Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Clinical Outcome of Atopic Dermatitis in Children
Abstract
1. Introduction
2. Materials and Methods
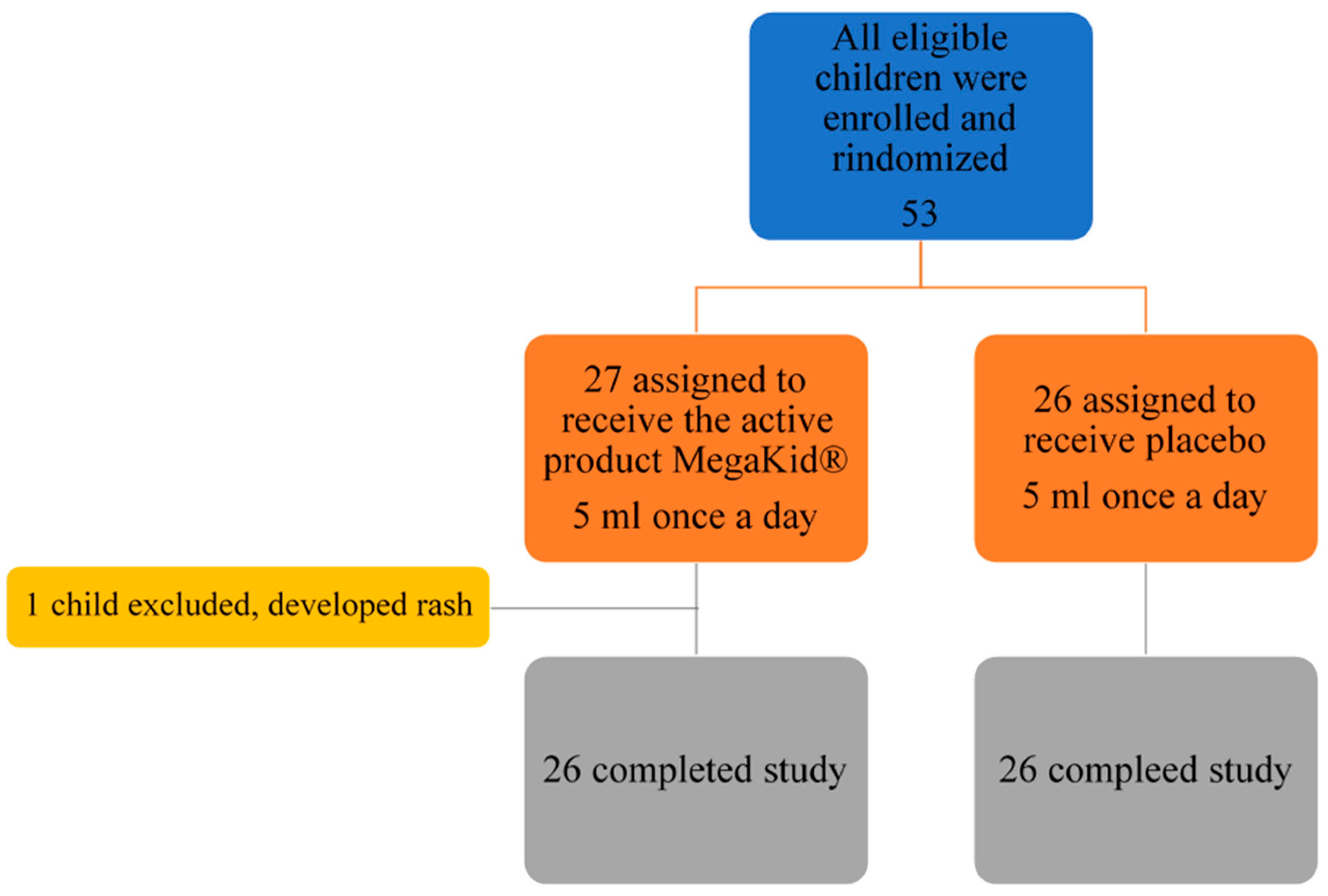
2.1. Participants
2.2. Study Product and Administration
2.3. Outcomes
2.4. Sample Size
2.5. Statistics
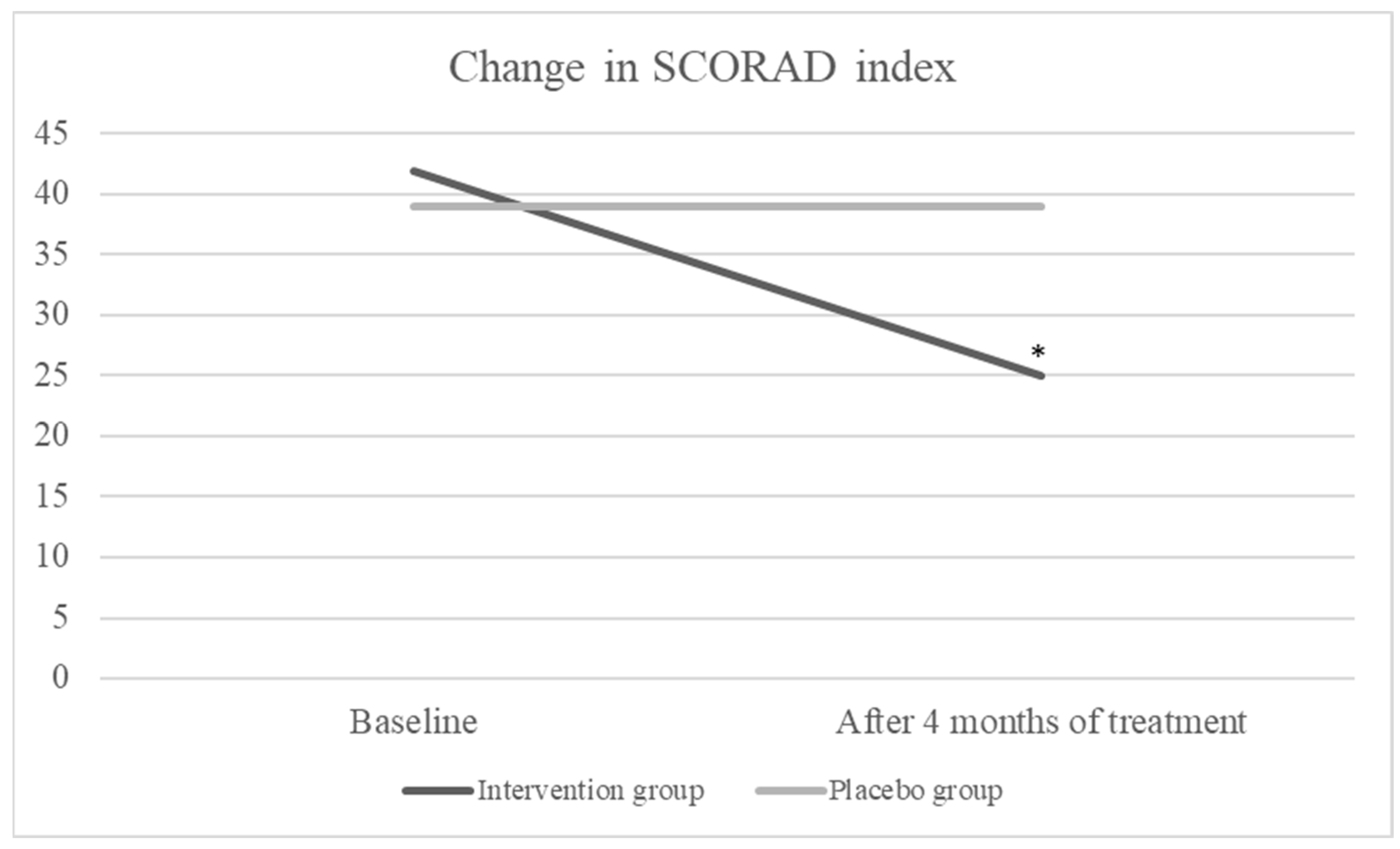
3. Results
Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, B.; Moazemi, M.; Eslami, N.; Jamshidi, E.; Mir, M.; Mohebbi, R.; Esmaily, H. Evaluating the Effect of Eicosapentaenoic Acid in Children With Atopic Dermatitis: A Randomized Triple-Blind Clinical Trial. J. Pediatr. Pharmacol. Ther. 2023, 28, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio: Health Implications. Oléagineux Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Mo. Med. 2021, 118, 453–459. [Google Scholar] [PubMed]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Mokos, Z.B. Omega-3 versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Yen, C.H.; Dai, Y.S.; Yang, Y.H.; Wang, L.C.; Lee, J.H.; Chiang, B.L. Linoleic Acid Metabolite Levels and Transepidermal Water Loss in Children with Atopic Dermatitis. Ann. Allergy Asthma Immunol. 2008, 100, 66–73. [Google Scholar] [CrossRef]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of Polyunsaturated Fatty Acids by Skin Epidermal Enzymes: Generation of Antiinflammatory and Antiproliferative Metabolites. Am. J. Clin. Nutr. 2000, 71, 361S–366S. [Google Scholar] [CrossRef] [PubMed]
- Strannegård, I.L.; Svennerholm, L.; Strannegård, Ö. Essential Fatty Acids in Serum Lecithin of Children with Atopic Dermatitis and in Umbilical Cord Serum of Infants with High or Low IgE Levels. Int. Arch. Allergy Immunol. 1987, 82, 422–423. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kwon, H.H.; Hong, J.S.; Yoon, J.Y.; Park, M.S.; Jang, M.Y.; Suh, D.H. Effect of Dietary Supplementation with Omega-3 Fatty Acid and Gamma-Linolenic Acid on Acne Vulgaris: A Randomised, Double-Blind, Controlled Trial. Acta Derm. Venereol. 2014, 94, 521–525. [Google Scholar] [CrossRef]
