Exploring the Geroprotective Potential of Nutraceuticals
Abstract
1. Introduction
2. Nutraceuticals
3. Nutraceuticals as Geroprotectors
3.1. Telomere Attrition
3.2. Epigenetics
3.3. Loss of Proteostasis
3.4. Disabled Macroautophagy
3.5. Deregulated Nutrient Sensing
3.6. Mitochondrial Dysfunction
3.7. Cellular Senescence
3.8. Stem Cell Exhaustion
3.9. Altered Intercellular Communication
3.10. Chronic Inflammation (Inflammaging)
3.11. Dysbiosis
3.12. Genomic Instability
4. State of the Art on Nutraceuticals Research
5. Concluding Remarks State of the Art on Nutraceuticals Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lean, M.E.J. Principles of Human Nutrition. Medicine 2015, 43, 61–65. [Google Scholar] [CrossRef]
- Longo, V.D.; Anderson, R.M. Nutrition, Longevity and Disease: From Molecular Mechanisms to Interventions. Cell 2022, 185, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Combet, E.; Buckton, C. Micronutrient Deficiencies, Vitamin Pills and Nutritional Supplements. Medicine 2015, 43, 66–72. [Google Scholar] [CrossRef]
- DeFelice, S.L. The Nutraceutical Revolution: Its Impact on Food Industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Aronson, J.K. Defining “Nutraceuticals”: Neither Nutritious nor Pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Nicoletti, M. Nutraceuticals and Botanicals: Overview and Perspectives. Int. J. Food Sci. Nutr. 2012, 63 (Suppl. S1), 2–6. [Google Scholar] [CrossRef]
- da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A Synopsis on Aging-Theories, Mechanisms and Future Prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Scheuren, A.C.; Potter, P.; Müller, R.; Bellantuono, I. Geroprotectors: A Role in the Treatment of Frailty. Mech. Ageing Dev. 2019, 180, 11–20. [Google Scholar] [CrossRef]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies; William Heinemann Ltd.: London, UK, 1910. [Google Scholar]
- Moskalev, A.; Chernyagina, E.; Kudryavtseva, A.; Shaposhnikov, M. Geroprotectors: A Unified Concept and Screening Approaches. Aging Dis. 2017, 8, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Hodjat, M.; Khalid, M.; Asghari, M.; Atri, S.; Rahimifard, M.; Nejad, S.M.; Baeeri, M. Nutrients and Nutraceuticals in Aging. In Nutrients and Nutraceuticals for Active & Healthy Ageing; Springer: Singapore, 2020; pp. 63–109. ISBN 9789811535512. [Google Scholar]
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Ho, Y.W. Probiotics: From Isolation to Application. J. Am. Coll. Nutr. 2017, 36, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Nutraceutical Industry 2024. Available online: https://www.reportlinker.com/market-report/Nutraceutical/11559/Nutraceutical?term=nutraceutical%20industry&matchtype=b&loc_interest=&loc_physical=9047091&utm_group=standard&utm_term=nutraceutical%20industry&utm_campaign=ppc&utm_source=google_ads&utm_medium=paid_ads&utm_content=transactionnel-4&hsa_acc=7082072004&hsa_cam=15072279998&hsa_grp=129918785895&hsa_ad=559945523670&hsa_src=g&hsa_tgt=kwd-1461105442248&hsa_kw=nutraceutical%20industry&hsa_mt=b&hsa_net=adwords&hsa_ver=3&gad_source=1&gclid=Cj0KCQjwh7K1BhCZARIsAKOrVqECD74ko4F-SUwa-XYfwmAbyFK14yPv6EIMRXEIuA377ovdzlPiEtkaAgNtEALw_wcB (accessed on 2 August 2024).
- Afroz, R.D.; Nurunabbi, A.S.M.; Khan, M.I. Effects of Wheatgrass (Triticum aestivum) Juice on Serum Cholesterol of Experimentally Induced Hypercholesterolaemic Male Long Evans Rat. Banglad. J. Physiol. Pharmacol. 2014, 27, 21–27. [Google Scholar] [CrossRef]
- Bagwe, S.M.; Kale, P.P.; Bhatt, L.K.; Prabhavalkar, K.S. Herbal Approach in the Treatment of Pancytopenia. J. Complement. Integr. Med. 2017, 14, 20160053. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sela, G.; Cohen, M.; Ben-Arye, E.; Epelbaum, R. The Medical Use of Wheatgrass: Review of the Gap Between Basic and Clinical Applications. Mini Rev. Med. Chem. 2015, 15, 1002–1010. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe Vera: A Short Review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Cho, S.; Lee, S.; Lee, M.-J.; Lee, D.H.; Won, C.-H.; Kim, S.M.; Chung, J.H. Dietary Aloe Vera Supplementation Improves Facial Wrinkles and Elasticity and It Increases the Type I Procollagen Gene Expression in Human Skin in Vivo. Ann. Dermatol. 2009, 21, 6–11. [Google Scholar] [CrossRef]
- Lee, H.; Choi, W.; Ro, H.; Kim, G.; Lee, H. Skin Antiaging Effects of the Fermented Outer Layers of Leaf Skin of Aloe Barbadensis Miller Associated with the Enhancement of Mitochondrial Activities of UVb-Irradiated Human Skin Fibroblasts. Appl. Sci. 2021, 11, 5660. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Panax Ginseng and Panax Quinquefolius: From Pharmacology to Toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Namkoong, S.; Yun, Y.-G.; Hong, H.-D.; Lee, Y.-C.; Ha, K.-S.; Lee, H.; Kwon, H.J.; Kwon, Y.-G.; Kim, Y.-M. Water Extract of Korean Red Ginseng Stimulates Angiogenesis by Activating the PI3K/Akt-Dependent ERK1/2 and eNOS Pathways in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2007, 30, 1674–1679. [Google Scholar] [CrossRef]
- Dattilo, S.; Mancuso, C.; Koverech, G.; Di Mauro, P.; Ontario, M.L.; Petralia, C.C.; Petralia, A.; Maiolino, L.; Serra, A.; Calabrese, E.J.; et al. Heat Shock Proteins and Hormesis in the Diagnosis and Treatment of Neurodegenerative Diseases. Immun. Ageing 2015, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Jannat, K.; Balakrishnan, R.; Han, J.-H.; Yu, Y.-J.; Kim, G.-W.; Choi, D.-K. The Neuropharmacological Evaluation of Seaweed: A Potential Therapeutic Source. Cells 2023, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Alves, C.; Pinteus, S.; Mendes, S.; Pedrosa, R. Seaweeds’ Neuroprotective Potential Set in Vitro on a Human Cellular Stress Model. Mol. Cell. Biochem. 2020, 473, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; Karadeniz, B.; Romero-Reyes, S.; Espín, J.C.; Pelvan, E.; González-Sarrías, A. Polyphenolic Characterization and Anti-Inflammatory Effect of In Vitro Digested Extracts of L. Plant Parts in an Inflammatory Model of Human Colon Cells. Int. J. Mol. Sci. 2024, 25, 1744. [Google Scholar] [CrossRef]
- Lee, S.-K.; Lee, D.-R.; Kim, H.-L.; Choi, B.-K.; Kwon, K.-B. A Randomized, Double-Blind, Placebo-Controlled Study on Immune Improvement Effects of Ethanolic Extract of Echinacea purpurea (L.) Moench in Korean Adults. Phytother. Res. 2024, 38, 3645–3659. [Google Scholar] [CrossRef]
- Ruíz-Salinas, A.K.; Vázquez-Roque, R.A.; Díaz, A.; Pulido, G.; Treviño, S.; Floran, B.; Flores, G. The Treatment of Goji Berry (Lycium barbarum) Improves the Neuroplasticity of the Prefrontal Cortex and Hippocampus in Aged Rats. J. Nutr. Biochem. 2020, 83. [Google Scholar] [CrossRef]
- Srinuanchai, W.; Nooin, R.; Pitchakarn, P.; Karinchai, J.; Suttisansanee, U.; Chansriniyom, C.; Jarussophon, S.; Temviriyanukul, P.; Nuchuchua, O. Inhibitory Effects of Gymnema inodorum (Lour.) Decne Leaf Extracts and Its Triterpene Saponin on Carbohydrate Digestion and Intestinal Glucose Absorption. J. Ethnopharmacol. 2021, 266, 113398. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Esteban-Muñoz, A.; Sánchez-González, C.; Rivas-García, L.; Llopis, J.; Cianciosi, D.; Giampieri, F.; Sumalla-Cano, S.; Battino, M.; et al. Strawberry (Fragaria × Ananassa Cv. Romina) Methanolic Extract Attenuates Alzheimer’s Beta Amyloid Production and Oxidative Stress by SKN-1/NRF and DAF-16/FOXO Mediated Mechanisms in C. Elegans. Food Chem. 2022, 372, 131272. [Google Scholar] [CrossRef]
