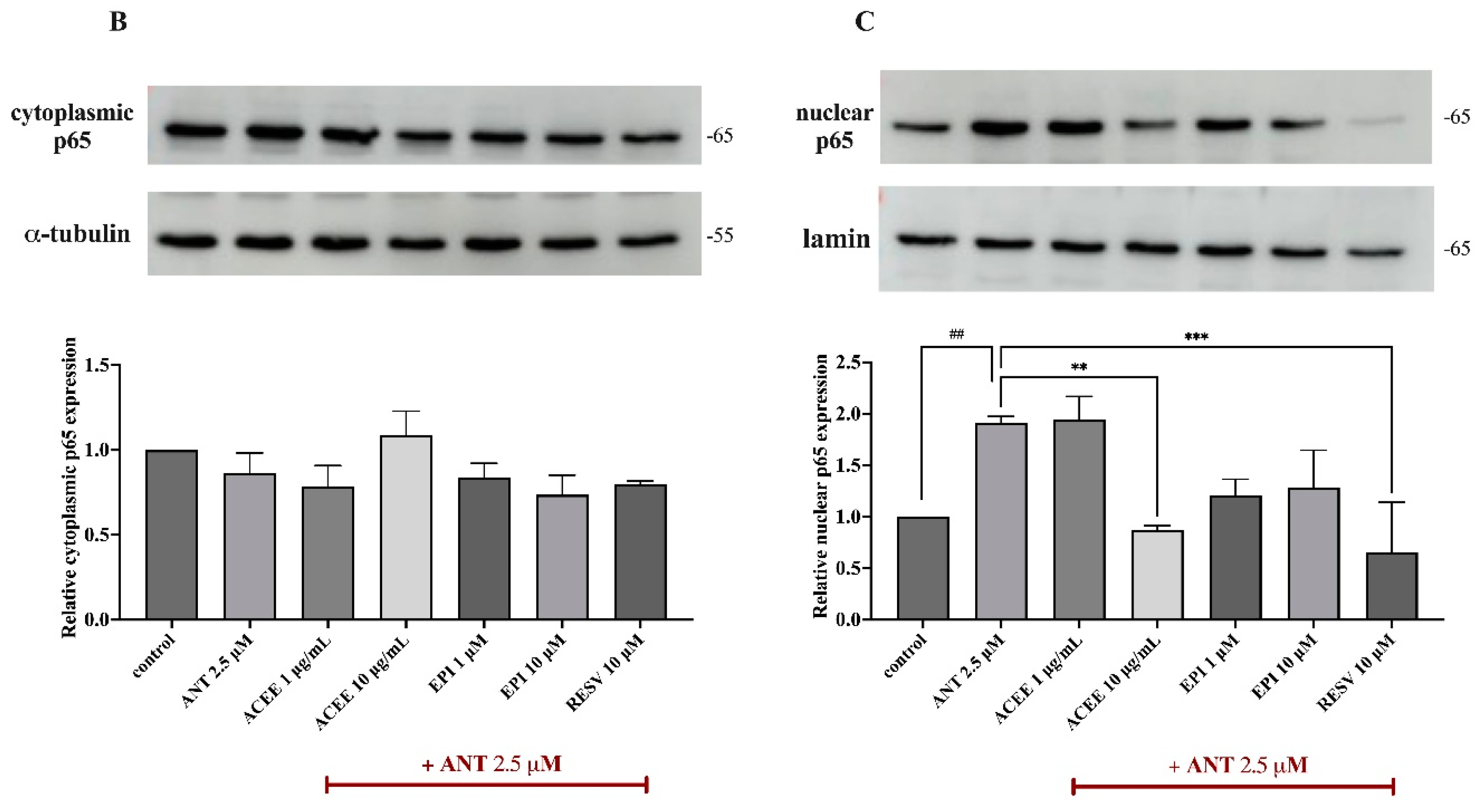
3.8. Effect of ACEE and (−)-Epicatechin on NF-κB Signaling Activation
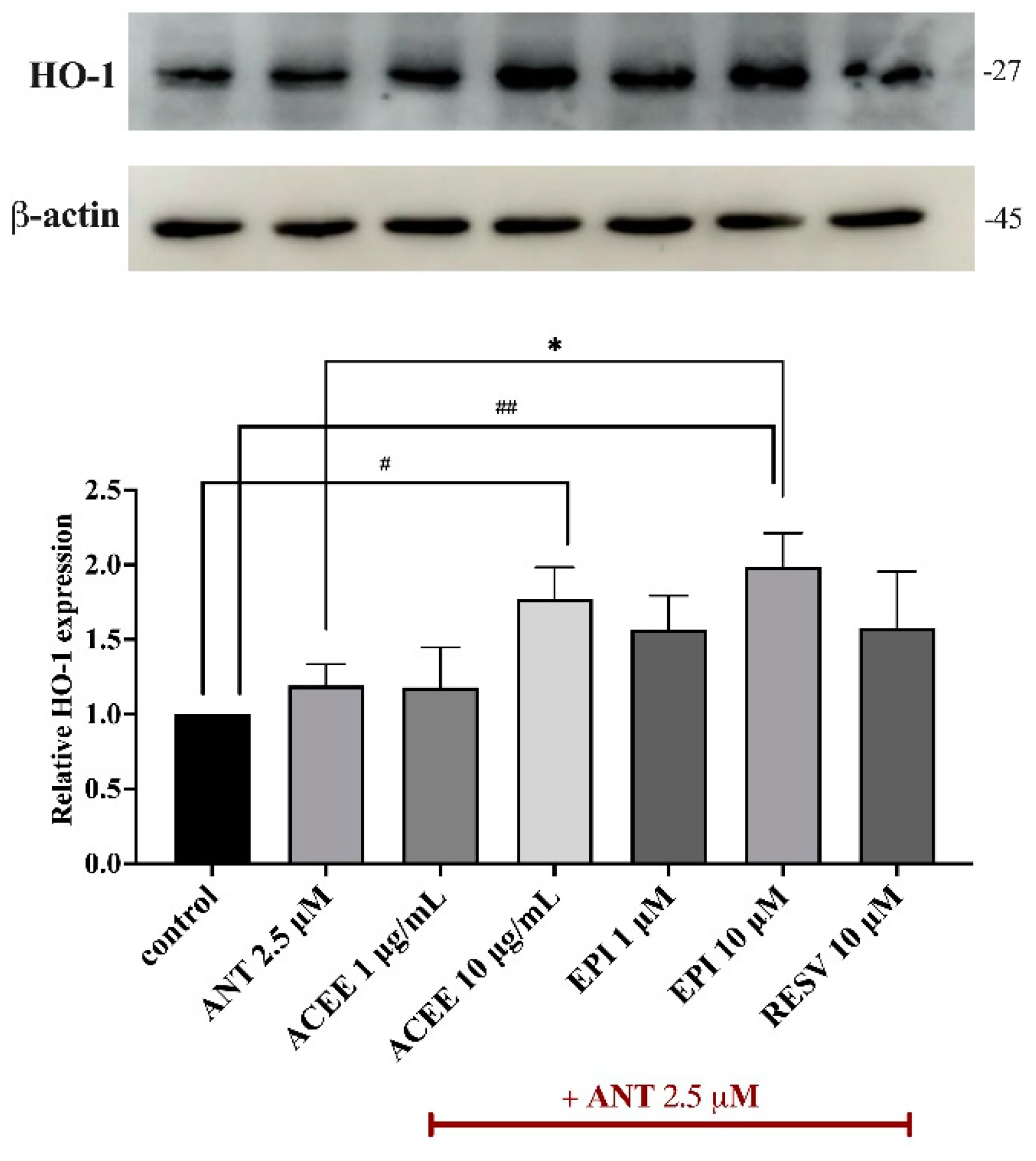
3.9. Effect of ACEE and (−)-Epicatechin on HO-1 Activation
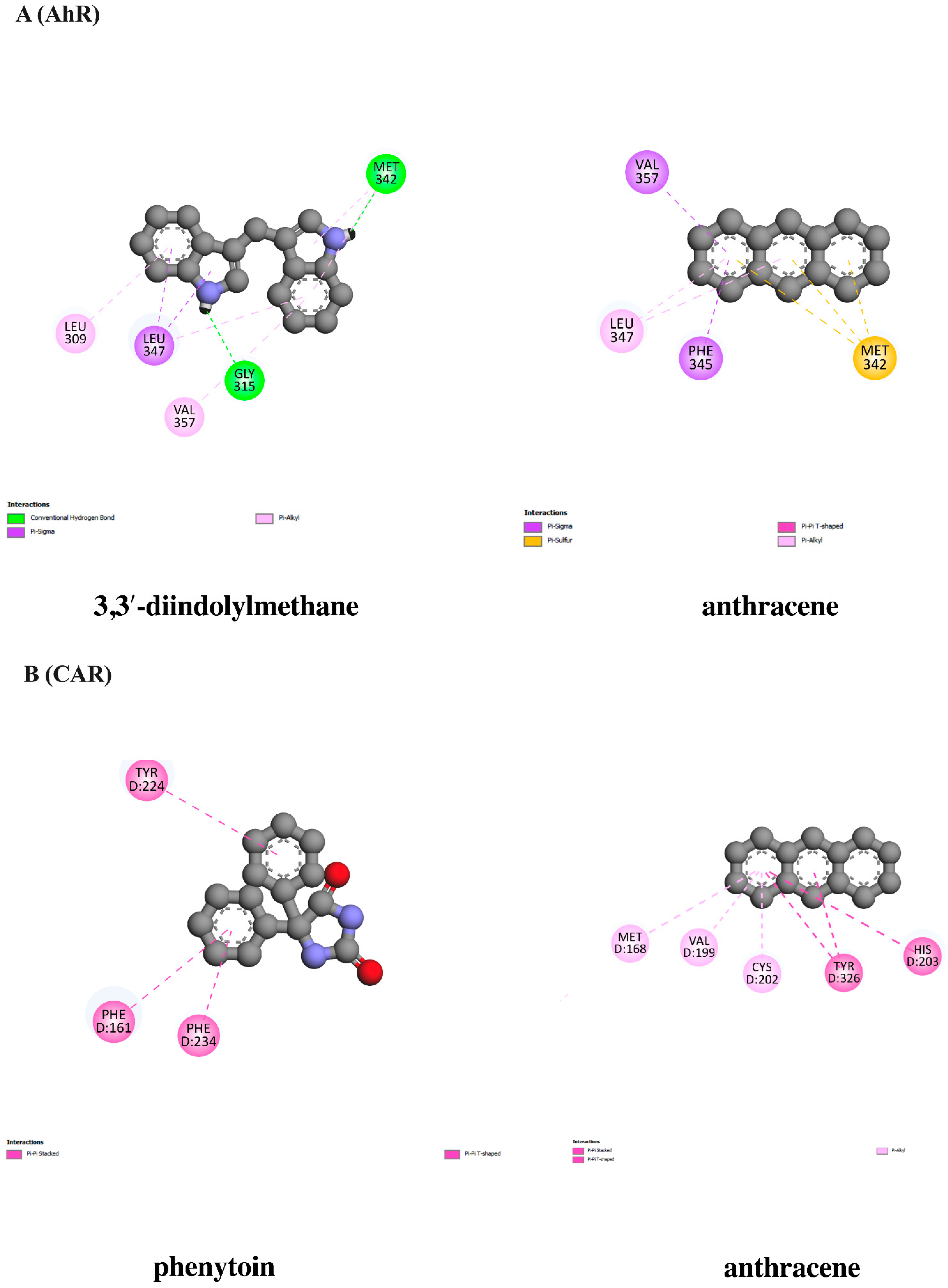
3.10. In Silico Evaluation of Anthracene against Xenobiotic Receptors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, T.; Sun, Y.; Shi, Y.; Ma, J.; Feng, C.; Chen, Z. Air pollution control policies and impacts: A review. Renew. Sustain. Energy Rev. 2024, 191, 114071. [Google Scholar] [CrossRef]
- Alahmad, B.; Khraishah, H.; Althalji, K.; Borchert, W.; Al-Mulla, F.; Koutrakis, P. Connections between Air Pollution, Climate Change, and Cardiovascular Health. Can. J. Cardiol. 2023, 39, 1182–1190. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Manavalan, B.; Shin, T.H.; Park, C.B.; Lee, W.S.; Kim, J.; Lee, G. The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer. Nanomaterials 2022, 12, 2656. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, R.; Zhang, J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol. Lett. 2018, 15, 7506–7514. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Guo, X.; Cheung, F.M.H.; Yung, K.K.L. The association between PM2.5 exposure and neurological disorders: A systematic review and meta-analysis. Sci. Total Environ. 2019, 655, 1240–1248. [Google Scholar] [CrossRef]
- Kang, Y.J.; Tan, H.-Y.; Lee, C.Y.; Cho, H. An Air Particulate Pollutant Induces Neuroinflammation and Neurodegeneration in Human Brain Models. Adv. Sci. 2021, 8, 2101251. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, F.; Yang, Y.; Yang, L.; Wu, Q.; Sun, H.; An, Z.; Li, J.; Wu, H.; Song, J.; et al. PM2.5 exposure upregulates pro-inflammatory protein expression in human microglial cells via oxidant stress and TLR4/NF-κB pathway. Ecotoxicol. Environ. Saf. 2024, 277, 116386. [Google Scholar] [CrossRef]
- Breton, C.V.; Song, A.Y.; Xiao, J.; Kim, S.J.; Mehta, H.H.; Wan, J.; Yen, K.; Sioutas, C.; Lurmann, F.; Xue, S.; et al. Effects of air pollution on mitochondrial function, mitochondrial DNA methylation, and mitochondrial peptide expression. Mitochondrion 2019, 46, 22–29. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef] [PubMed]
- Grabato, J.R.H.; Hizon-Fradejas, A.B.; Federico, S.A.P.; Mojica, E.-R.E. Anthracene. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2024; pp. 515–520. [Google Scholar] [CrossRef]
- Kottuparambil, S.; Park, J. Anthracene phytotoxicity in the freshwater flagellate alga Euglena agilis Carter. Sci. Rep. 2019, 9, 15323. [Google Scholar] [CrossRef]
