Circulating Amino Acid Concentration after the Consumption of Pea or Whey Proteins in Young and Older Adults Affects Protein Synthesis in C2C12 Myotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
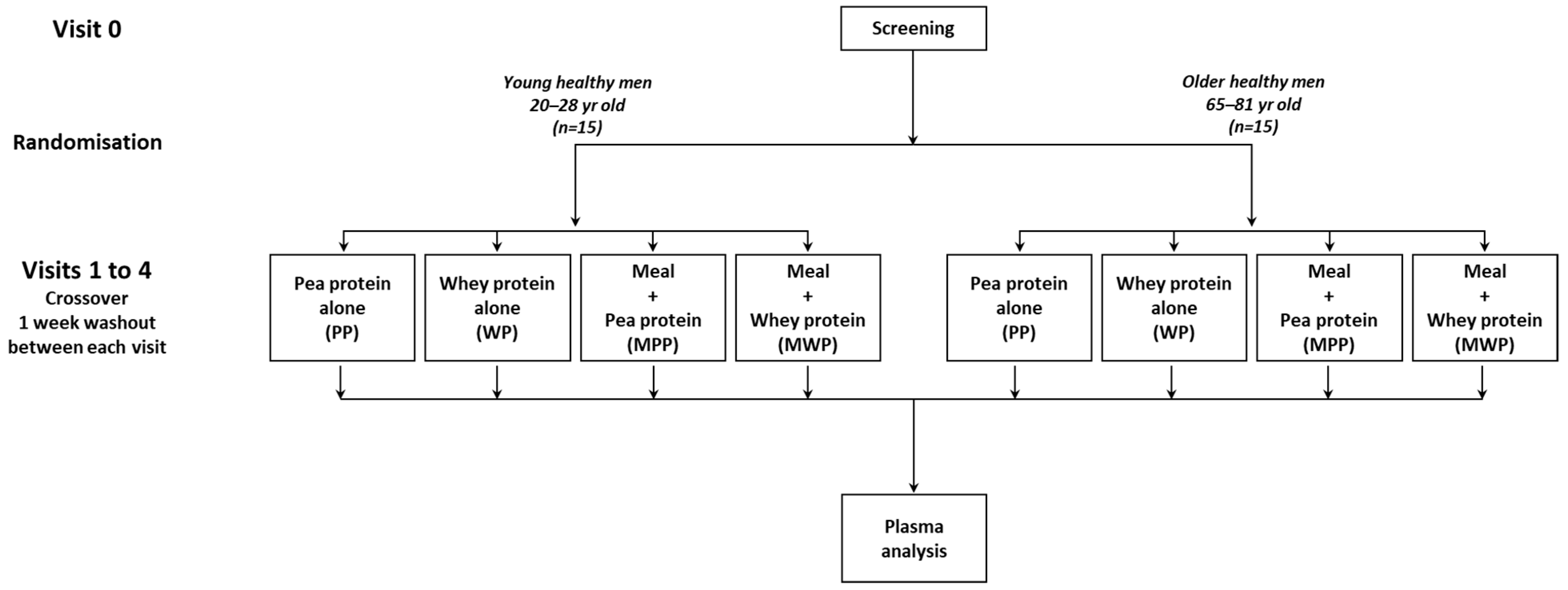
2.2. Study Design
2.3. Analytical Procedures
2.3.1. Determination of Plasma AA Concentrations
2.3.2. Measurement of Plasma Biomarkers and Markers of Inflammation
2.3.3. Muscle Cell Culture
2.3.4. Measurement of Protein Synthesis and Immunoblot Analysis
2.4. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Plasma Amino Acid Concentration Changes
3.2.1. Leucine
3.2.2. Arginine
3.2.3. Sulfur-Containing Amino Acids (SCAA)
3.2.4. Essential Amino Acids (EAA)
3.2.5. Non Essential Amino Acids (NEAA)
3.3. Changes in Plasma Glucose and Insulin Concentration
3.3.1. Glucose
3.3.2. Insulin
3.4. Treatment of C2C12 with Plasmas from the Human Dietary Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, M.A.; Ma, E.B.; Huh, J.Y. Pathophysiology of sarcopenia: Genetic factors and their interplay with environmental factors. Metab. Clin. Exp. 2023, 149, 155711. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Regulation of muscle protein by amino acids. J. Nutr. 2002, 132, 3219S–3224S. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.; Lyons, M.; Piasecki, M.; Mc Phee, J.; Hind, K.; Jakeman, P. Measurement of muscle health in aging. Biogerontology 2017, 18, 901–911. [Google Scholar] [CrossRef]
- Moro, T.; Ebert, S.M.; Adams, C.M.; Rasmussen, B.B. Amino Acid Sensing in Skeletal Muscle. Trends Endocrinol. Metab. TEM 2016, 27, 796–806. [Google Scholar] [CrossRef]
- Boirie, Y.; Gachon, P.; Beaufrere, B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am. J. Clin. Nutr. 1997, 65, 489–495. [Google Scholar] [CrossRef]
- Tezze, C.; Sandri, M.; Tessari, P. Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients 2023, 15, 4073. [Google Scholar] [CrossRef]
- Cailleaux, P.E.; Dechelotte, P.; Coeffier, M. Novel dietary strategies to manage sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 234–243. [Google Scholar] [CrossRef]
- Dardevet, D.; Remond, D.; Peyron, M.A.; Papet, I.; Savary-Auzeloux, I.; Mosoni, L. Muscle wasting and resistance of muscle anabolism: The “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. Sci. World J. 2012, 2012, 269531. [Google Scholar] [CrossRef] [PubMed]
- WHO. Sustainable Healthy Diets—Guiding Principles; FAO: Rome, Italy, 2019. [Google Scholar]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- Ferrari, L.; Panaite, S.A.; Bertazzo, A.; Visioli, F. Animal- and Plant-Based Protein Sources: A Scoping Review of Human Health Outcomes and Environmental Impact. Nutrients 2022, 14, 5115. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, C.; Yan, Q.; Tan, Z.; Kang, J.; Tang, S. Elucidating the underlying mechanism of amino acids to regulate muscle protein synthesis: Effect on human health. Nutrition 2022, 103–104, 111797. [Google Scholar] [CrossRef] [PubMed]