- Mougin, C.; Chataigner, M.; Lucas, C.; Leyrolle, Q.; Pallet, V.; Layé, S.; Bouvret, E.; Dinel, A.-L.; Joffre, C. Dietary Marine Hydrolysate Improves Memory Performance and Social Behavior through Gut Microbiota Remodeling during Aging. Foods 2023, 12, 4199. [Google Scholar] [CrossRef]
- Xu, D.; Lu, Y.; Yang, X.; Pan, D.; Wang, Y.; Yin, S.; Wang, S.; Sun, G. Effects of Fish Oil-Derived N-3 Polyunsaturated Fatty Acid on Body Composition, Muscle Strength and Physical Performance in Older People: A Secondary Analysis of a Randomised, Double-Blind, Placebo-Controlled Trial. Age Ageing 2022, 51, afac274. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yang, H.P.; Pang, W.; Lu, H.; Hu, Y.D.; Li, J.; Lu, S.J.; Zhang, W.Q.; Jiang, Y.G. Cyanidin-3-O-Galactoside and Blueberry Extracts Supplementation Improves Spatial Memory and Regulates Hippocampal ERK Expression in Senescence-Accelerated Mice. Biomed. Environ. Sci. 2014, 27, 186–196. [Google Scholar]
- Boespflug, E.L.; Eliassen, J.C.; Dudley, J.A.; Shidler; Kalt, W.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Enhanced Neural Activation with Blueberry Supplementation in Mild Cognitive Impairment. Nutr. Neurosci. 2018, 21, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-C.; Lee, I.-T.; Wang, M.-F.; Yeh, W.-C.; Liang, B.-C. Tempeh Attenuates Cognitive Deficit, Antioxidant Imbalance, and Amyloid β of Senescence-Accelerated Mice by Modulating Nrf2 Expression via MAPK Pathway. J. Funct. Foods 2018, 50, 112–119. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented Soybean Foods: A Review of Their Functional Components, Mechanism of Action and Factors Influencing Their Health Benefits. Food Res. Int. 2022, 158, 111575. [Google Scholar] [CrossRef]
- Das, G.; Paramithiotis, S.; Sundaram Sivamaruthi, B.; Wijaya, C.H.; Suharta, S.; Sanlier, N.; Shin, H.-S.; Patra, J.K. Traditional Fermented Foods with Anti-Aging Effect: A Concentric Review. Food Res. Int. 2020, 134, 109269. [Google Scholar] [CrossRef] [PubMed]
- Tamam, B.; Syah, D.; Suhartono, M.T.; Kusuma, W.A.; Tachibana, S.; Lioe, H.N. Proteomic Study of Bioactive Peptides from Tempe. J. Biosci. Bioeng. 2019, 128, 241–248. [Google Scholar] [CrossRef]
- Selim, A.M.; Nooh, M.M.; El-Sawalhi, M.M.; Ismail, N.A. Amelioration of Age-Related Alterations in Rat Liver: Effects of Curcumin C3 Complex, Astragalus Membranaceus and Blueberry. Exp. Gerontol. 2020, 137, 110982. [Google Scholar] [CrossRef]
- Asyakina, L.; Atuchin, V.; Drozdova, M.; Kozlova, O.; Prosekov, A. Ex Vivo and In Vitro Antiaging and Antioxidant Extract Activity of the Amelanchier Ovalis from Siberia. Int. J. Mol. Sci. 2022, 23, 15156. [Google Scholar] [CrossRef]
- Singlár, Z.; Szentesi, P.; Fodor, J.; Angyal, Á.; Csernoch, L.; Sztretye, M. Assessing the Potential of Nutraceuticals as Geroprotectors on Muscle Performance and Cognition in Aging Mice. Antioxidants 2021, 10, 1415. [Google Scholar] [CrossRef]
- Cui, B.; Liu, L.; Shi, T.; Yin, M.; Feng, X.; Shan, Y. The Ethanolic Extract of Lycium Ruthenicum Ameliorates Age-Related Physiological Damage in Mice. Molecules 2023, 28, 7615. [Google Scholar] [CrossRef]
- Koh, Y.C.; Kuo, L.H.; Chang, Y.Y.; Tung, Y.C.; Lo, Y.C.; Pan, M.H. Modulatory Effect of Fermented Black Soybean and Adlay on Gut Microbiota Contributes to Healthy Aging. Mol. Nutr. Food Res. 2023, 67, 2200700. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Hsu, B.-Y.; Wu, N.-L.; Tsui, W.-H.; Lin, T.-J.; Su, C.-C.; Hung, C.-F. Anti-Photoaging Effects of Soy Isoflavone Extract (aglycone and Acetylglucoside Form) from Soybean Cake. Int. J. Mol. Sci. 2010, 11, 4782–4795. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nakamura, F.; Kanzato, H.; Sawada, D.; Hirata, H.; Nishimura, A.; Kajimoto, O.; Fujiwara, S. Clinical Effects of Lactobacillus Acidophilus Strain L-92 on Perennial Allergic Rhinitis: A Double-Blind, Placebo-Controlled Study. J. Dairy Sci. 2005, 88, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Liao, T.-W.; Chen, Y.-H.; Chiang, Y.-C.; Tsai, Y.-C. Oral Administration of Heat-Inactivated Lactobacillus Plantarum K37 Modulated Airway Hyperresponsiveness in Ovalbumin-Sensitized BALB/c Mice. PLoS ONE 2014, 9, e100105. [Google Scholar] [CrossRef][Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2017, 19, 13. [Google Scholar] [CrossRef]
- Jacczak, B.; Rubiś, B.; Totoń, E. Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging. Int. J. Mol. Sci. 2021, 22, 6381. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Renieri, E.; Tsoukalas, D.; Buga, A.M.; Sarandi, E.; Vakonaki, E.; Fragkiadaki, P.; Alegakis, A.; Nikitovic, D.; Calina, D.; et al. A Novel Nutraceutical Formulation Increases Telomere Length and Activates Telomerase Activity in Middle-aged Rats. Mol. Med. Rep. 2023, 28, 232. [Google Scholar] [CrossRef]
- You, Y.O.; Lee, G.; Min, B.M. Retinoic Acid Extends the in Vitro Life Span of Normal Human Oral Keratinocytes by Decreasing p16(INK4A) Expression and Maintaining Telomerase Activity. Biochem. Biophys. Res. Commun. 2000, 268, 268–274. [Google Scholar] [CrossRef]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2020, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Schellnegger, M.; Hofmann, E.; Carnieletto, M.; Kamolz, L.-P. Unlocking Longevity: The Role of Telomeres and Its Targeting Interventions. Front. Aging 2024, 5, 1339317. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.; Singaravelu, G.; Harley, C.B.; Flom, P.; Suram, A.; Raffaele, J.M. A Natural Product Telomerase Activator Lengthens Telomeres in Humans: A Randomized, Double Blind, and Placebo Controlled Study. Rejuvenation Res. 2016, 19, 478. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Raghuram, G.V.; Dsouza, J.; Shinde, S.; Jadhav, V.; Shaikh, A.; Rane, B.; Tandel, H.; Kondhalkar, D.; Chaudhary, S.; et al. A pro-Oxidant Combination of Resveratrol and Copper down-Regulates Multiple Biological Hallmarks of Ageing and Neurodegeneration in Mice. Sci. Rep. 2022, 12, 17209. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.; Parletta, N.; Milte, C.M.; Benassi-Evans, B.; Fenech, M.; Howe, P.R. Telomere Shortening in Elderly Individuals with Mild Cognitive Impairment May Be Attenuated with ω-3 Fatty Acid Supplementation: A Randomized Controlled Pilot Study. Nutrition 2014, 30, 489–491. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Pal, S.; Tyler, J.K. Epigenetics and Aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef]
- Greer, E.L.; Maures, T.J.; Ucar, D.; Hauswirth, A.G.; Mancini, E.; Lim, J.P.; Benayoun, B.A.; Shi, Y.; Brunet, A. Transgenerational Epigenetic Inheritance of Longevity in C. Elegans. Nature 2011, 479, 365. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Naidoo, V.; Carrera, I.; Corzo, L.; Cacabelos, R. Natural Bioactive Products as Epigenetic Modulators for Treating Neurodegenerative Disorders. Pharmaceuticals 2023, 16, 216. [Google Scholar] [CrossRef]