- Muggli, R. Systemic Evening Primrose Oil Improves the Biophysical Skin Parameters of Healthy Adults. Int. J. Cosmet. Sci. 2005, 27, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, S.; Newman, S.A. Review of Evidence for Dietary Influences on Atopic Dermatitis. Ski. Ther. Lett. 2014, 19, 5–7. [Google Scholar]
- Huang, X.W.; Pang, S.W.; Yang, L.Z.; Han, T.; Chen, J.M.; Huang, C.W.; Liao, L.; Xie, P.J. TNFSF14 Mediates the Impact of Docosahexaenoic Acid on Atopic Dermatitis: A Mendelian Randomization Study. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Reese, I.; Werfel, T. Do Long-Chain Omega-3 Fatty Acids Protect from Atopic Dermatitis? JDDG J. Ger. Soc. Dermatol. 2015, 13, 879–885. [Google Scholar] [CrossRef]
- Abdelnour, A.M.; Shareef, S.J.; Kreuser, K.; Ashack, K. 319 Effects of Omega-3 Fatty Acid Supplementation on Atopic Dermatitis. Br. J. Dermatol. 2023, 189, e62. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Barbarot, S.; Bieber, T.; Brough, H.A.; Pinton, P.C.; Christen-Zaech, S.; Deleuran, M.; et al. First Update of the Living European Guideline (EuroGuiDerm) on Atopic Eczema. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1283–e1287. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Rajka, G. Diagnostic Features of Atopic Dermatitis. Acta Derm. Venereol. 1980, 60, 44–47. [Google Scholar] [CrossRef]
- Kunz, B.; Oranje, A.P.; Labréze, L.; Stabler, J.F.; Ring, J.; Taïeb, A. Clinical Validation and Guidelines for the Scorad Index: Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1997, 195, 10–19. [Google Scholar] [CrossRef]
- Stalder, J.F.; Barbarot, S.; Wollenberg, A.; Holm, E.A.; De Raeve, L.; Seidenari, S.; Oranje, A.; Deleuran, M.; Cambazard, F.; Svensson, A.; et al. Patient-Oriented SCORAD (PO-SCORAD): A New Self-Assessment Scale in Atopic Dermatitis Validated in Europe. Allergy: Eur. J. Allergy Clin. Immunol. 2011, 66, 1114–1121. [Google Scholar] [CrossRef]
- Basra, M.K.A.; Edmunds, O.; Salek, M.S.; Finlay, A.Y. Measurement of Family Impact of Skin Disease: Further Validation of the Family Dermatology Life Quality Index (FDLQI). J. Eur. Acad. Dermatol. Venereol. 2008, 22, 813–821. [Google Scholar] [CrossRef]
