Effect of Arthrospira platensis (Spirulina) Fortification on Physicochemical, Nutritional, Bioactive, Textural, and Sensory Properties of Vegan Basil Pesto
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pesto Preparation
2.3. Color and pH Measurement
2.4. Nutrient Composition
2.5. In Vitro Protein Digestibility and Protein Availability
In Vitro Digestion
2.6. Minerals Content
2.7. Bioactive Properties
2.7.1. Bioactive Compounds Extraction
2.7.2. Total Polyphenol Content (TPC)
2.7.3. Total Flavonoids Content (TFC)
2.7.4. DPPH Radical Scavenging Activity
2.7.5. ABTS Radical Scavenging Activity
2.8. Texture Profile Analysis (TPA)
2.9. Semi-Consumer Evaluation
2.10. Statistical Analysis
3. Results
3.1. Color and pH Measurements
3.2. Nutritional Value
3.3. In Vitro Protein Digestibility
3.4. Minerals Content
3.5. Bioactive Properties
Total Polyphenol Content, Total Flavonoids Content, and Antioxidant Activity
3.6. TPA
3.7. Semi-Consumer Evaluation
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Bruno:, A.; Gattuso, A.; Romeo, R.; Santacaterina, S.; Piscopo, A. Functional and Sustainable Application of Natural Antioxidant Extract Recovered from Olive Mill Wastewater on Shelf-Life Extension of “Basil Pesto”. Appl. Sci. 2022, 12, 10965. [Google Scholar] [CrossRef]
- Durazzo, A.; Camilli, E.; Marconi, S.; Lisciani, S.; Gabrielli, P.; Gambelli, L.; Aguzzi, A.; Lucarini, M.; Kiefer, J.; Marletta, L. Nutritional Composition and Dietary Intake of Composite Dishes Traditionally Consumed in Italy. J. Food Compos. Anal. 2019, 77, 115–124. [Google Scholar] [CrossRef]
- Turini, E.; Sarsale, M.; Petri, D.; Totaro, M.; Lucenteforte, E.; Tavoschi, L.; Baggiani, A. Efficacy of Plant Sterol-Enriched Food for Primary Prevention and Treatment of Hypercholesterolemia: A Systematic Literature Review. Foods 2022, 11, 839. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.d.A.A.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional Properties of Bioactive Compounds from Spirulina spp.: Current Status and Future Trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef] [PubMed]
- Sözeri Atik, D.; Gürbüz, B.; Bölük, E.; Palabıyık, İ. Development of Vegan Kefir Fortified with Spirulina platensis. Food Biosci. 2021, 42, 101050. [Google Scholar] [CrossRef]
- Janda-Milczarek, K.; Szymczykowska, K.; Jakubczyk, K.; Kupnicka, P.; Skonieczna-Żydecka, K.; Pilarczyk, B.; Tomza-Marciniak, A.; Ligenza, A.; Stachowska, E.; Dalewski, B. Spirulina Supplements as a Source of Mineral Nutrients in the Daily Diet. Appl. Sci. 2023, 13, 1011. [Google Scholar] [CrossRef]
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients 2022, 14, 676. [Google Scholar] [CrossRef]
- Hussein, A.; Ibrahim, G.; Kamil, M.; El-Shamarka, M.; Mostafa, S.; Mohamed, D. Spirulina-Enriched Pasta as Functional Food Rich in Protein and Antioxidant. Biointerface Res. Appl. Chem. 2021, 11, 14736–14750. [Google Scholar] [CrossRef]
- Rodríguez De Marco, E.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of Spirulina Biomass on the Technological and Nutritional Quality of Bread Wheat Pasta. LWT-Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- Hernández-López, I.; Alamprese, C.; Cappa, C.; Prieto-Santiago, V.; Abadias, M.; Aguiló-Aguayo, I. Effect of Spirulina in Bread Formulated with Wheat Flours of Different Alveograph Strength. Foods 2023, 12, 3724. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis Fortification on Physicochemical, Textural, Antioxidant and Sensory Properties of Yogurt During Fermentation and Storage. LWT-Food Sci. Technol. 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. Üseyin Influence of Incorporated Spirulina Platensis on the Growth of Microflora and Physicochemical Properties of Ayran as a Functional Food. Algal Res. 2019, 44, 101710. [Google Scholar] [CrossRef]