- Huang, D.; Cui, L.; Ahmed, S.; Zainab, F.; Wu, Q.; Wang, X.; Yuan, Z. An Overview of Epigenetic Agents and Natural Nutrition Products Targeting DNA Methyltransferase, Histone Deacetylases and microRNAs. Food Chem. Toxicol. 2019, 123, 574–594. [Google Scholar] [CrossRef]
- Bar-El Dadon, S.; Reifen, R. Vitamin A and the Epigenome. Crit. Rev. Food Sci. Nutr. 2017, 57, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Khajebishak, Y.; Alivand, M.; Faghfouri, A.H.; Moludi, J.; Payahoo, L. The Effects of Vitamins and Dietary Pattern on Epigenetic Modification of Non-Communicable Diseases. Int. J. Vitam. Nutr. Res. 2023, 93, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.-U.; Rehman, M.S.-U.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Silva, G.D.B.; Pavan, A.R.; Chiba, D.E.; Chin, C.M.; Dos Santos, J.L. Epigenetic Regulatory Mechanisms Induced by Resveratrol. Nutrients 2017, 9, 1201. [Google Scholar] [CrossRef]
- Joven, J.; Micol, V.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A. Polyphenols and the Modulation of Gene Expression Pathways: Can We Eat Our Way out of the Danger of Chronic Disease? Crit. Rev. Food Sci. Nutr. 2014, 54, 985–1001. [Google Scholar] [CrossRef]
- McGee, K.C.; Sullivan, J.; Hazeldine, J.; Schmunk, L.J.; Martin-Herranz, D.E.; Jackson, T.; Lord, J.M. A Combination Nutritional Supplement Reduces DNA Methylation Age Only in Older Adults with a Raised Epigenetic Age. GeroScience 2024, 46, 4333–4347. [Google Scholar] [CrossRef]
- Park, L.K.; Friso, S.; Choi, S.W. Nutritional Influences on Epigenetics and Age-Related Disease. Proc. Nutr. Soc. 2012, 71, 75–83. [Google Scholar] [CrossRef]
- Julien, C.; Tremblay, C.; Émond, V.; Lebbadi, M.; Salem, N., Jr.; Bennett, D.A.; Calon, F. SIRT1 Decrease Parallels the Accumulation of Tau in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2009, 68, 48. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and Aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef]
- Brehme, M.; Sverchkova, A.; Voisine, C. Proteostasis Network Deregulation Signatures as Biomarkers for Pharmacological Disease Intervention. Curr. Opin. Syst. Biol. 2019, 15, 74–81. [Google Scholar] [CrossRef]
- Cuanalo-Contreras, K.; Moreno-Gonzalez, I. Natural Products as Modulators of the Proteostasis Machinery: Implications in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 4666. [Google Scholar] [CrossRef]
- Kato, K.; Ito, H.; Kamei, K.; Iwamoto, I. Stimulation of the Stress-Induced Expression of Stress Proteins by Curcumin in Cultured Cells and in Rat Tissues in Vivo. Cell Stress Chaperones 1998, 3, 152. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Veleri, S.; Frautschy, S. Molecular Chaperone Dysfunction in Neurodegenerative Diseases and Effects of Curcumin. BioMed Res. Int. 2014, 2014, 495091. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.J.; Pan, Y.; Singh, S.; Powell, D.W.; Wu, R.; Cummins, T.; Chen, Q.; McLeish, K.R.; Klein, J.B. Heat Shock Protein 27 Controls Apoptosis by Regulating Akt Activation. J. Biol. Chem. 2003, 278, 27828–27835. [Google Scholar] [CrossRef]
- Guo, H.; Cao, M.; Zou, S.; Ye, B.; Dong, Y. Cranberry Extract Standardized for Proanthocyanidins Alleviates β-Amyloid Peptide Toxicity by Improving Proteostasis Through HSF-1 in Caenorhabditis Elegans Model of Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1564. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Gonos, E.S. Proteasome Activation as a Novel Antiaging Strategy. IUBMB Life 2008, 60, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, M.; Chondrogianni, N.; Chinou, I.; Rivett, A.J.; Gonos, E.S. The Olive Constituent Oleuropein Exhibits Proteasome Stimulatory Properties in Vitro and Confers Life Span Extension of Human Embryonic Fibroblasts. Rejuvenation Res. 2007, 10, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Corpas, R.; Griñán-Ferré, C.; Rodríguez-Farré, E.; Pallàs, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol. Neurobiol. 2019, 56, 1502–1516. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; Hansen, M. Macroautophagy and Aging: The Impact of Cellular Recycling on Health and Longevity. Mol. Aspects Med. 2021, 82, 101020. [Google Scholar] [CrossRef]
- Wong, S.Q.; Kumar, A.V.; Mills, J.; Lapierre, L.R. Autophagy in Aging and Longevity. Hum. Genet. 2020, 139, 277. [Google Scholar] [CrossRef]
- Cao, H.; Jia, Q.; Shen, D.; Yan, L.; Chen, C.; Xing, S. Quercetin Has a Protective Effect on Atherosclerosis via Enhancement of Autophagy in ApoE Mice. Exp. Ther. Med. 2019, 18, 2451–2458. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin Induces Autophagy, Inhibits Proliferation and Invasion by Downregulating AKT/mTOR Signaling Pathway in Human Melanoma Cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, Z.; Li, Y.; Ou, C.; Huang, Z.; Zhang, J.; Liu, P.; Luo, C.; Chen, M. Puerarin Prevents Cardiac Hypertrophy Induced by Pressure Overload through Activation of Autophagy. Biochem. Biophys. Res. Commun. 2015, 464, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.-H.; Li, G.-H.; Zhang, X.; Zhang, P.; Dong, M.-Q.; Zhao, Z.-J.; Zhang, Y.; Dong, L.; Gao, F. Berberine Improves Mesenteric Artery Insulin Sensitivity through up-Regulating Insulin Receptor-Mediated Signalling in Diabetic Rats. Br. J. Pharmacol. 2016, 173, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, C.; Zhang, X.; Li, X.; Chen, Z.; Yang, C.; Liang, X.; Zhu, G.; Xu, Z. Neuroprotective Effect of Curcumin Against Cerebral Ischemia-Reperfusion Via Mediating Autophagy and Inflammation. J. Mol. Neurosci. 2018, 64, 129–139. [Google Scholar] [CrossRef]
- Zhong, C.; Pu, L.-Y.; Fang, M.-M.; Gu, Z.; Rao, J.-H.; Wang, X.-H. Retinoic Acid Receptor α Promotes Autophagy to Alleviate Liver Ischemia and Reperfusion Injury. World J. Gastroenterol. 2015, 21, 12381–12391. [Google Scholar] [CrossRef]
- Kim, S.-H.; Chung, D.-K.; Lee, Y.J.; Song, C.-H.; Ku, S.-K. Neuroprotective Effects of Danggui-Jakyak-San on Rat Stroke Model through Antioxidant/antiapoptotic Pathway. J. Ethnopharmacol. 2016, 188, 123–133. [Google Scholar] [CrossRef]
- Ahn, S.M.; Kim, H.N.; Kim, Y.R.; Choi, Y.W.; Kim, C.M.; Shin, H.K.; Choi, B.T. Emodin from Polygonum Multiflorum Ameliorates Oxidative Toxicity in HT22 Cells and Deficits in Photothrombotic Ischemia. J. Ethnopharmacol. 2016, 188, 13–20. [Google Scholar] [CrossRef]
- Sun, R.; Song, Y.; Li, S.; Ma, Z.; Deng, X.; Fu, Q.; Qu, R.; Ma, S. Levo-Tetrahydropalmatine Attenuates Neuron Apoptosis Induced by Cerebral Ischemia-Reperfusion Injury: Involvement of c-Abl Activation. J. Mol. Neurosci. 2018, 65, 391–399. [Google Scholar] [CrossRef]
- Pang, Q.; Zhao, Y.; Chen, X.; Zhao, K.; Zhai, Q.; Tu, F. Apigenin Protects the Brain against Ischemia/Reperfusion Injury via Caveolin-1/VEGF In Vitro and In Vivo. Oxidative Med. Cell. Longev. 2018, 2018, 7017204. [Google Scholar] [CrossRef]