- Guillemin, F.; Bombardier, C.; Beaton, D. Cross-Cultural Adaptation of Health-Related Quality of Life Measures: Literature Review and Proposed Guidelines. J. Clin. Epidemiol. 1993, 46, 1417–1432. [Google Scholar] [CrossRef] [PubMed]
- Bjørneboe, A.; Smith, A.K.; Bjørneboe, G.A.; Thune, P.O.; Drevon, C.A. Effect of Dietary Supplementation with N-3 Fatty Acids on Clinical Manifestations of Psoriasis. Br. J. Dermatol. 1988, 118, 77–83. [Google Scholar] [CrossRef]
- Bittiner, S.B.; Cartwright, I.; Tucker, W.F.G.; Bleehen, S.S. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet 1988, 331, 378–380. [Google Scholar] [CrossRef]
- Soyland, E.; Funk, J.; Rajka, G.; Sandberg, M.; Thune, P.; Rustad, L.; Helland, S.; Middelfart, K.; Odu, S.; Falk, E.S.; et al. Effect of Dietary Supplementation with Very-Long-Chain n-3 Fatty Acids in Patients with Psoriasis. N. Engl. J. Med. 1993, 328, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarski, M.; Cudowska, B.; Sawicka-Zukowska, M.; Bobrus-Chociej, A. Supplementation with Long Chain Polyunsaturated Fatty Acids in Treatment of Atopic Dermatitis in Children. Postep. Dermatol. I Alergol. 2013, 30, 103–107. [Google Scholar] [CrossRef]
- Lin, Z.; Niu, Y.; Jiang, Y.; Chen, B.; Peng, L.; Mi, T.; Huang, N.; Li, W.; Xu, D.; Chen, R.; et al. Protective Effects of Dietary Fish-Oil Supplementation on Skin Inflammatory and Oxidative Stress Biomarkers Induced by Fine Particulate Air Pollution: A Pilot Randomized, Double-Blind, Placebo-Controlled Trial. Br. J. Dermatol. 2021, 184, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Dölle, S.; Metzger, M.; Rasche, C.; Jungclas, H.; Rühl, R.; Renz, H.; Worm, M. Docosahexaenoic Acid (DHA) Supplementation in Atopic Eczema: A Randomized, Double-Blind, Controlled Trial. Br. J. Dermatol. 2008, 158, 786–792. [Google Scholar] [CrossRef]

| Intervention Group (n = 26) | Placebo Group (n = 26) | |
|---|---|---|
| Age (years), median (range) | 1.8 (1.1–5.9) | 2.3 (1–5.7) |
| Sex, female N (%) | 10 (38.5) | 11 (42.3) |
| Food allergy N (%) | 18 (69.2) | 12 (46.2) |
| SCORAD N (%) | ||
| 1 mild | 0 (0) | 0 (0) |
| 2 moderate | 21 (80.8) | 23 (88.5) |