- Mujtaba, S.F.; Dwivedi, A.; Mudiam, M.K.; Ali, D.; Yadav, N.; Ray, R.S. Production of ROS by photosensitized anthracene under sunlight and UV-R at ambient environmental intensities. Photochem. Photobiol. 2011, 87, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O. Neurotoxicity of anthracene and benz[a]anthracene involves oxidative stress-induced neuronal damage, cholinergic dysfunction and disruption of monoaminergic and purinergic enzymes. Toxicol. Res. 2022, 38, 365–377. [Google Scholar] [CrossRef]
- Ayyubova, G.; Fazal, N. Beneficial versus Detrimental Effects of Complement–Microglial Interactions in Alzheimer’s Disease. Brain Sci. 2024, 14, 434. [Google Scholar] [CrossRef]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef]
- Kaminska, B.; Gozdz, A.; Zawadzka, M.; Ellert-Miklaszewska, A.; Lipko, M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. 2009, 292, 1902–1913. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-κB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Deshmukh, P.S.; Patil, P.G.; Shahare, P.U.; Bhanage, G.B.; Dhekale, J.S.; Dhande, K.G.; Aware, V.V. Effect of mechanical and chemical treatments of arecanut (Areca catechu L.) fruit husk on husk and its fibre. Waste Manag. 2019, 95, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yu, W.; Li, H.; Hu, X.; Wang, X. Bioactive Components of Areca Nut: An Overview of Their Positive Impacts Targeting Different Organs. Nutrients 2024, 16, 695. [Google Scholar] [CrossRef] [PubMed]
- Jam, N.; Hajimohammadi, R.; Gharbani, P.; Mehrizad, A. Evaluation of Antibacterial Activity of Aqueous, Ethanolic and Methanolic Extracts of Areca Nut Fruit on Selected Bacteria. BioMed Res. Int. 2021, 2021, 6663399. [Google Scholar] [CrossRef]
- Chaikhong, K.; Chumpolphant, S.; Rangsinth, P.; Sillapachaiyaporn, C.; Chuchawankul, S.; Tencomnao, T.; Prasansuklab, A. Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction. Plants 2022, 12, 65. [Google Scholar] [CrossRef]
- Zou, L.; Yi, S.; Wang, Y. Ameliorative Effect of Areca Nut Polyphenols on Adverse Effects Induced by Lipopolysaccharides in RAW264.7 Cells. Molecules 2024, 29, 1329. [Google Scholar] [CrossRef]
- Janpaijit, S.; Sillapachaiyaporn, C.; Theerasri, A.; Charoenkiatkul, S.; Sukprasansap, M.; Tencomnao, T. Cleistocalyx nervosum var. paniala Berry Seed Protects against TNF-α-Stimulated Neuroinflammation by Inducing HO-1 and Suppressing NF-κB Mechanism in BV-2 Microglial Cells. Molecules 2023, 28, 3057. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.; Chen, L.; Dai, Y.; Xia, Y. Discovery of Norisoboldine Analogue III11 as a Novel and Potent Aryl Hydrocarbon Receptor Agonist for the Treatment of Ulcerative Colitis. J. Med. Chem. 2023, 66, 6869–6888. [Google Scholar] [CrossRef] [PubMed]