- Kaspy, M.S.; Hannaian, S.J.; Bell, Z.W.; Churchward-Venne, T.A. The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: An update. Nutr. Res. Rev. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef]
- Combaret, L.; Dardevet, D.; Rieu, I.; Pouch, M.N.; Bechet, D.; Taillandier, D.; Grizard, J.; Attaix, D. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J. Physiol. 2005, 569, 489–499. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Pinckaers, P.J.M.; Smeets, J.S.J.; Kouw, I.W.K.; Goessens, J.P.B.; Gijsen, A.P.B.; de Groot, L.; Verdijk, L.B.; van Loon, L.J.C.; Snijders, T. Post-prandial muscle protein synthesis rates following the ingestion of pea-derived protein do not differ from ingesting an equivalent amount of milk-derived protein in healthy, young males. Eur. J. Nutr. 2024, 63, 893–904. [Google Scholar] [CrossRef] [PubMed]
- West, S.; Monteyne, A.J.; Whelehan, G.; van der Heijden, I.; Abdelrahman, D.R.; Murton, A.J.; Finnigan, T.J.A.; Stephens, F.B.; Wall, B.T. Ingestion of mycoprotein, pea protein, and their blend support comparable postexercise myofibrillar protein synthesis rates in resistance-trained individuals. Am. J. Physiology. Endocrinol. Metab. 2023, 325, E267–E279. [Google Scholar] [CrossRef]
- Ozdemir, O.; Erten, F.; Er, B.; Orhan, C.; Komorowski, J.R.; Sylla, S.; Perez Ojalvo, S.; Sahin, K. Evaluation of pea/rice and amylopectin/chromium as an alternative protein source to improve muscle protein synthesis in rats. Eur. J. Nutr. 2023, 62, 2293–2302. [Google Scholar] [CrossRef]
- Bos, C.; Metges, C.C.; Gaudichon, C.; Petzke, K.J.; Pueyo, M.E.; Morens, C.; Everwand, J.; Benamouzig, R.; Tome, D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J. Nutr. 2003, 133, 1308–1315. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Benazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Giraudet, C.; Patrac, V.; Pierre, P.; Jourdan, M.; Luiking, Y.C.; Verlaan, S.; Migne, C.; Boirie, Y.; et al. 1,25(OH)2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol. Nutr. Food Res. 2013, 57, 2137–2146. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Guillet, C.; Vaes, A.M.; Brouwer-Brolsma, E.M.; Rocher, C.; Giraudet, C.; Patrac, V.; Meugnier, E.; Montaurier, C.; et al. Vitamin D status modulates mitochondrial oxidative capacities in skeletal muscle: Role in sarcopenia. Commun. Biol. 2022, 5, 1288. [Google Scholar] [CrossRef]
- Goodman, C.A.; Mabrey, D.M.; Frey, J.W.; Miu, M.H.; Schmidt, E.K.; Pierre, P.; Hornberger, T.A. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 1028–1039. [Google Scholar] [CrossRef]
- Crossland, H.; Smith, K.; Atherton, P.J.; Wilkinson, D.J. A novel puromycin decorporation method to quantify skeletal muscle protein breakdown: A proof-of-concept study. Biochem. Biophys. Res. Commun. 2017, 494, 608–614. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Berry, A.; Giraudet, C.; Patrac, V.; Domingues-Faria, C.; Rocher, C.; Guillet, C.; Denis, P.; Pouyet, C.; et al. Fast digestive, leucine-rich, soluble milk proteins improve muscle protein anabolism, and mitochondrial function in undernourished old rats. Mol. Nutr. Food Res. 2017, 61, 1700287. [Google Scholar] [CrossRef]