- Zheng, W.-X.; Cao, X.-L.; Wang, F.; Wang, J.; Ying, T.-Z.; Xiao, W.; Zhang, Y.; Xing, H.; Dong, W.; Xu, S.-Q.; et al. Baicalin Inhibiting Cerebral Ischemia/hypoxia-Induced Neuronal Apoptosis via MRTF-A-Mediated Transactivity. Eur. J. Pharmacol. 2015, 767, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Ho, Y.H.; Hung, C.F.; Kuo, J.R.; Wang, S.J. Xanthohumol, an Active Constituent from Hope, Affords Protection against Kainic Acid-Induced Excitotoxicity in Rats. Neurochem. Int. 2020, 133, 104629. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-Sensing Mechanisms and Pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-C.; Chin, C.-Y.; Zheng, Y.-X.; Lee, I. The Attenuation of Insulin/IGF-1 Signaling Pathway Plays a Crucial Role in the Myo-Inositol-Alleviated Aging in Caenorhabditis Elegans. Int. J. Mol. Sci. 2023, 24, 6194. [Google Scholar] [CrossRef]
- Franceschi, C.; Passarino, G.; Mari, D.; Monti, D. Centenarians as a 21st Century Healthy Aging Model: A Legacy of Humanity and the Need for a World-Wide Consortium (WWC100+). Mech. Ageing Dev. 2017, 165, 55–58. [Google Scholar] [CrossRef]
- Paolisso, G.; Ammendola, S.; Del Buono, A.; Gambardella, A.; Riondino, M.; Tagliamonte, M.R.; Rizzo, M.R.; Carella, C.; Varricchio, M. Serum Levels of Insulin-Like Growth Factor-I (IGF-I) and IGF-Binding Protein-3 in Healthy Centenarians: Relationship with Plasma Leptin and Lipid Concentrations, Insulin Action, and Cognitive Function. J. Clin. Endocrinol. Metab. 1997, 82, 2204–2209. [Google Scholar] [CrossRef]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. ROLE of IGF-1 System in the Modulation of Longevity: Controversies and New Insights From a Centenarians’ Perspective. Front. Endocrinol. 2019, 10, 431907. [Google Scholar] [CrossRef]
- Chang, C.-H.; Bijian, K.; Wernic, D.; Su, J.; da Silva, S.D.; Yu, H.; Qiu, D.; Asslan, M.; Alaoui-Jamali, M.A. A Novel Orally Available Seleno-Purine Molecule Suppresses Triple-Negative Breast Cancer Cell Proliferation and Progression to Metastasis by Inducing Cytostatic Autophagy. Autophagy 2019, 15, 1376–1390. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Teng, Z.; Zhang, T.; Li, Y. Downregulation of PI3K/Akt/mTOR Signaling Pathway in Curcumin-Induced Autophagy in APP/PS1 Double Transgenic Mice. Eur. J. Pharmacol. 2014, 740, 312–320. [Google Scholar] [CrossRef]
- Ji, Y.; Li, L.; Ma, Y.-X.; Li, W.-T.; Li, L.; Zhu, H.-Z.; Wu, M.-H.; Zhou, J.-R. Quercetin Inhibits Growth of Hepatocellular Carcinoma by Apoptosis Induction in Part via Autophagy Stimulation in Mice. J. Nutr. Biochem. 2019, 69, 108–119. [Google Scholar] [CrossRef]
- Pignatti, C.; D’Adamo, S.; Stefanelli, C.; Flamigni, F.; Cetrullo, S. Nutrients and Pathways That Regulate Health Span and Life Span. Geriatrics 2020, 5, 95. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Borrás, C.; Viña, J. Genistein, a Tool for Geroscience. Mech. Ageing Dev. 2022, 204, 111665. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yao, L.; Ma, X.; Xu, X. Small Molecules as SIRT Modulators. Mini-Rev. Med. Chem. 2018, 18, 1151–1157. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, J.; Hu, K.; He, X.; Yun, D.; Tong, T.; Han, L. Sirtuins and Their Biological Relevance in Aging and Age-Related Diseases. Aging Dis. 2020, 11, 927. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Mitochondrial Dysfunction and Chronic Disease: Treatment With Natural Supplements. Integr. Med. A Clin. J. 2014, 13, 35. [Google Scholar]
- van der Rijt, S.; Molenaars, M.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Integrating the Hallmarks of Aging Throughout the Tree of Life: A Focus on Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2020, 8, 594416. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin Attenuates Oxidative Stress and Neuronal Apoptosis after Ischemia/reperfusion Injury via Nrf2-Mediated Inhibition of the NOX4/ROS/NF-κB Pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef]
- Leung, S.W.; Lai, J.H.; Wu, J.C.-C.; Tsai, Y.-R.; Chen, Y.-H.; Kang, S.-J.; Chiang, Y.-H.; Chang, C.-F.; Chen, K.-Y. Neuroprotective Effects of Emodin against Ischemia/Reperfusion Injury through Activating ERK-1/2 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 2899. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yu, H.; Li, M.; Hang, L.; Xu, X. Apigenin Protects Mouse Retina against Oxidative Damage by Regulating the Nrf2 Pathway and Autophagy. Oxidative Med. Cell. Longev. 2020, 2020, 9420704. [Google Scholar] [CrossRef]
- Lippi, L.; Uberti, F.; Folli, A.; Turco, A.; Curci, C.; d’Abrosca, F.; de Sire, A.; Invernizzi, M. Impact of Nutraceuticals and Dietary Supplements on Mitochondria Modifications in Healthy Aging: A Systematic Review of Randomized Controlled Trials. Aging Clin. Exp. Res. 2022, 34, 2659–2674. [Google Scholar] [CrossRef] [PubMed]
- Shanaida, M.; Lysiuk, R.; Mykhailenko, O.; Hudz, N.; Abdulsalam, A.; Gontova, T.; Oleshchuk, O.; Ivankiv, Y.; Shanaida, V.; Lytkin, D.; et al. Alpha-Lipoic Acid: An Antioxidant with Anti-Aging Properties for Disease Therapy. Curr. Med. Chem. 2024, 31, e190424229159. [Google Scholar] [CrossRef] [PubMed]
- Lewis Luján, L.M.; McCarty, M.F.; Di Nicolantonio, J.J.; Gálvez Ruiz, J.C.; Rosas-Burgos, E.C.; Plascencia-Jatomea, M.; Iloki Assanga, S.B. Nutraceuticals/Drugs Promoting Mitophagy and Mitochondrial Biogenesis May Combat the Mitochondrial Dysfunction Driving Progression of Dry Age-Related Macular Degeneration. Nutrients 2022, 14, 1985. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Kifley, A.; Flood, V.M.; Joachim, N.; Lewis, J.R.; Hodgson, J.M.; Mitchell, P. Dietary Flavonoids and the Prevalence and 15-Y Incidence of Age-Related Macular Degeneration. Am. J. Clin. Nutr. 2018, 108, 381–387. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Lee, Y.-J.; Hsu, M.-Y.; Wang, M.; Tsou, S.-C.; Chen, C.-C.; Lin, J.-A.; Hsiao, Y.-P.; Lin, H.-W. Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway. Int. J. Mol. Sci. 2021, 22, 4056. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular Senescence: A Key Therapeutic Target in Aging and Diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Regulski, M.J. Cellular Senescence: What, Why, and How. Wounds A Compend. Clin. Res. Pract. 2017, 29, 168–174. [Google Scholar]
- Biazi, B.I.; Zanetti, T.A.; Baranoski, A.; Corveloni, A.C.; Mantovani, M.S. Cis-Nerolidol Induces Endoplasmic Reticulum Stress and Cell Death in Human Hepatocellular Carcinoma Cells through Extensive CYP2C19 and CYP1A2 Oxidation. Basic Clin. Pharmacol. Toxicol. 2017, 121, 334–341. [Google Scholar] [CrossRef]
- Xu, Q.; Fu, Q.; Li, Z.; Liu, H.; Wang, Y.; Lin, X.; He, R.; Zhang, X.; Ju, Z.; Campisi, J.; et al. The Flavonoid Procyanidin C1 Has Senotherapeutic Activity and Increases Lifespan in Mice. Nat. Metab. 2021, 3, 1706–1726. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin Is a Senotherapeutic That Extends Health and Lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic Drugs: From Discovery to Translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Mbara, K.C.; Devnarain, N.; Owira, P.M.O. Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection. Pharmaceut. Med. 2022, 36, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.I.; Sheng, M.H.; Wasnik, S.; Baylink, D.J.; Lau, K.-H.W. Effect of Aging on Stem Cells. World J. Exp. Med. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Stover, P.J.; Field, M.S.; Brawley, H.N.; Angelin, B.; Iversen, P.O.; Frühbeck, G. Nutrition and Stem Cell Integrity in Aging. J. Intern. Med. 2022, 292, 587–603. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Biton, M.; Haber, A.L.; Gunduz, N.; Eng, G.; Gaynor, L.T.; Tripathi, S.; Calibasi-Kocal, G.; Rickelt, S.; Butty, V.L.; et al. Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell 2019, 178, 1115–1131.e15. [Google Scholar] [CrossRef]