- Sundhani, E.; Nugroho, A.; Nurrochmad, A.; Lukitaningsih, E. Molecular interactions of Andrographis paniculata Burm. f. Active Compound with Nuclear Receptor (CAR and PXR): An In Silico Assessment Approach. Indones. J. Chem. 2022, 22, 126. [Google Scholar] [CrossRef]
- Malar, D.S.; Prasanth, M.I.; Brimson, J.M.; Verma, K.; Prasansuklab, A.; Tencomnao, T. Hibiscus sabdariffa extract protects HT-22 cells from glutamate-induced neurodegeneration by upregulating glutamate transporters and exerts lifespan extension in C. elegans via DAF-16 mediated pathway. Nutr. Healthy Aging 2021, 6, 229–247. [Google Scholar] [CrossRef]
- Kunwittaya, S.; Nantasenamat, C.; Treeratanapiboon, L.; Srisarin, A.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. Influence of logBB cut-off on the prediction of blood-brain barrier permeability. Biomed. Appl. Technol. J. 2013, 1, 16–34. [Google Scholar]
- Flood-Garibay, J.; Angulo Molina, A.; Méndez-Rojas, M. Particulate matter and ultrafine particles in urban air pollution and their effect on the Nervous System. Environ. Sci. Process. Impacts 2023, 25, 704–726. [Google Scholar] [CrossRef]
- Li, W.; Lin, G.; Xiao, Z.; Zhang, Y.; Li, B.; Zhou, Y.; Ma, Y.; Chai, E. A review of respirable fine particulate matter (PM2.5)-induced brain damage. Front. Mol. Neurosci. 2022, 15, 967174. [Google Scholar] [CrossRef]
- Peters, A.; Veronesi, B.; Calderón-Garcidueñas, L.; Gehr, P.; Chen, L.C.; Geiser, M.; Reed, W.; Rothen-Rutishauser, B.; Schürch, S.; Schulz, H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part. Fibre Toxicol. 2006, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.C.; Dao, K.; Roqué, P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology 2017, 59, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Hussar, E.; Richards, S.; Lin, Z.Q.; Dixon, R.P.; Johnson, K.A. Human Health Risk Assessment of 16 Priority Polycyclic Aromatic Hydrocarbons in Soils of Chattanooga, Tennessee, USA. Water Air Soil. Pollut. 2012, 223, 5535–5548. [Google Scholar] [CrossRef] [PubMed]
- Blazkova, B.; Pastorkova, A.; Solansky, I.; Veleminsky, M.; Veleminsky, M.; Urbancova, K.; Vondraskova, V.; Hajslova, J.; Pulkrabova, J.; Sram, R.J. Effect of Polycyclic Aromatic Hydrocarbons Exposure on Cognitive Development in 5 Years Old Children. Brain Sci. 2020, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Abd El Naby, W.S.; Zong, C.; Fergany, A.; Ekuban, F.A.; Ahmed, S.; Reda, Y.; Sato, H.; Ichihara, S.; Kubota, N.; Yanagita, S.; et al. Exposure to Benzo[a]pyrene Decreases Noradrenergic and Serotonergic Axons in Hippocampus of Mouse Brain. Int. J. Mol. Sci. 2023, 24, 9895. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, N.; Kodidela, S.; Zhou, L.; Singh, U.P.; Kumar, S. Effect of benzo(a)pyrene on oxidative stress and inflammatory mediators in astrocytes and HIV-infected macrophages. PLoS ONE 2022, 17, e0275874. [Google Scholar] [CrossRef]