- Salles, J.; Gueugneau, M.; Patrac, V.; Malnero-Fernandez, C.; Guillet, C.; Le Bacquer, O.; Giraudet, C.; Sanchez, P.; Collin, M.L.; Hermet, J.; et al. Associating Inulin with a Pea Protein Improves Fast-Twitch Skeletal Muscle Mass and Muscle Mitochondrial Activities in Old Rats. Nutrients 2023, 15, 3766. [Google Scholar] [CrossRef]
- Murakami, H.; Guillet, C.; Tardif, N.; Salles, J.; Migne, C.; Boirie, Y.; Walrand, S. Cumulative 3-nitrotyrosine in specific muscle proteins is associated with muscle loss during aging. Exp. Gerontol. 2012, 47, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.; Luiking, Y.C.; van Helvoort, A.; Boirie, Y.; Schols, J.; de Groot, C. Low Levels of Branched Chain Amino Acids, Eicosapentaenoic Acid and Micronutrients Are Associated with Low Muscle Mass, Strength and Function in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 27–34. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.K.; Ankarfeldt, M.Z.; Capra, S.; Bauer, J.; Raymond, K.; Heitmann, B.L. Lean body mass change over 6 years is associated with dietary leucine intake in an older Danish population. Br. J. Nutr. 2016, 115, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Ummels, M.; JanssenDuijghuijsen, L.; Mes, J.J.; van der Aa, C.; Wehrens, R.; Esser, D. Evaluating Brewers’ Spent Grain Protein Isolate Postprandial Amino Acid Uptake Kinetics: A Randomized, Cross-Over, Double-Blind Controlled Study. Nutrients 2023, 15, 3196. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef]
- Darmaun, D.; Cynober, L.A. Approaches to studying amino acid metabolism: From quantitative assays to flux assessment using stable isotopes. In Metabolic & Therapeutic Aspects of Amino Acids in Clinical Nutrition, 2nd ed.; Cynober, L.A., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Chanet, A.; Salles, J.; Guillet, C.; Giraudet, C.; Berry, A.; Patrac, V.; Domingues-Faria, C.; Tagliaferri, C.; Bouton, K.; Bertrand-Michel, J.; et al. Vitamin D supplementation restores the blunted muscle protein synthesis response in deficient old rats through an impact on ectopic fat deposition. J. Nutr. Biochem. 2017, 46, 30–38. [Google Scholar] [CrossRef]
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, G.E.; Jahoor, F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853. [Google Scholar] [CrossRef]
- Mattson, M.P.; Kruman, I.I.; Duan, W. Folic acid and homocysteine in age-related disease. Ageing Res. Rev. 2002, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.M.; Ganzevles, R.A.; Kudla, U.; Kardinaal, A.F.; van den Borne, J.J.; Huppertz, T. Postprandial blood amino acid concentrations in older adults after consumption of dairy products: The role of the dairy matrix. Int. Dairy J. 2021, 113, 104890. [Google Scholar] [CrossRef]