- Richer, S.; Patel, S.; Sockanathan, S.; Ulanski, L.J., 2nd; Miller, L.; Podella, C. Resveratrol Based Oral Nutritional Supplement Produces Long-Term Beneficial Effects on Structure and Visual Function in Human Patients. Nutrients 2014, 6, 4404–4420. [Google Scholar] [CrossRef]
- Dai, J.; Chen, L.; Qiu, Y.-M.; Li, S.-Q.; Xiong, W.-H.; Yin, Y.-H.; Jia, F.; Jiang, J.-Y. Activations of GABAergic Signaling, HSP70 and MAPK Cascades Are Involved in Baicalin’s Neuroprotection against Gerbil Global Ischemia/reperfusion Injury. Brain Res. Bull. 2013, 90, 1–9. [Google Scholar] [CrossRef]
- Ruggiero, C.; Tafaro, L.; Cianferotti, L.; Tramontana, F.; Macchione, I.G.; Caffarelli, C.; Virdis, A.; Ferracci, M.; Rinonapoli, G.; Mecocci, P.; et al. Targeting the Hallmarks of Aging with Vitamin D: Starting to Decode the Myth. Nutrients 2024, 16, 906. [Google Scholar] [CrossRef]
- McDonald, C.M.; Ramirez-Sanchez, I.; Oskarsson, B.; Joyce, N.; Aguilar, C.; Nicorici, A.; Dayan, J.; Goude, E.; Abresch, R.T.; Villarreal, F.; et al. (-)-Epicatechin Induces Mitochondrial Biogenesis and Markers of Muscle Regeneration in Adults with Becker Muscular Dystrophy. Muscle Nerve 2021, 63, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Long, H.-Z.; Cheng, Y.; Zhou, Z.-W.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Xia, J.; Li, H.; Zhang, Z.; Yang, Y.; Huang, X.; He, Z.; Liu, J.; Yang, X. Ginsenoside Rg1 Ameliorates Behavioral Abnormalities and Modulates the Hippocampal Proteomic Change in Triple Transgenic Mice of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2017, 2017, 6473506. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Haigis, M.C.; Guarente, L.P. Mammalian Sirtuins--Emerging Roles in Physiology, Aging, and Calorie Restriction. Genes Dev. 2006, 20, 2913–2921. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, Flavones and Flavanols—Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin Suppresses Cyclooxygenase-2 Expression and Angiogenesis through Inactivation of P300 Signaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of Naturally-Occurring Flavonoids and Biflavonoids on Epidermal Cyclooxygenase and Lipoxygenase from Guinea-Pigs. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Lee, K.M.; Hwang, M.K.; Lee, D.E.; Lee, K.W.; Lee, H.J. Protective Effect of Quercetin against Arsenite-Induced COX-2 Expression by Targeting PI3K in Rat Liver Epithelial Cells. J. Agric. Food Chem. 2010, 58, 5815–5820. [Google Scholar] [CrossRef]
- Seo, M.-J.; Lee, Y.-J.; Hwang, J.-H.; Kim, K.-J.; Lee, B.-Y. The Inhibitory Effects of Quercetin on Obesity and Obesity-Induced Inflammation by Regulation of MAPK Signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef]
- Flores-Soto, E.; Romero-Martínez, B.S.; Solís-Chagoyán, H.; Estrella-Parra, E.A.; Avila-Acevedo, J.G.; Gomez-Verjan, J.C.; Reyes-García, J.; Casas-Hernández, M.F.; Sommer, B.; Montaño, L.M. Chamaecyparis Lawsoniana and Its Active Compound Quercetin as Ca2+ Inhibitors in the Contraction of Airway Smooth Muscle. Molecules 2024, 29, 2284. [Google Scholar] [CrossRef] [PubMed]
- Peairs, A.; Dai, R.; Gan, L.; Shimp, S.; Rylander, M.N.; Li, L.; Reilly, C.M. Epigallocatechin-3-Gallate (EGCG) Attenuates Inflammation in MRL/lpr Mouse Mesangial Cells. Cell. Mol. Immunol. 2010, 7, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, L.; Tang, P.; Chen, D.; Li, Y.; Li, J.; Bao, C. Carthamin Yellow Improves Cerebral Ischemia-reperfusion Injury by Attenuating Inflammation and Ferroptosis in Rats. Int. J. Mol. Med. 2021, 47, 52. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, H.; Yang, Y.; Wang, R.; Wang, Y.; Wu, C.; Du, G. Baicalein Administered in the Subacute Phase Ameliorates Ischemia-Reperfusion-Induced Brain Injury by Reducing Neuroinflammation and Neuronal Damage. Biomed. Pharmacother. 2019, 117, 109102. [Google Scholar] [CrossRef]
- Chen, H.-L.; Jia, W.-J.; Li, H.-E.; Han, H.; Li, F.; Zhang, X.-L.-N.; Li, J.-J.; Yuan, Y.; Wu, C.-Y. Scutellarin Exerts Anti-Inflammatory Effects in Activated Microglia/Brain Macrophage in Cerebral Ischemia and in Activated BV-2 Microglia Through Regulation of MAPKs Signaling Pathway. Neuromol. Med. 2020, 22, 264–277. [Google Scholar] [CrossRef]
- Mo, Z.-T.; Liao, Y.-L.; Zheng, J.; Li, W.-N. Icariin Protects Neurons from Endoplasmic Reticulum Stress-Induced Apoptosis after OGD/R Injury via Suppressing IRE1α-XBP1 Signaling Pathway. Life Sci. 2020, 255, 117847. [Google Scholar] [CrossRef]
- Dai, M.; Chen, B.; Wang, X.; Gao, C.; Yu, H. Icariin Enhance Mild Hypothermia-Induced Neuroprotection via Inhibiting the Activation of NF-κB in Experimental Ischemic Stroke. Metab. Brain Dis. 2021, 36, 1779–1790. [Google Scholar] [CrossRef]
- Wei, P.; Wang, K.; Luo, C.; Huang, Y.; Misilimu, D.; Wen, H.; Jin, P.; Li, C.; Gong, Y.; Gao, Y. Cordycepin Confers Long-Term Neuroprotection via Inhibiting Neutrophil Infiltration and Neuroinflammation after Traumatic Brain Injury. J. Neuroinflamm. 2021, 18, 137. [Google Scholar] [CrossRef]
- Li, Z.; Geng, Y.-N.; Jiang, J.-D.; Kong, W.-J. Antioxidant and Anti-Inflammatory Activities of Berberine in the Treatment of Diabetes Mellitus. Evid.-Based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wang, W.; Okla, M.; Kang, I.; Moreau, R.; Chung, S. Suppression of NLRP3 Inflammasome by γ-Tocotrienol Ameliorates Type 2 Diabetes. J. Lipid Res. 2016, 57, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the Immune System. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Chen, X.; Lu, Z.; Meng, L.; Huang, Y.; Yu, X.; Huang, L.; Ye, P.; Chen, X.; Liang, J.; et al. Longevity of Centenarians Is Reflected by the Gut Microbiome with Youth-Associated Signatures. Nat. Aging 2023, 3, 436–449. [Google Scholar] [CrossRef]
- Johansen, J.; Atarashi, K.; Arai, Y.; Hirose, N.; Sørensen, S.J.; Vatanen, T.; Knip, M.; Honda, K.; Xavier, R.J.; Rasmussen, S.; et al. Centenarians Have a Diverse Gut Virome with the Potential to Modulate Metabolism and Promote Healthy Lifespan. Nat. Microbiol. 2023, 8, 1064–1078. [Google Scholar] [CrossRef]
- Milshteyn, A.; Colosimo, D.A.; Brady, S.F. Accessing Bioactive Natural Products from the Human Microbiome. Cell Host Microbe 2018, 23, 725–736. [Google Scholar] [CrossRef]
- López-Gil, L.; Pascual-Ahuir, A.; Proft, M. Genomic Instability and Epigenetic Changes during Aging. Int. J. Mol. Sci. 2023, 24, 14279. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Muñoz-Espada, A.C.; Wood, K.V.; Bordelon, B.; Watkins, B.A. Anthocyanin Quantification and Radical Scavenging Capacity of Concord, Norton, and Marechal Foch Grapes and Wines. J. Agric. Food Chem. 2004, 52, 6779–6786. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Wang, J.Y.; Nemzer, B. Antioxidant and Antiradical Activity of Coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small Molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Rahmah, N.N.; Tama, N.D.; Wulandari, N.R.; Nurcholis, W. Potential of β-Carotene as Anti-Aging Serum: A Narrative Review. Int. J. Life Sci. Pharma Res. 2021, 12, 1999–2004. [Google Scholar] [CrossRef]