- Liu, P.-F.; Chang, Y.-F. The Controversial Roles of Areca Nut: Medicine or Toxin? Int. J. Mol. Sci. 2023, 24, 8996. [Google Scholar] [CrossRef]
- Oliveira, N.; Ramos, D.; Dinis-Oliveira, R. Genetic toxicology and toxicokinetics of arecoline and related areca nut compounds: An updated review. Arch. Toxicol. 2021, 95, 375–393. [Google Scholar] [CrossRef]
- Ho, T.-J.; Chi-Kang Tsai, B.; Kuo, C.-H.; Luk, H.-N.; Day, C.H.; Jine-Yuan Hsieh, D.; Chen, R.-J.; Kuo, W.-W.; Kumar, V.B.; Yao, C.-H.; et al. Arecoline induces cardiotoxicity by upregulating and activating cardiac hypertrophy-related pathways in Sprague–Dawley rats. Chem.-Biol. Interact. 2022, 354, 109810. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.M.; Tu, H.-P.; Ko, Y.-C. Systematic Review of Roles of Arecoline and Arecoline N-Oxide in Oral Cancer and Strategies to Block Carcinogenesis. Cells 2023, 12, 1208. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-T.; Chen, P.S.; Wu, C.-H.; Tseng, Y.-T.; Wu, Y.-C.; Lo, Y.-C. Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free Radic. Biol. Med. 2010, 49, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Shou-Hong, Z.; Hong-Yan, L.; Qi-Xin, Y.A.O.; Zhu-Qing, Q.I.; Guang, W.; Hu, B. Arecoline Repressed Inflammation Factor Expression of Macrophages Stimulated by Oxidized Low Density Lipoprotein and Its Mechanism. Editor. Off. Chin. J. Arterioscler. 2009, 17, 269–272. [Google Scholar]
- Kim, Y.W.; Zhao, R.J.; Park, S.J.; Lee, J.R.; Cho, I.J.; Yang, C.H.; Kim, S.G.; Kim, S.C. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-κB-dependent iNOS and proinflammatory cytokines production. Br. J. Pharmacol. 2008, 154, 165–173. [Google Scholar] [CrossRef]
- Shaki, F.; Shayeste, Y.; Karami, M.; Akbari, E.; Rezaei, M.; Ataee, R. The effect of epicatechin on oxidative stress and mitochondrial damage induced by homocycteine using isolated rat hippocampus mitochondria. Res. Pharm. Sci. 2017, 12, 119–127. [Google Scholar] [CrossRef]
- Prince, P.D.; Lanzi, C.R.; Toblli, J.E.; Elesgaray, R.; Oteiza, P.I.; Fraga, C.G.; Galleano, M. Dietary (−)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic. Biol. Med. 2016, 90, 35–46. [Google Scholar] [CrossRef]