| 3 severe | 5 (19.2) | 3 (11.5) |
| Corticosteroid use (mg/month), median (range) | 30 (0–100) | 30 (0–100) |
| PO-SCORAD, median (range) | 35 (10–51) | 47 (34–65) |
| Itching, median (range) | 8 (3–10) | 8 (5–10) |
| Sleeping, median (range) | 7 (0–10) | 7 (0–10) |
| Supplementation vitamin D, N (%) | 18 (69.2) | 15 (57.7) |
| Median (Range) | Intervention Group | Placebo Group | p Value |
|---|---|---|---|
| PO-SCORAD baseline | 46 | 47 | 0.293 |
| PO-SCORAD after 4 months of intervention | 35 | 46 | <0.001 * |
| Difference PO-SCORAD | 13 ((−2)–29) | −1 ((−12)–20) | <0.001 * |
| Itching score baseline | 8 (3–10) | 8 (5–10) | 0.653 |
| Itching score after 4 months of intervention | 4 (0–7) | 8 (4–10) | <0.001 * |
| Itching score difference | 3 (0–9) | 0 ((−2)–3) | <0.001 * |
| Sleep disturbance baseline | 7 (0–10) | 7 (0–10) | 0.516 |
| Sleep disturbance after 4-month intervention | 3 (0–6) | 8 (0–10) | 0.002 * |
| Sleep disturbance difference | 3 (0–10) | 0 ((−2)–6) | 0.001 * |
| FLDQI baseline | 17 | 15 | 0.496 |
| FLDQI after 4 months of intervention | 11 | 15 | <0.001 * |
| Difference FLDQI | 5 (1–14) | 0 ((−3)–7) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niseteo, T.; Hojsak, I.; Ožanić Bulić, S.; Pustišek, N. Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Clinical Outcome of Atopic Dermatitis in Children. Nutrients 2024, 16, 2829. https://doi.org/10.3390/nu16172829
Niseteo T, Hojsak I, Ožanić Bulić S, Pustišek N. Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Clinical Outcome of Atopic Dermatitis in Children. Nutrients. 2024; 16(17):2829. https://doi.org/10.3390/nu16172829
Chicago/Turabian StyleNiseteo, Tena, Iva Hojsak, Suzana Ožanić Bulić, and Nives Pustišek. 2024. "Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Clinical Outcome of Atopic Dermatitis in Children" Nutrients 16, no. 17: 2829. https://doi.org/10.3390/nu16172829
APA StyleNiseteo, T., Hojsak, I., Ožanić Bulić, S., & Pustišek, N. (2024). Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Clinical Outcome of Atopic Dermatitis in Children. Nutrients, 16(17), 2829. https://doi.org/10.3390/nu16172829