- Huang, A.; Huo, Y.; Zhong, Y.; Yang, W. AI Technology for Anti-Aging: An Overview. In Proceedings of the 2023 International Conference on Intelligent Supercomputing and BioPharma (ISBP), Zhuhai, China, 6–8 January 2023; IEEE: New York, NY, USA. [Google Scholar]
- Carpio, L.E.; Sanz, Y.; Gozalbes, R.; Barigye, S.J. Computational Strategies for the Discovery of Biological Functions of Health Foods, Nutraceuticals and Cosmeceuticals: A Review. Mol. Divers. 2021, 25, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhavoronkov, A.; Bischof, E.; Lee, K.-F. Artificial Intelligence in Longevity Medicine. Nat. Aging 2021, 1, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C. Supplementation of the Pure Flavonoids Epicatechin and Quercetin Affects Some Biomarkers of Endothelial Dysfunction and Inflammation in (Pre)Hypertensive Adults: A Randomized Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxidative Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Hong, C.Y.; Lee, S.H. The Phytoestrogenic Effect of Daidzein in Human Dermal Fibroblasts. J. Soc. Cosmet. Sci. Korea 2014, 40, 279–287. [Google Scholar]
- Guo, J.M.; Kang, G.Z.; Xiao, B.X.; Liu, D.H.; Zhang, S. Effect of Daidzein on Cell Growth, Cell Cycle, and Telomerase Activity of Human Cervical Cancer in Vitro. Int. J. Gynecol. Cancer 2004, 14, 882–888. [Google Scholar] [CrossRef]
- Yoshino, M.; Naka, A.; Sakamoto, Y.; Shibasaki, A.; Toh, M.; Tsukamoto, S.; Kondo, K.; Iida, K. Dietary Isoflavone Daidzein Promotes Tfam Expression That Increases Mitochondrial Biogenesis in C2C12 Muscle Cells. J. Nutr. Biochem. 2015, 26, 1193–1199. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Bilotto, S.; Spagnuolo, C.; Durante, M.; Lenucci, M.S.; Mita, G.; Volpe, M.G.; Aquino, R.P.; Russo, G.L. A Carotenoid Extract from a Southern Italian Cultivar of Pumpkin Triggers Nonprotective Autophagy in Malignant Cells. Oxidative Med. Cell. Longev. 2017, 2017, 7468538. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxidative Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef] [PubMed]
- Bartali, B.; Semba, R.D. Carotenoids and Healthy Aging: The Fascination Continues. Am. J. Clin. Nutr. 2021, 113, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cheng, J.; Min, Z.; Yin, T.; Zhang, R.; Zhang, W.; Hu, L.; Cui, Z.; Gao, C.; Xu, S.; et al. Effects of Fucoxanthin on Autophagy and Apoptosis in SGC-7901cells and the Mechanism. J. Cell. Biochem. 2018, 119, 7274–7284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Fan, Y.; Gao, Y.; Li, X.; Hu, Z.; Ding, K.; Wang, Y.; Wang, X. Fucoxanthin Provides Neuroprotection in Models of Traumatic Brain Injury via the Nrf2-ARE and Nrf2-Autophagy Pathways. Sci. Rep. 2017, 7, 46763. [Google Scholar] [CrossRef]
- Lee, A.-H.; Shin, H.-Y.; Park, J.-H.; Koo, S.Y.; Kim, S.M.; Yang, S.-H. Fucoxanthin from Microalgae Phaeodactylum Tricornutum Inhibits pro-Inflammatory Cytokines by Regulating Both NF-κB and NLRP3 Inflammasome Activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef]
- Cheng, Y.; Pan, X.; Wang, J.; Li, X.; Yang, S.; Yin, R.; Ma, A.; Zhu, X. Fucoidan Inhibits NLRP3 Inflammasome Activation by Enhancing p62/SQSTM1-Dependent Selective Autophagy to Alleviate Atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 3186306. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.H.; Choi, S.H.; Asahara, T.; Kwon, S.M. The Sulfated Polysaccharide Fucoidan Rescues Senescence of Endothelial Colony-Forming Cells for Ischemic Repair. Stem Cells 2015, 33, 1939–1951. [Google Scholar] [CrossRef]
- Yu, W.-C.; Chen, Y.-L.; Hwang, P.-A.; Chen, T.-H.; Chou, T.-C. Fucoidan Ameliorates Pancreatic β-Cell Death and Impaired Insulin Synthesis in Streptozotocin-Treated β Cells and Mice via a Sirt-1-Dependent Manner. Mol. Nutr. Food Res. 2017, 61, 1700136. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, M.; He, Z.Z. Low-Molecular-Weight Fucoidan Attenuates Mitochondrial Dysfunction and Improves Neurological Outcome After Traumatic Brain Injury in Aged Mice: Involvement of Sirt3. Cell. Mol. Neurobiol. 2016, 36, 1257–1268. [Google Scholar] [CrossRef]
- Li, J.; Chen, K.; Li, S.; Feng, J.; Liu, T.; Wang, F.; Zhang, R.; Xu, S.; Zhou, Y.; Zhou, S.; et al. Protective Effect of Fucoidan from Fucus Vesiculosus on Liver Fibrosis via the TGF-β1/Smad Pathway-Mediated Inhibition of Extracellular Matrix and Autophagy. Drug Des. Devel. Ther. 2016, 10, 619–630. [Google Scholar]
- Oh, J.M.; Kim, E.; Chun, S. Ginsenoside Compound K Induces Ros-Mediated Apoptosis and Autophagic Inhibition in Human Neuroblastoma Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2019, 20, 4279. [Google Scholar] [CrossRef] [PubMed]
- Arafa, E.-S.A.; Refaey, M.S.; Abd El-Ghafar, O.A.; Hassanein, E.H.M.; Sayed, A.M. The Promising Therapeutic Potentials of Ginsenosides Mediated through p38 MAPK Signaling Inhibition. Heliyon 2021, 7, e08354. [Google Scholar] [CrossRef] [PubMed]
- Baik, I.-H.; Kim, K.-H.; Lee, K.-A. Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts. Molecules 2021, 26, 4102. [Google Scholar] [CrossRef]
- Wang, S.; Qiao, J.; Jiang, C.; Pan, D.; Yu, S.; Chen, J.; Liu, S.; Zhang, P.; Zhao, D.; Liu, M. Ginsenoside Rg1 Delays Chronological Aging in a Yeast Model via CDC19- and SDH2-Mediated Cellular Metabolism. AntiOxidative Redox Signal. 2023, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, J.; Cai, S.; Liu, D.; Jiang, R.; Wang, Y. Protective Effects of Ginsenoside Rg1 on Aging Sca-1+ Hematopoietic Cells. Mol. Med. Rep. 2015, 12, 3621–3628. [Google Scholar] [CrossRef]
- Sun, M.; Ji, Y.; Zhou, S.; Chen, R.; Yao, H.; Du, M. Ginsenoside Rb3 Inhibits Osteoclastogenesis via ERK/NF-κB Signaling Pathway in Vitro and in Vivo. Oral Dis. 2023, 29, 3460–3471. [Google Scholar] [CrossRef]
- Zhou, S.; Ji, Y.; Yao, H.; Guo, H.; Zhang, Z.; Wang, Z.; Du, M. Application of Ginsenoside Rd in Periodontitis With Inhibitory Effects on Pathogenicity, Inflammation, and Bone Resorption. Front. Cell. Infect. Microbiol. 2022, 12, 813953. [Google Scholar] [CrossRef]
- Yin, L.H.; Cheng, W.X.; Qin, Z.S.; Sun, K.M.; Zhong, M.; Wang, J.K.; Gao, W.Y.; Yu, Z.H. Effects of Ginsenoside Rg-1 on the Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells. Chin. J. Integr. Med. 2015, 21, 676–681. [Google Scholar] [CrossRef]
- Park, M.Y.; Kwon, H.J.; Sung, M.K. Evaluation of Aloin and Aloe-Emodin as Anti-Inflammatory Agents in Aloe by Using Murine Macrophages. Biosci. Biotechnol. Biochem. 2009, 73, 828–832. [Google Scholar] [CrossRef]
- Zimbone, S.; Romanucci, V.; Zarrelli, A.; Giuffrida, M.L.; Sciacca, M.F.M.; Lanza, V.; Campagna, T.; Maugeri, L.; Petralia, S.; Consoli, G.M.L.; et al. Exploring the Therapeutic Potential of Aloin: Unraveling Neuroprotective and Anticancer Mechanisms, and Strategies for Enhanced Stability and Delivery. Sci. Rep. 2024, 14, 16731. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, W.; Zhou, X.; Yan, W.; Wang, Z. Aloin Induced Apoptosis by Enhancing Autophagic Flux through the PI3K/AKT Axis in Osteosarcoma. Chin. Med. 2021, 16, 1–21. [Google Scholar] [CrossRef]