- Navarrete-Yañez, V.; Garate-Carrillo, A.; Rodriguez, A.; Mendoza-Lorenzo, P.; Ceballos, G.; Calzada-Mendoza, C.; Hogan, M.C.; Villarreal, F.; Ramirez-Sanchez, I. Effects of (−)-epicatechin on neuroinflammation and hyperphosphorylation of tau in the hippocampus of aged mice. Food Funct. 2020, 11, 10351–10361. [Google Scholar] [CrossRef]
- Somade, O.T.; Oyinloye, B.E.; Ajiboye, B.O.; Osukoya, O.A. Syringic acid demonstrates an anti-inflammatory effect via modulation of the NF-κB-iNOS-COX-2 and JAK-STAT signaling pathways in methyl cellosolve-induced hepato-testicular inflammation in rats. Biochem. Biophys. Rep. 2023, 34, 101484. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tsai, T.-H.; Chuang, L.-T.; Li, Y.-Y.; Zouboulis, C.C.; Tsai, P.-J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Serrano-Vega, R.; Pérez Gutiérrez, S.; Isiordia-Espinoza, M.A.; Solorio-Alvarado, C.R. Myristic acid reduces skin inflammation and nociception. J. Food Biochem. 2022, 46, e14013. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Q.-L.; Zhang, X.-Y.; Lv, C.; Li, J.; Yuan, Y.; Yin, F.-X. Pharmacokinetics and Blood–Brain Barrier Penetration of (+)-Catechin and (−)-Epicatechin in Rats by Microdialysis Sampling Coupled to High-Performance Liquid Chromatography with Chemiluminescence Detection. J. Agric. Food Chem. 2012, 60, 9377–9383. [Google Scholar] [CrossRef] [PubMed]
- Senevirathna, K.; Pradeep, R.; Jayasinghe, Y.A.; Jayawickrama, S.M.; Illeperuma, R.; Warnakulasuriya, S.; Jayasinghe, R.D. Carcinogenic Effects of Areca Nut and Its Metabolites: A Review of the Experimental Evidence. Clin. Pract. 2023, 13, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Sudjarwo, G.W.; Kim, J.S.; Dirgantara, S.; Maeng, W.J.; Hong, H. The anti-inflammatory effect of Indonesian Areca catechu leaf extract in vitro and in vivo. Nutr. Res. Pract. 2014, 8, 267–271. [Google Scholar] [CrossRef]
- Bourgognon, J.-M.; Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 2020, 4, 2398212820979802. [Google Scholar] [CrossRef]
- Farzan, S.F.; Chen, Y.; Trachtman, H.; Trasande, L. Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003-2008. Environ. Res. 2016, 144, 149–157. [Google Scholar] [CrossRef]
- Faouzi, M.; Neupane, R.P.; Yang, J.; Williams, P.; Penner, R. Areca nut extracts mobilize calcium and release pro-inflammatory cytokines from various immune cells. Sci. Rep. 2018, 8, 1075. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Z.-R. Anti-inflammatory Effects of (−)-Epicatechin in Lipopolysaccharide-Stimulated Raw 264.7 Macrophages. Trop. J. Pharm. Res. 2014, 13, 1415. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Jeng, H.A.; Pan, C.H.; Diawara, N.; Chang-Chien, G.P.; Lin, W.Y.; Huang, C.T.; Ho, C.K.; Wu, M.T. Polycyclic aromatic hydrocarbon-induced oxidative stress and lipid peroxidation in relation to immunological alteration. Occup. Environ. Med. 2011, 68, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Zou, L.; Li, Z.; Sakao, K.; Wang, Y.; Hou, D.X. In Vitro Antioxidant Activity of Areca Nut Polyphenol Extracts on RAW264.7 Cells. Foods 2022, 11, 3607. [Google Scholar] [CrossRef]
- Ahmed, T.; Zulfiqar, A.; Arguelles, S.; Rasekhian, M.; Nabavi, S.F.; Silva, A.S.; Nabavi, S.M. Map kinase signaling as therapeutic target for neurodegeneration. Pharmacol. Res. 2020, 160, 105090. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fu, X.; Fan, Y.; Zhao, C.; Wang, Q.; Feng, L.; Shen, X.; Han, Z.; Fan, J. Effect of epicatechin on inflammatory cytokines and MAPK/NF-κB signaling pathway in lipopolysaccharideinduced acute lung injury of BALB/c mice. Gen. Physiol. Biophys. 2022, 41, 299–308. [Google Scholar] [CrossRef]
- Li, Y.C.; Cheng, A.J.; Lee, L.Y.; Huang, Y.C.; Chang, J.T. Multifaceted Mechanisms of Areca Nuts in Oral Carcinogenesis: The Molecular Pathology from Precancerous Condition to Malignant Transformation. J. Cancer 2019, 10, 4054–4062. [Google Scholar] [CrossRef]
- Lin, S.C.; Lu, S.Y.; Lee, S.Y.; Lin, C.Y.; Chen, C.H.; Chang, K.W. Areca (betel) nut extract activates mitogen-activated protein kinases and NF-κB in oral keratinocytes. Int. J. Cancer 2005, 116, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.Y.; Vankova, D.G.; Madjova, V.H.; Bekyarov, N.A.; Salim, A.S.; Ivanova, D.G.; Stoeva, S.M.; Gerova, D.I.; Kiselova-Kaneva, Y.D. Association between Nfr2, HO-1, NF-kB Expression, Plasma ADMA, and Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 17067. [Google Scholar] [CrossRef]
- Goedtke, L.; Sprenger, H.; Hofmann, U.; Schmidt, F.F.; Hammer, H.S.; Zanger, U.M.; Poetz, O.; Seidel, A.; Braeuning, A.; Hessel-Pras, S. Polycyclic Aromatic Hydrocarbons Activate the Aryl Hydrocarbon Receptor and the Constitutive Androstane Receptor to Regulate Xenobiotic Metabolism in Human Liver Cells. Int. J. Mol. Sci. 2020, 22, 372. [Google Scholar] [CrossRef]
- O’Driscoll, C.A.; Gallo, M.E.; Hoffmann, E.J.; Fechner, J.H.; Schauer, J.J.; Bradfield, C.A.; Mezrich, J.D. Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro. PLoS ONE 2018, 13, e0209690. [Google Scholar] [CrossRef]
- Kim, H.-B.; Um, J.-Y.; Chung, B.-Y.; Kim, J.-C.; Kang, S.-Y.; Park, C.-W.; Kim, H.-O. Aryl Hydrocarbon Receptors: Evidence of Therapeutic Targets in Chronic Inflammatory Skin Diseases. Biomedicines 2022, 10, 1087. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| IL-1β | GAAATGCCACCTTTTGACAGTG | CTGGATGCTCTCATCAGGACA |