- van Dam, L.; Kardinaal, A.; Troupin, J.; Boulier, A.; Hiolle, M.; Wehrens, R.; Mensink, M. Postprandial amino acid response after the ingestion of pea protein, milk protein, casein and a casein-pea blend, in healthy older adults. Int. J. Food Sci. Nutr. 2024, 75, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Declan Fleming, R.Y.; Wolfe, R.R. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J. Clin. Investig. 1995, 95, 811–819. [Google Scholar] [CrossRef]
- Tessari, P. Stepwise Discovery of Insulin Effects on Amino Acid and Protein Metabolism. Nutrients 2023, 16, 119. [Google Scholar] [CrossRef]
- Guillet, C.; Boirie, Y. Insulin resistance: A contributing factor to age-related muscle mass loss? Diabetes Metab. 2005, 31, 5S20–25S26. [Google Scholar] [CrossRef]
- Guillet, C.; Masgrau, A.; Walrand, S.; Boirie, Y. Impaired protein metabolism: Interlinks between obesity, insulin resistance and inflammation. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2012, 13 (Suppl. S2), 51–57. [Google Scholar] [CrossRef]
- Unger, R.H.; Ohneda, A.; Aguilar-Parada, E.; Eisentraut, A.M. The role of aminogenic glucagon secretion in blood glucose homeostasis. J. Clin. Investig. 1969, 48, 810–822. [Google Scholar] [CrossRef]
- Boden, G.; Tappy, L.; Jadali, F.; Hoeldtke, R.D.; Rezvani, I.; Owen, O.E. Role of glucagon in disposal of an amino acid load. Am. J. Physiol. 1990, 259, E225–E232. [Google Scholar] [CrossRef]
- Charlton, M.R.; Adey, D.B.; Nair, K.S. Evidence for a catabolic role of glucagon during an amino acid load. J. Clin. Investig. 1996, 98, 90–99. [Google Scholar] [CrossRef]
- Riddle, E.S.; Stipanuk, M.H.; Thalacker-Mercer, A.E. Amino acids in healthy aging skeletal muscle. Front. Biosci. 2016, 8, 326–350. [Google Scholar] [CrossRef]
- Hurson, M.; Regan, M.C.; Kirk, S.J.; Wasserkrug, H.L.; Barbul, A. Metabolic effects of arginine in a healthy elderly population. JPEN J. Parenter. Enter. Nutr. 1995, 19, 227–230. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Rodionov, R.N.; McEvoy, M.; Zinellu, A.; Carru, C.; Sotgia, S. New horizons in arginine metabolism, ageing and chronic disease states. Age Ageing 2019, 48, 776–782. [Google Scholar] [CrossRef]
- Wang, R.; Jiao, H.; Zhao, J.; Wang, X.; Lin, H. L-Arginine Enhances Protein Synthesis by Phosphorylating mTOR (Thr 2446) in a Nitric Oxide-Dependent Manner in C2C12 Cells. Oxidative Med. Cell. Longev. 2018, 2018, 7569127. [Google Scholar] [CrossRef]
- Berrazaga, I.; Salles, J.; Laleg, K.; Guillet, C.; Patrac, V.; Giraudet, C.; Le Bacquer, O.; Gueugneau, M.; Denis, P.; Pouyet, C.; et al. Anabolic Properties of Mixed Wheat-Legume Pasta Products in Old Rats: Impact on Whole-Body Protein Retention and Skeletal Muscle Protein Synthesis. Nutrients 2020, 12, 1596. [Google Scholar] [CrossRef]