- Vidakovic, M.; Marinello, J.; Lahtela-Kakkonen, M.; Matulis, D.; Linkuvienė, V.; Michel, B.Y.; Navakauskiene, R.; Christodoulou, M.S.; Passarella, D.; Klimasauskas, S.; et al. New Insights into the Epigenetic Activities of Natural Compounds. OBM Genet. 2018, 2, 029. [Google Scholar] [CrossRef]
- Li, P.; Kong, J.; Chen, Z.; Huang, S.; Lv, G.; Wei, B.; Wei, J.; Jing, K.; Quan, J.; Chu, J. Aloin Promotes Osteogenesis of Bone-Marrow-Derived Mesenchymal Stem Cells via the ERK1/2-Dependent Runx2 Signaling Pathway. J. Nat. Med. 2019, 73, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, P.; Qiu, J.; Hu, W.; Li, S.; Shi, H.; Qiu, X.; Huang, D.; Gao, W.; Liang, A. Aloin Regulates Matrix Metabolism and Apoptosis in Human Nucleus Pulposus Cells via the TAK1/NF-κB/NLRP3 Signaling Pathway. Stem Cells Int. 2022, 2022, 5865011. [Google Scholar] [CrossRef]
- Moskalev, A.; Chernyagina, E.; de Magalhães, J.P.; Barardo, D.; Thoppil, H.; Shaposhnikov, M.; Budovsky, A.; Fraifeld, V.E.; Garazha, A.; Tsvetkov, V.; et al. Geroprotectors.org: A New, Structured and Curated Database of Current Therapeutic Interventions in Aging and Age-Related Disease. Aging 2015, 7, 616–628. [Google Scholar] [CrossRef] [PubMed]
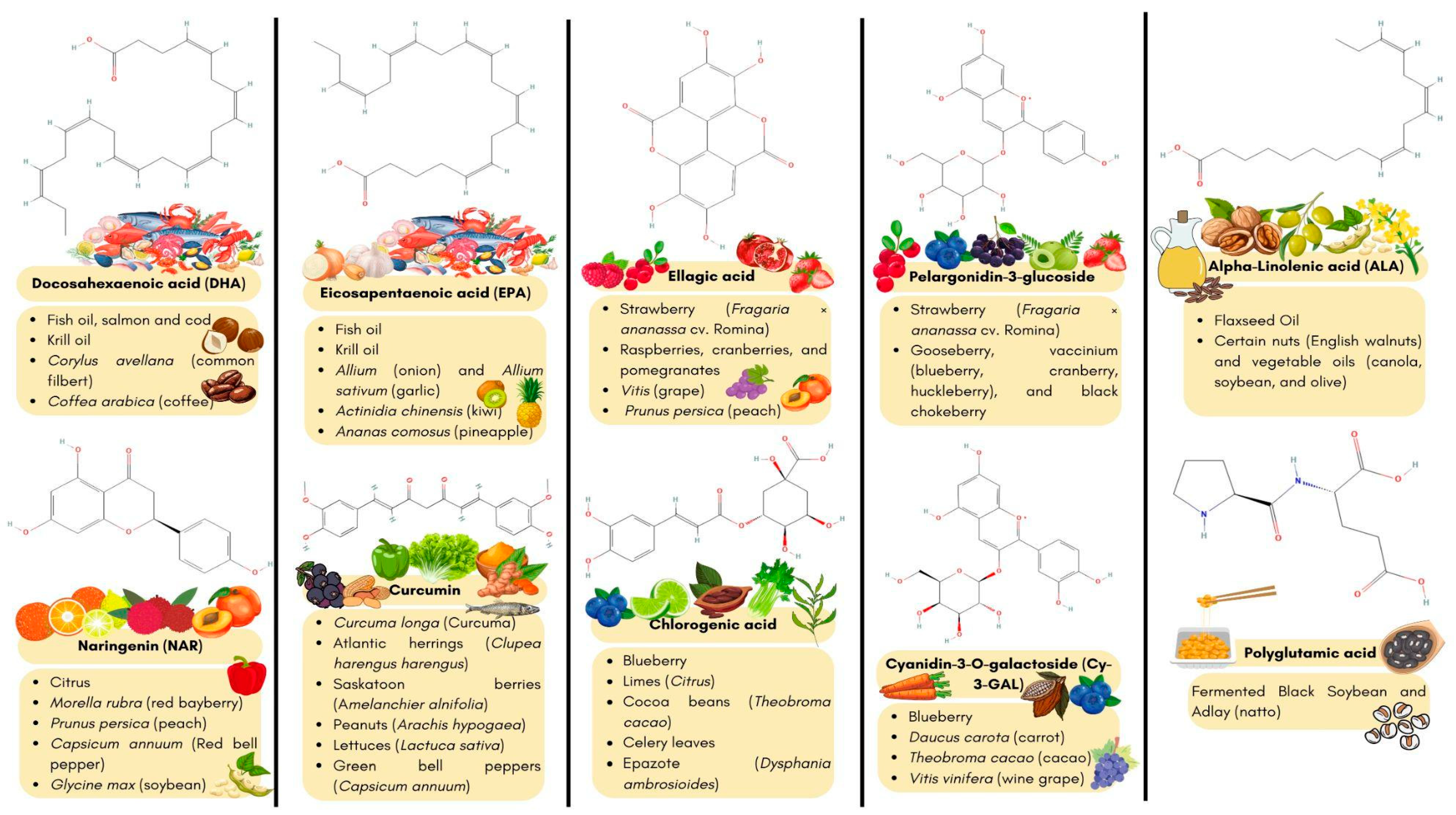
| Nutraceutical Source | Bioactive Compounds or Organisms | Nutraceutical Classification | Age-Related Target (Pharmacological or Biological Activities) | Refs. |
|---|---|---|---|---|
| Wheatgrass (Triticum aestivum) | Chlorophyll Flavonoids Vitamin C Vitamin E | Phytochemicals Antioxidants Vitamins | Decreases triglycerides in blood. Inhibits growth of leukemia cells. Benefits immunological activity. Decreases oxidative stress. | [17,18,19] |
| Aloe vera extract | Quercetin Myricetin Aloin Vanillic acid Palmitic acid Vitamin E Polysaccharides Phenolic compounds | Phytochemicals Antioxidants | Aloe is useful for photoaging since it stimulates fibroblast, which produces collagen and elastin fibers, making the skin more elastic and less wrinkled. Additionally, it inhibits the cyclooxygenase pathway and reduces prostaglandin E2 production from arachidonic acid. Quercetin, which exists in the outer layers of aloe leaf, has a cytoprotective effect on mitochondrial pathways by inhibiting oxidative stress. | [20,21,22] |
| Ginseng extract Panax ginseng | Ginsenoside C-K Oleanolic acid Ginsenoside Rg1-Rb1, Rd Oleanane Polysaccharides Peptides Phenolic compounds | Phytochemicals | Ginseng exhibited a remarkable antioxidant effect through the enhancement of the cell stress response, mainly by up-regulating heme oxygenase-1. In a rat model of high-fructose diet-induced metabolic disorder, fermented red ginseng reduced hyperlipidemia and hypertension. An aqueous extract of Korean red ginseng rapidly up-regulated endothelial NO synthase (eNOS) via the phosphoinositide 3-kinase (PI3K)/Akt-pathway in human umbilical vein endothelial cells (HUVEC). | [23,24,25] |
| Seaweed species Hypnea musiformis, Ochtodes secundiramea, Padina gymnospora, Codium tomentosum, and Pterocladiella capillacea | Fucoidan Fucoxanthin Phycoerythin Alginic acid Polysaccharides Carotenoids Taurine | Phytochemicals Amino acid | Seaweed is reported to ameliorate or prevent Aβ25–35 aggregation and inhibit AChE and BuChE levels in vitro. MeOH extracts of seaweed S. muticum and S. polyschides exhibited the highest neuroprotective effects against dopamine-induced neurotoxicity in SH-SY5Y cells. | [26,27] |
| Echinacea purpurea extracts | Caffeic acid β-sitosterol Phenolic compounds | Phytochemicals | After 8 weeks of Echinacea consumption, a significant increase in NK cell cytotoxic activity was observed. Serum cytokine levels of IL-2, IFN-γ, and TNF-α also significantly increased. In vitro gastrointestinal digestion on the phenolic composition of Echinacea extracts showed significant reductions in IL-6, IL-8, and PGE2 levels in vitro. | [28,29] |
| Goji berry (Lycium barbarum) extract | L. barbarum polysaccharides (LBPs) Pectic polysaccharides Lycopene Beta-carotene Lutein Zeaxanthin Phenolic compounds Rutin | Phytochemicals Antioxidants | Improve mitochondrial function and decrease oxidative stress via Nrf2-Maf and NOS signaling pathways. Improve cognitive performance in aged rats by decreased astrogliosis. | [30] |
| Chiang-Da (Gymnema inodorum) leaf extracts | (3β, 16β)-16,28-dihydroxyolean-12-en-3-yl-O-β-d-glucopyranosyl-β-d-glucopyranosiduronic acid (GIA1) | Phytochemicals | Induces anti-hyperglycemic mechanisms by reducing α-glucosidase activity and glucose transport of SGLT. | [31] |
| Strawberry (Fragaria x ananassa cv. Romina) extracts | Ellagic acid Pelargonidin-3-glucoside (Phenolic compounds) K+, Mg+, P+ and Ca2+ (Minerals) | Phytochemicals Essential trace elements | Induces DAF-16/FOXO and SKN-1/NRF2 pathways. Delay β-amyloid induced paralysis Reduced β-amyloid aggregation Prevents oxidative stress in C. elegans. | [32] |
| Fish hydrolysate | Eicosapentaenoic acid (EPA) Docosahexaenoic acid (DHA) | Fatty acids | Improved memory performance in aged mice. Regulates gut microbiota. Regulates corticosterone levels. Increased the expression of the mitochondrial respiratory chain (ND1, ND2, ND5, and ND6). Improving total skeletal muscle mass, muscle strength and physical performance in older adults. | [33,34] |
| Blueberry (Vaccinium uliginosum L.) extracts | Polyphenolic compounds Cyaniding-3-O-galactoside (Antocyanin) Pyruvic acid Chlorogenic acid | Phytochemicals | Promotes recovery from cell injury and improves survival of hippocampal pyramidal neurons. Increases antioxidant defenses via ERK signaling pathway in the hippocampus of a senescence-accelerated mouse model. | [35,36] |
| Tempeh (soybean fermentation) | Daidzein Genistein Polyphenols Low-molecular-weight soluble dietary fiber Tempeh isoflavone Peptides: Ala-Val, Gly-Leu, Gly-Phe, Pro-Leu, Ala-Phe, Asp-Met, Asp-Tyr, Pro-Ala-Pro, Ile-Ala-Lys, Arg-Ile-Tyr and Val-Ile-Lys-Pro. | Phytochemicals Dietary fiber Antioxidants Proteins and amino acids | Induces Anti-inflammatory and immunomodulatory components. Improve antioxidative activity and increase both SOD and CAT gene expression. Induces anti-hypertensive activity via ACE inhibitor peptide Induces neuroprotection and GABA synthesis in six-month-old senescence-accelerated mice. | [37,38,39,40] |
| Curcumin C3 complex | Polyphenolic orange-yellow pigments: curcumin, demethoxycurcuminbis-demethoxycurcumin | Phytochemicals | Decreased IL-6 concentration and gene expression. Prevents senescent cell accumulation. Improve antioxidant capacity. Upregulate TERT gene expression Increased telomere length in aged rats. Upregulate TERT gene expression Increased telomere length in aged rats (17 months old). | [41] |
| Blueberry (Vaccinium uliginosum L.) extract | Flavonoids (anthocyanidins) Polyphenols (procyanidin) Phenolic acid Pyruvic acid Chlorogenic acid | |||
| Astragalus membranaceus | Astragaloside IV Kaempferol Quercetin Isorhamnetin Triterpene saponins | |||