| TNF-α | GATCGGTCCCCAAAGGGATG | TAGCAAATCGGCTGACGGTG |
| IL-6 | TCTTGGGACTGATGCTGGTG | CAGGTCTGTTGGGAGTGGTA |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| NO. | Compound Name | Molecular Formula | Retention Time (min) | m/z | ppm |
|---|---|---|---|---|---|
| 1. | quinic acid | C7H12O6 | 0.68 | 193.0707 [M+H]+ | 2.55 |
| 2. | liquiritigenin | C15H12O4 | 0.73 | 279.0707 [M+Na]+ | −28.63 |
| 3. | homoarecoline | C9H15NO2 | 0.85 | 170.1179 [M+H]+ | 1.06 |
| 4. | ethyl nicotinate | C8H9NO2 | 0.89 | 152.0700 [M+H]+ | 7.47 |
| 5. | arecoline | C8H13NO2 | 0.9 | 156.1021 [M+H]+ | 1.92 |
| 6. | (−)-epicatechin | C15H14O6 | 6.16 | 291.0892 [M+H]+ | −8.34 |
| 7. | syringic acid | C9H10O5 | 6.47 | 199.0583 [M+H]+ | 11.62 |
| 8. | 3-carene | C10H16 | 12.66 | 159.1182 [M+Na]+ | −23.75 |
| 9. | lauric acid | C12H24O2 | 12.95 | 223.1702 [M+Na]+ | −14 |
| 10. | capric acid (decanoic acid) | C10H20O2 | 13.19 | 195.1394 [M+Na]+ | −19.19 |
| 11. | alpha-terpineol | C10H18O | 13.2 | 177.1294 [M+Na]+ | −25.10 |
| 12. | procurcumenol | C15H22O2 | 13.58 | 235.1701 [M+H]+ | −1.37 |
| 13. | myristoleic acid | C14H26O2 | 13.63 | 249.1869 [M+Na]+ | −17.04 |
| 14. | myristic acid (tetradecanoic acid) | C14H28O2 | 14.57 | 251.1970 [M+Na]+ | 0.88 |
| 15. | octadecanoic acid | C18H36O2 | 19.94 | 307.2481 [M+Na]+ | −11.27 |
| Ligand | Binding Energy (kcal/mol) | Inhibition Constant (μM) | Amino Acid Interaction | ||
|---|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Bond | Other | |||
| 3,5-dimethyl-4- [(2nitrophenyl)diazenyl]pyrazole-1- carbothioamide (native inhibitor) | −6.47 | 18.12 | ARG246(3) GLN247 DA8 | ALA192 LYS218 DG7 | LYS218 DA9 |
| (−)-epicatechin | −6.08 | 34.75 | ARG33 ARG246(2) GLN247 | GLN263 | ASP382(2) |
| arecoline | −4.84 | 281.76 | ARG33(2) GLY31 | ARG33 | - |
| syringic acid | −4.11 | 970.76 | ARG33(2) LYS218 ARG187 ASN186 | ARG187(2) | ARG33 |
| Compound | Molecular Weight (≤500) | #H-Bond Acceptors (≤10) | #H-Bond Donors (≤5) | MLOGP (≤4.15) | Lipinski #Violations (≤1) |
|---|---|---|---|---|---|
| Arecoline | 155.19 | 3 | 0 | 0.58 | 0 |
| (−)-Epicatechin | 290.27 | 6 | 5 | 0.24 | 0 |
| Syringic acid | 198.17 | 5 | 2 | 0.49 | 0 |
| Pharmacokinetic Property | Arecoline | (−)-Epicatechin | Syringic Acid |
|---|---|---|---|
| GI absorption | High | High | High |
| Pgp substrate | No | No | No |
| log Kp (skin permeation) (cm/s) | −7.00 | −7.82 | −6.77 |
| BBB permeant (log BB) | 0.033 | −1.054 | −0.191 |
| CYP1A2 inhibitor | No | No | No |
| CYP2C19 inhibitor | No | No | No |
| CYP2C9 inhibitor | No | No | No |