- Salles, J.; Guillet, C.; Le Bacquer, O.; Malnero-Fernandez, C.; Giraudet, C.; Patrac, V.; Berry, A.; Denis, P.; Pouyet, C.; Gueugneau, M.; et al. Pea Proteins Have Anabolic Effects Comparable to Milk Proteins on Whole Body Protein Retention and Muscle Protein Metabolism in Old Rats. Nutrients 2021, 13, 4234. [Google Scholar] [CrossRef]
- Nieman, D.C.; Zwetsloot, K.A.; Simonson, A.J.; Hoyle, A.T.; Wang, X.; Nelson, H.K.; Lefranc-Millot, C.; Guerin-Deremaux, L. Effects of Whey and Pea Protein Supplementation on Post-Eccentric Exercise Muscle Damage: A Randomized Trial. Nutrients 2020, 12, 2382. [Google Scholar] [CrossRef]
- Lanng, S.K.; Oxfeldt, M.; Pedersen, S.S.; Johansen, F.T.; Risikesan, J.; Lejel, T.; Bertram, H.C.; Hansen, M. Influence of protein source (cricket, pea, whey) on amino acid bioavailability and activation of the mTORC1 signaling pathway after resistance exercise in healthy young males. Eur. J. Nutr. 2023, 62, 1295–1308. [Google Scholar] [CrossRef]


| Pronativ® 95 | Nutralys®S85 | |
|---|---|---|
| Composition (g/100 g powder) | ||
| Moisture | 6.2 | 6.0 |
| Proteins | 90.2 | 80.2 |
| Carbohydrates | 0.2 | 0.4 |
| Fat | 0.3 | 9.1 |
| Amino acid content (g/100 g protein) | ||
| Alanine | 4.7 | 4.3 |
| Arginine | 2.4 | 8.5 |
| Aspartic acid | 11.2 | 11.6 |
| Cystine | 2.8 | 1 |
| Glutamic acid | 16.9 | 17.5 |
| Glycine | 1.8 | 4.1 |
| Histidine | 2.0 | 2.4 |
| Isoleucine | 5.4 | 4.8 |
| Leucine | 11.9 | 8.4 |
| Lysine | 9.6 | 7.5 |
| Méthionine | 2.1 | 1 |
| Phenylalanine | 3.5 | 5.4 |
| Proline | 4.5 | 4.5 |
| Serine | 4.3 | 5.2 |
| Threonine | 4.9 | 3.9 |
| Tryptophan | 2.2 | 0.8 |
| Tyrosine | 3.7 | 4.0 |
| Valine | 5.0 | 5.2 |
| Young (n = 15) | Older (n = 15) | p-Value | |
|---|---|---|---|
| Physical characteristics | |||
| Age (years) | 23.8 ± 0.7 | 70.5 ± 1.2 | <0.001 |
| Weight (kg) | 79.7 ± 2.4 | 73.1 ± 2.1 | <0.05 |
| Height (m) | 1.78 ± 0.02 | 1.71 ± 0.01 | <0.001 |
| BMI (kg/m2) | 24.9 ± 0.4 | 25.0 ± 0.5 | |
| Biochemical characteristics | |||
| Glucose (g/L) | 0.792 ± 0.022 | 0.867 ± 0.023 | <0.05 |
| Cholesterol (mmol/L) | 4.22 ± 0.21 | 5.62 ± 0.16 | <0.001 |
| Triglycerides (g/L) | 0.90 ± 0.08 | 1.03 ± 0.09 | |
| Young (n = 15) | Old (n = 15) | p-Value | |
|---|---|---|---|
| TNFα (pg/mL) | 0.864 ± 0.056 | 0.985 ± 0.101 | |
| IL-6 (pg/mL) | 1.215 ± 0.167 | 2.789 ± 0.612 | <0.05 |
| Main Effects and Interactions | p-Value |
|---|---|
| Age | <0.001 |
| Protein source | <0.001 |
| TPI | <0.001 |
| Time | <0.001 |
| Age × Protein source | 0.611 |
| Age × TPI | 0.213 |
| Age × Time | <0.05 |
| Protein source × TPI | <0.05 |
| Protein source × Time | <0.001 |
| TPI × Time | <0.001 |
| Age × Protein Source × TPI | 0.502 |
| Age × Protein source × Time | 0.500 |
| Age × TPI × Time | 0.588 |
| Protein source × TPI × Time | <0.05 |
| Age × Protein source × TPI × Time | 0.818 |
| Young | Older | Main Effects and Interactions Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PP | WP | MPP | MWP | PP | WP | MPP | MWP | Age | Protein Source | TPI | Age × Protein Source | Age × TPI | Protein Source × TPI | Age × Protein Source × TPI | |