| Amelanchier ovalis berries ethanolic extract | Gallic acid p-hydroxybenzoic acid Protocatechinic acid | Phytochemicals | Promotes proliferation, lifespan and survival rate of Saccharomyces cerevisiae Y-564 exposed to oxidative stress. | [42] |
| Krill oil | Astaxanthin Choline Omega-3 DHA EPA | Phytochemicals Vitamin precursors Fatty acids | mTOR-p70s6k/Muscular strength and cognitive function The administration of krill oil to a mixed-sex aged C57BL/6 mouse model increased force production (increased grip strength, increased contraction and tetanic strength in the extensor digitorum longus muscle) without altering Ca2+ homeostasis in the excitation-contraction coupling mechanism or mitochondrial Ca2+ uptake processes. | [43] |
| Lycium ruthenicum Murr ethanolic extract | Anthocyanins Lycibarbar spermidine B N1-Dihydrocaffeoyl N10-trans-caffeoyl-spermidine) | Phytochemicals | Prevents oxidative damage by increasing SOD and glutathione peroxidase concentration in a murine model of accelerated aging induced by D-galactose. | [44] |
| Fermented Black Soybean and Adlay (FBA) | Nattokinase Polyglutamic acid Isoflavones | Proteins and amino acids Phytochemicals | Improves body composition in aged mice (increased gastrocnemius muscle and decreased fat accumulation). Interestingly, it reduced the expression of GLB1 and p16INK4A genes involved in senescence. Counteracts oxidative stress. Decrease inflammation markers MCP-1, IL-6 and IL-10 in aged mice. Improves aging-related gut microbial dysbiosis promoting the growth of beneficial microbes (Alistipes, Anaeroplasma, Coriobacteriaceae UCG002, and Parvibacter). | [45] |
| Soybean | Daidzein Genistein Glycitein Acetyldaidzin Acetylgenistin Acetylglycitin | Phytochemicals | Induces anti-photoaging in murine models exposed to UVB radiation. | [46] |
| Fermented milk | Lactobacillus paracasei Lactobacillus plantarum | Prebiotics or Probiotics | Improve symptoms associated with allergic rhinitis. Reduces airway hyperresponsiveness, asthma and systemic proinflammatory factors (IL-4, IL-5, and IL-3). | [47,48] |
| Nutraceutical Source | Bioactive Compounds | Hallmarks of Aging | Refs. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T.A. | EP. | L.P. | D.M. | D.N.S. | M.D. | C.S. | S.C.E. | A.I.C. | C.I. | Dys. | G.I. | |||
| Dairy products, Cod liver oil, Fish oil, Beef liver, Carrot seed oil, Palm fruit oil | Vitamin A (retinoic acid) | ✔ | ✔ | ✔ | [53,68,89] | |||||||||
| Wheatgrass, Acerola cherry extract, Rosehip extract, Camu camu extract, Sea buckthorn oil | Vitamin C | ✔ | ✔ | [52,54,63,69] | ||||||||||
| Cod liver oil, Fish oil, Lanolin | Vitamin D | ✔ | ✔ | ✔ | ✔ | [52,54,69,131,133] | ||||||||
| Wheat seed oil, Sunflower oil, Almond oil, Soybean oil, Acai berry extract | Vitamin E | ✔ | ✔ | ✔ | [54,63,155] | |||||||||
| Turmeric extract, Curcumin C3 complex, | Curcumin | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | [51,63,70,74,75,85,88,102,135] | |||||
| Grape seed extract Red wine extract Blueberry extract Cranberry extract Peanut extract | Resveratrol | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | [51,57,63,69,70,81,104,106,107,117,131,139,164] | ||||
| Aloe vera extract Quercetin supplements Multi-antioxidant formulas Onion extract Apple extract Broccoli extract | Quercetin | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | [84,103,106,118,125,127,145,169] | ||||||
| Fish oil Krill oil Seaweed oil | EPA | ✔ | ✔ | ✔ | [55,58,69,114] | |||||||||
| Fish oil Krill oil Seaweed oil | DHA | ✔ | ✔ | ✔ | [55,58,69,114] | |||||||||
| Sunflower oil Corn oil Soybean oil Grape seed oil Hemp seed oil | Linoleic Acid | ✔ | ✔ | [58,74] | ||||||||||
| Chamomile extract Parsley extract Celery seed extract Citrus bioflavonoid complex | Apigenin | ✔ | ✔ | ✔ | [93,113] | |||||||||
| Tempeh Red clover extract Soybean Soy isoflavone supplements | Genistein | ✔ | ✔ | ✔ | ✔ | [104,105] | ||||||||
| Amelanchier ovalis berries ethanolic extract Pomegranate extract Green tea extract Grape seed extract Acai berry extract | Gallic acid | ✔ | [104] | |||||||||||
| Wheat germ Quinoa | Betaine | ✔ | [104] | |||||||||||
| Grape seed extract Pine bark extract Cocoa extracct | Procyanidin C1 | ✔ | [123] | |||||||||||
| Carrots, peppers, thyme, broccoli, onion leaves, cabbages, apple skins, rosemary, parsley, and spinach | Luteolin | ✔ | [127] | |||||||||||
| Krill oil Seaweed-based supplemnts | Astaxanthin | ✔ | [69] | |||||||||||
| Olive leaf extract Olive oil | Oleuropein | ✔ | [80] | |||||||||||
| Scutellaria baicalensis root extract | Baicalin | ✔ | ✔ | ✔ | [94,132,149] | |||||||||
| Multivitamin/mineral supplements α-lipoic acid supplements | α-lipoic acid | ✔ | [115] | |||||||||||
| Inulin supplements Prebiotic supplements Probiotic and prebiotic combination Chicory root extract | Inulin | ✔ | [116] | |||||||||||
| Cocoa extract Dark Chocolate Green tea extract | Epicatechin | ✔ | [134] | |||||||||||
| Soy isoflavone supplements Soy-based products | Daidzein | ✔ | ✔ | ✔ | ✔ | ✔ | [170,171,172,173] | |||||||
| Seaweed oil Tomato extract Palm fruit oil Carrot seed oil Mixed carotenoid supplements | Carotenoids | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | [174,175,176,177] | ||||||
| Brown algae extract Seaweed-based supplements | Fucoxanthin | ✔ | ✔ | [178,179,180] | ||||||||||
| Brown algae extract Seaweed-based supplements | Fucoidan | ✔ | ✔ | ✔ | ✔ | [181,182,183,184,185] | ||||||||
| Ginseng root extract Ginseng containing supplements | Ginsenosides C-K | ✔ | ✔ | [186,187,188] | ||||||||||
| Korean Red Ginseng extract American Ginseng extract | Ginsenosides Rg1-Rb1, Rd | ✔ | ✔ | ✔ | ✔ | ✔ | [189,190,191,192,193] | |||||||
| Aloe vera gel Aloe vera extract | Aloin | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | [194,195,196,197,198,199] | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Segura, N.A.; Zepeda-Arzate, E.A.; Castillo-Vazquez, S.K.; Fleischmann-delaParra, P.; Hernández-Pineda, J.; Flores-Soto, E.; García-delaTorre, P.; Estrella-Parra, E.A.; Gomez-Verjan, J.C. Exploring the Geroprotective Potential of Nutraceuticals. Nutrients 2024, 16, 2835. https://doi.org/10.3390/nu16172835
Rivero-Segura NA, Zepeda-Arzate EA, Castillo-Vazquez SK, Fleischmann-delaParra P, Hernández-Pineda J, Flores-Soto E, García-delaTorre P, Estrella-Parra EA, Gomez-Verjan JC. Exploring the Geroprotective Potential of Nutraceuticals. Nutrients. 2024; 16(17):2835. https://doi.org/10.3390/nu16172835
Chicago/Turabian StyleRivero-Segura, Nadia Alejandra, Emmanuel Alejandro Zepeda-Arzate, Selma Karime Castillo-Vazquez, Patrick Fleischmann-delaParra, Jessica Hernández-Pineda, Edgar Flores-Soto, Paola García-delaTorre, Edgar Antonio Estrella-Parra, and Juan Carlos Gomez-Verjan. 2024. "Exploring the Geroprotective Potential of Nutraceuticals" Nutrients 16, no. 17: 2835. https://doi.org/10.3390/nu16172835
APA StyleRivero-Segura, N. A., Zepeda-Arzate, E. A., Castillo-Vazquez, S. K., Fleischmann-delaParra, P., Hernández-Pineda, J., Flores-Soto, E., García-delaTorre, P., Estrella-Parra, E. A., & Gomez-Verjan, J. C. (2024). Exploring the Geroprotective Potential of Nutraceuticals. Nutrients, 16(17), 2835. https://doi.org/10.3390/nu16172835