| CYP2D6 inhibitor | No | No | No |
| CYP3A4 inhibitor | No | Yes | No |
| Carcinogenicity (Mouse) | Positive | Negative | Negative |
| Carcinogenicity (Rat) | Negative | Negative | Positive |
| Hepatotoxicity | Yes | No | No |
| AMES toxicity | No | No | No |
| hERG inhibition | Low risk | Medium risk | Low risk |
| Ligand | Binding Energy (kcal/mol) | Inhibition Constant (μM) | Amino Acid Interaction | ||
|---|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Bond | Other | |||
| 3,3-Diindolylmethane (AhR agonist) | −7.71 | 2.24 | MET342 GLY315 | LEU347(3) MET342(2) VAL357 LEU309 | - |
| Anthracene | −6.82 | 10.09 | - | PHE345(2) VAL357 LEU347(2) | MET342(3) |
| Ligand | Binding Energy (kcal/mol) | Inhibition Constant (μM) | Amino Acid Interaction | ||
|---|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Bond | Other | |||
| Phenytoin (CAR agonist) | −6.99 | 7.56 | - | TYR224 PHE234 PHE161 | - |
| Anthracene | −7.14 | 5.84 | - | TYR326(2) HIS203 MET168 VAL199 CYS202 | - |
| Ligand | Binding Energy (kcal/mol) | Inhibition Constant (μM) | Amino Acid Interaction | ||
|---|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Bond | Other | |||
| Hyperforin (PXR agonist) | −10.37 | 0.025 | GLN285 | TYR306 MET243(2) MET246 CYS284 LEU209(4) LEU239 LEU206 LEU240(2) LEU411 ILE414 VAL211(2) PHE251(2) PHE281 TRP299 HIS407(2) PHE420 PHE429 | - |
| Anthracene | −6.86 | 9.41 | - | LEU308 TRP299(5) PHE288 VAL199 MET323(2) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janpaijit, S.; Sukprasansap, M.; Tencomnao, T.; Prasansuklab, A. Anti-Neuroinflammatory Potential of Areca Nut Extract and Its Bioactive Compounds in Anthracene-Induced BV-2 Microglial Cell Activation. Nutrients 2024, 16, 2882. https://doi.org/10.3390/nu16172882
Janpaijit S, Sukprasansap M, Tencomnao T, Prasansuklab A. Anti-Neuroinflammatory Potential of Areca Nut Extract and Its Bioactive Compounds in Anthracene-Induced BV-2 Microglial Cell Activation. Nutrients. 2024; 16(17):2882. https://doi.org/10.3390/nu16172882
Chicago/Turabian StyleJanpaijit, Sakawrat, Monruedee Sukprasansap, Tewin Tencomnao, and Anchalee Prasansuklab. 2024. "Anti-Neuroinflammatory Potential of Areca Nut Extract and Its Bioactive Compounds in Anthracene-Induced BV-2 Microglial Cell Activation" Nutrients 16, no. 17: 2882. https://doi.org/10.3390/nu16172882
APA StyleJanpaijit, S., Sukprasansap, M., Tencomnao, T., & Prasansuklab, A. (2024). Anti-Neuroinflammatory Potential of Areca Nut Extract and Its Bioactive Compounds in Anthracene-Induced BV-2 Microglial Cell Activation. Nutrients, 16(17), 2882. https://doi.org/10.3390/nu16172882