| Leucine | |||||||||||||||
| Baseline (µmol/L) | 159 ± 6 ac | 150 ± 7 ab | 151 ± 6 ac | 157 ± 7 ac | 133 ± 4 b | 142 ± 11 bc | 131 ± 3 b | 140 ± 8 bc | <0.05 | - | - | - | - | - | - |
| iCmax (µmol/L) | 187 ± 15 | 395 ± 17 | 149 ± 11 | 329 ± 23 | 217 ± 11 | 464 ± 23 | 157 ± 13 | 326 ± 25 | 0.152 | <0.001 | <0.001 | 0.491 | 0.07 | <0.01 | 0.161 |
| iAUC (µMol/L × 180 min) | 19,771 ± 1242 | 39,547 ± 1969 | 13,546 ± 923 | 31,259 ± 1568 | 24,125 ± 1025 | 46,149 ± 1795 | 16,511 ± 1303 | 33,251 ± 2057 | <0.05 | <0.001 | <0.001 | 0.723 | 0.183 | <0.05 | 0.328 |
| Arginine | |||||||||||||||
| Baseline (µmol/L) | 93 ± 4 | 84 ± 4 | 91 ± 6 | 90 ± 5 | 95 ± 5 | 94 ± 5 | 94 ± 5 | 96 ± 5 | 0.315 | - | - | - | - | - | - |
| iCmax (µmol/L) | 151 ± 17 | 66 ± 3 | 120 ± 11 | 57 ± 5 | 165 ± 7 | 75 ± 4 | 124 ± 10 | 60 ± 3 | 0.354 | <0.001 | <0.001 | 0.793 | 0.494 | <0.05 | 0.828 |
| iAUC (µMol/L × 180 min) | 15,791 ± 1263 | 6385 ± 335 | 12,033 ± 940 | 4083 ± 367 | 17,294 ± 862 | 6680 ± 588 | 13,268 ± 929 | 4187 ± 359 | 0.289 | <0.001 | <0.001 | 0.342 | 0.787 | <0.05 | 0.958 |
| SCAA | |||||||||||||||
| Baseline (µmol/L) | 89 ± 2 a | 85 ± 2 a | 89 ± 2 a | 89 ± 2 a | 105 ± 2 b | 104 ± 3 b | 104 ± 3 b | 104 ± 2 b | <0.001 | - | - | - | - | - | - |
| iCmax (µmol/L) | 10 ± 2 | 58 ± 2 | 10 ± 1 | 56 ± 4 | 15 ± 3 | 76 ± 5 | 14 ± 2 | 65 ± 5 | <0.01 | <0.001 | 0.090 | 0.058 | 0.280 | 0.175 | 0.353 |
| iAUC (µMol/L × 180 min) | 567 ± 132 | 4157 ± 159 | 550 ± 114 | 4055 ± 351 | 743 ± 116 | 5397 ± 425 | 928 ± 248 | 4896 ± 386 | <0.01 | <0.001 | 0.600 | <0.05 | 0.812 | 0.264 | 0.382 |
| EAA | |||||||||||||||
| Baseline (µmol/L) | 1072 ± 34 a | 1002 ± 34 ac | 1048 ± 33 ab | 1050 ± 40 ac | 926 ± 22 b | 955 ± 50 b | 909 ± 19 bc | 948 ± 35 bc | <0.01 | - | - | - | - | - | - |
| iCmax (µmol/L) | 822 ± 65 | 1358 ± 55 | 720 ± 50 | 1200 ± 78 | 967 ± 44 | 1594 ± 78 | 750 ± 57 | 1200 ± 87 | 0.139 | <0.001 | <0.001 | 0.662 | 0.07 | 0.05 | 0.300 |
| iAUC (µMol/L × 180 min) | 89,090 ± 5122 | 137,753 ± 6133 | 71,382 ± 4080 | 116,165 ± 5676 | 105,487 ± 4197 | 157,004 ± 6764 | 82,356 ± 6037 | 123,419 ± 7617 | <0.05 | <0.001 | <0.001 | 0.944 | 0.308 | 0.232 | 0.580 |
| NEAA | |||||||||||||||
| Baseline (µmol/L) | 1846 ± 58 | 1721 ± 64 | 1809 ± 73 | 1738 ± 59 | 1679 ± 48 | 1712 ± 64 | 1654 ± 42 | 1694 ± 48 | 0.184 | - | - | - | - | - | - |
| iCmax (µmol/L) | 754 ± 66 | 678 ± 31 | 787 ± 63 | 733 ± 57 | 869 ± 34 | 786 ± 48 | 846 ± 76 | 763 ± 52 | 0.163 | <0.05 | 0.793 | 0.770 | 0.405 | 0.839 | 0.850 |
| iAUC (µMol/L × 180 min) | 71,350 ± 4648 | 63,688 ± 4158 | 80,023 ± 6328 | 71,868 ± 6841 | 79,100 ± 4742 | 68,556 ± 6925 | 90,418 ± 9146 | 75,043 ± 7353 | 0.301 | <0.01 | 0.08 | 0.478 | 0.961 | 0.670 | 0.729 |