- Ismail, H.A.; El-Sawah, T.H.; Ayyash, M.; Adhikari, B.; Elkot, W.F. Functionalization of Ricotta Cheese with Powder of Spirulina platensis: Physicochemical, Sensory, and Microbiological Properties. Int. J. Food Prop. 2023, 26, 1968–1983. [Google Scholar] [CrossRef]
- Szmejda, K.; Duliński, R.; Byczyński, Ł.; Karbowski, A.; Florczak, T.; Żyła, K. Analysis of the Selected Antioxidant Compounds in Ice Cream Supplemented with Spirulina (Arthrospira platensis) Extract. Biotechnol. Food Sci. 2018, 82, 41–48. [Google Scholar]
- Paula da Silva, S.; Ferreira do Valle, A.; Perrone, D. Microencapsulated Spirulina Maxima Biomass as an Ingredient for the Production of Nutritionally Enriched and Sensorially Well-Accepted Vegan Biscuits. LWT-Food Sci. Technol. 2021, 142, 110997. [Google Scholar] [CrossRef]
- Batista de Oliveira, T.T.; Miranda dos Reis, I.; Bastos de Souza, M.; da Silva Bispo, E.; Fonseca Maciel, L.; Druzian, J.I.; Lordelo Guimarães Tavares, P.P.; de Oliveira Cerqueira, A.; dos Santos Boa Morte, E.; Abreu Glória, M.B.; et al. Microencapsulation of Spirulina Sp. LEB-18 and Its Incorporation in Chocolate Milk: Properties and Functional Potential. LWT-Food Sci. Technol. 2021, 148, 4–11. [Google Scholar] [CrossRef]
- El-Anany, A.M.; Althwab, S.A.; Alhomaid, R.M.; Ali, R.F.M.; Mousa, H.M. Effect of Spirulina (Arthrospira platensis) Powder Addition on Nutritional and Sensory Attributes of Chicken Mortadella. Ital. J. Food Sci. 2023, 35, 1–11. [Google Scholar] [CrossRef]
- Gromek, W.; Kołdej, N.; Kurowski, M.; Majsiak, E. Spirulina (Arthrospira platensis): Antiallergic Agent or Hidden Allergen? A Literature Review. Foods 2024, 13, 1052. [Google Scholar] [CrossRef]
- Prete, V.; Abate, A.C.; Di Pietro, P.; De Lucia, M.; Vecchione, C.; Carrizzo, A. Beneficial Effects of Spirulina Supplementation in the Management of Cardiovascular Diseases. Nutrients 2024, 16, 642. [Google Scholar] [CrossRef]
- Torres-Duran, P.V.; Ferreira-Hermosillo, A.; Juarez-Oropeza, M.A. Antihyperlipemic and Antihypertensive Effects of Spirulina Maxima in an Open Sample of Mexican Population: A Preliminary Report. Lipids Health Dis. 2007, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the Food and Functional Food Industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Fogarasi, M.; Urs, M.J.; Socaciu, M.I.; Ranga, F.; Semeniuc, C.A.; Vodnar, D.C.; Mureșan, V.; Țibulcă, D.; Fogarasi, S.; Socaciu, C. Polyphenols-Enrichment of Vienna Sausages Using Microcapsules Containing Acidic Aqueous Extract of Boletus Edulis Mushrooms. Foods 2024, 13, 979. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Jakubczyk, A. Antioxidant Activity of Predigested Protein Obtained From a Range of Farmed Edible Insects. Int. J. Food Sci. Technol. 2017, 52, 306–312. [Google Scholar] [CrossRef]
- Jorhem, L.; Engman, J.; Collaborators; Arvidsson, B.-M.; Åsman, B.; Åstrand, C.; Gjerstad, K.O.; Haugsnes, J.; Heldal, V.; Holm, K.; et al. Determination of Lead, Cadmium, Zinc, Copper, and Iron in Foods by Atomic Absorption Spectrometry after Microwave Digestion: NMKL1 Collaborative Study. J. AOAC Int. 2000, 83, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Implementing Regulation-EU-2024/771-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg_impl/2024/771/oj (accessed on 14 July 2024).