| Young | Older | Main Effects and Interactions Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PP | WP | MPP | MWP | PP | WP | MPP | MWP | Age | Protein Source | TPI | Age × Protein Source | Age × TPI | Protein Source × TPI | Age × Protein Source × TPI | |
| Glucose | |||||||||||||||
| Baseline (g/L) | 0.940 ± 0.015 | 0.938 ± 0.019 | 0.920 ± 0.014 | 0.863 ± 0.027 | 0.965 ± 0.022 | 0.966 ± 0.0.017 | 0.933 ± 0.018 | 0.960 ± 0.022 | 0.064 | - | - | - | - | - | - |
| Cmax (g/L) | 0.977 ± 0.015 d | 0.993 ± 0.018 d | 1.169 ± 0.034 abcd | 1.237 ± 0.040 abc | 1.017 ± 0.023 cd | 1.042 ± 0.031 bcd | 1.347 ± 0.058 a | 1.248 ± 0.047 ab | 0.098 | 0.861 | <0.001 | <0.01 | 0.254 | 0.333 | <0.05 |
| AUC (g/L × 360 min) | 162.2 ± 2.7 | 161.8 ± 2.5 | 170.7 ± 5.1 | 166.5 ± 4.4 | 170.7 ± 3.8 | 172.2 ± 3.9 | 193.3 ± 7.1 | 186.3 ± 6.9 | <0.05 | 0.179 | <0.001 | 0.907 | <0.05 | 0.070 | 0.488 |
| Insulin | |||||||||||||||
| Baseline (µiU/mL) | 6.44 ± 1.03 | 5.57 ± 0.96 | 4.56 ± 0.98 | 5.03 ± 0.62 | 4.07 ± 0.69 | 4.01 ± 0.66 | 4.10 ± 0.80 | 4.78 ± 0.82 | 0.181 | - | - | - | - | - | - |
| Cmax (µiU/mL) | 18.80 ± 2.11 | 25.3 ± 2.1 | 62.4 ± 6.5 | 82.0 ± 8.3 | 17.2 ± 1.9 | 20.6 ± 1.9 | 65.5 ± 11.7 | 57.0 ± 6.2 | 0.240 | 0.100 | <0.001 | <0.05 | 0.410 | 0.929 | 0.067 |
| AUC (µiU/mL × 360 min) | 2253 ± 170 a | 2657 ± 249 a | 6825 ± 548 b | 8194 ± 729 b | 1998 ± 266 a | 2249 ± 289 a | 7760 ± 995 b | 7322 ± 783 b | 0.819 | 0.107 | <0.001 | <0.05 | 0.646 | 0.720 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salles, J.; Gueugneau, M.; Laleg, K.; Giraudet, C.; Sanchez, P.; Blot, A.; Richard, R.; Neveux, N.; Lefranc-Millot, C.; Perreau, C.; et al. Circulating Amino Acid Concentration after the Consumption of Pea or Whey Proteins in Young and Older Adults Affects Protein Synthesis in C2C12 Myotubes. Nutrients 2024, 16, 2870. https://doi.org/10.3390/nu16172870
Salles J, Gueugneau M, Laleg K, Giraudet C, Sanchez P, Blot A, Richard R, Neveux N, Lefranc-Millot C, Perreau C, et al. Circulating Amino Acid Concentration after the Consumption of Pea or Whey Proteins in Young and Older Adults Affects Protein Synthesis in C2C12 Myotubes. Nutrients. 2024; 16(17):2870. https://doi.org/10.3390/nu16172870
Chicago/Turabian StyleSalles, Jérôme, Marine Gueugneau, Karima Laleg, Christophe Giraudet, Phelipe Sanchez, Adeline Blot, Ruddy Richard, Nathalie Neveux, Catherine Lefranc-Millot, Caroline Perreau, and et al. 2024. "Circulating Amino Acid Concentration after the Consumption of Pea or Whey Proteins in Young and Older Adults Affects Protein Synthesis in C2C12 Myotubes" Nutrients 16, no. 17: 2870. https://doi.org/10.3390/nu16172870
APA StyleSalles, J., Gueugneau, M., Laleg, K., Giraudet, C., Sanchez, P., Blot, A., Richard, R., Neveux, N., Lefranc-Millot, C., Perreau, C., Guérin-Deremaux, L., Boirie, Y., & Walrand, S. (2024). Circulating Amino Acid Concentration after the Consumption of Pea or Whey Proteins in Young and Older Adults Affects Protein Synthesis in C2C12 Myotubes. Nutrients, 16(17), 2870. https://doi.org/10.3390/nu16172870