- Karadeniz, F.; Burdurlu, H.S.; Koca, N.; Soyer, Y. Antioxidant Activity of Selected Fruits and Vegetables Grown in Turkey. Turk. J. Agric. For. 2005, 29, 297–303. [Google Scholar]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Szafrańska, J.; Muszyński, S.; Sołowiej, B. Ocena Właściwości Fizykochemicznych Sosów Serowych Otrzymanych Na Bazie Kazeiny Kwasowej i Oleju Rzepakowego z Dodatkiem Koncentratu Białek Serwatkowych. Przemysł Spożywczy 2019, 1, 38–44. [Google Scholar] [CrossRef]
- Almohtadi, R.M.; Aldarabah, I.T. University Students’ Attitudes toward the Formal Integration of Facebook in Their Education: Investigation Guided by Rogers’ Attributes of Innovation. World J. Educ. 2021, 11, 20. [Google Scholar] [CrossRef]
- Arwanto, V.; Buschle-Diller, G.; Mukti, Y.P.; Dewi, A.D.R.; Mumpuni, C.; Purwanto, M.G.M.; Sukweenadhi, J. The State of Plant-Based Food Development and Its Prospects in the Indonesia Market. Heliyon 2022, 8, e11062. [Google Scholar] [CrossRef]
- Hassoun, A.; Cropotova, J.; Trif, M.; Rusu, A.V.; Bobiş, O.; Nayik, G.A.; Jagdale, Y.D.; Saeed, F.; Afzaal, M.; Mostashari, P.; et al. Consumer Acceptance of New Food Trends Resulting From the Fourth Industrial Revolution Technologies: A Narrative Review of Literature and Future Perspectives. Front. Nutr. 2022, 9, 972154. [Google Scholar] [CrossRef] [PubMed]
- Morsy, O.M.; Sharoba, A.M.; El-Desouky, A.I.; Bahlol HE, M.; Abd El Mawla, E.M. Production and Evaluation of Some Extruded Food Products Using Spirulina Algae. Ann. Agric. Sci. Moshtohor 2014, 52, 495–510. [Google Scholar] [CrossRef]
- Carnauba, R.A.; Baptistella, A.B.; Paschoal, V.; Hübscher, G.H. Diet-Induced Low-Grade Metabolic Acidosis and Clinical Outcomes: A Review. Nutrients 2017, 9, 538. [Google Scholar] [CrossRef]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential Application of Microalga Spirulina Platensis as a Protein Source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Sánchez-Zurano, A.; Villaró, S.; Morillas-España, A.; Acién, G. Industrial Production of Spirulina as a Protein Source for Bioactive Peptide Generation. Trends Food Sci. Technol. 2021, 116, 176–185. [Google Scholar] [CrossRef]
- Bueschke, M.; Gramza-Michałowska, A.; Kubiak, T.; Kulczyński, B. Alternatywne Źródła Białka w Żywieniu Człowieka. Zesz. Nauk. SGGW Warszawie-Probl. Rol. Swiat. 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Ramírez-Rodrigues, M.M.; Estrada-Beristain, C.; Metri-Ojeda, J.; Pérez-Alva, A.; Baigts-Allende, D.K. Spirulina Platensis Protein as Sustainable Ingredient for Nutritional Food Products Development. Sustainability 2021, 13, 6849. [Google Scholar] [CrossRef]
- Cader, P.; Lesiów, T. Weganizm i Wegetarianizm Jako Diety We Współczesnym Społeczeństwie Konsumpcyjnym. Nauk. Inżynierskie Technol. 2021, 37, 9–33. [Google Scholar] [CrossRef]
- Kumar, S.B.; Arnipalli, S.R.; Mehta, P.; Carrau, S.; Ziouzenkova, O. Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies. Nutrients 2022, 14, 2976. [Google Scholar] [CrossRef]
- Sordini, B.; Urbani, S.; Esposto, S.; Selvaggini, R.; Daidone, L.; Veneziani, G.; Servili, M.; Taticchi, A. Evaluation of the Effect of an Olive Phenolic Extract on the Secondary Shelf Life of a Fresh Pesto. Antioxidants 2024, 13, 128. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Bürck, M.; Fratelli, C.; Assis, M.; Braga, A.R.C. Naturally Colored Ice Creams Enriched with C-Phycocyanin and Spirulina Residual Biomass: Development of a Fermented, Antioxidant, Tasty and Stable Food Product. Fermentation 2024, 10, 304. [Google Scholar] [CrossRef]
- Barakat, E.H.; El-Kewaisny, N.M.; Salama, A.A. Chemical and Nutritional Evaluation of Fortified Biscuits with Dried Spirulina Algae. J. Food Dairy Sci. 2016, 7, 167–177. [Google Scholar] [CrossRef]
- Turrini, F.; Farinini, E.; Leardi, R.; Grasso, F.; Orlandi, V.; Boggia, R. A Preliminary Color Study of Different Basil-Based Semi-Finished Products during Their Storage. Molecules 2022, 27, 2059. [Google Scholar] [CrossRef]
- Boyanova, P.; Gradinarska, D.; Dobreva, V.; Panayotov, P.; Momchilova, M.; Zsivanovits, G. Effect of Spirulina platensis on the Quality and Antioxidants Characteristics of Ice Cream. BIO Web Conf. 2022, 45, 1–6. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef]
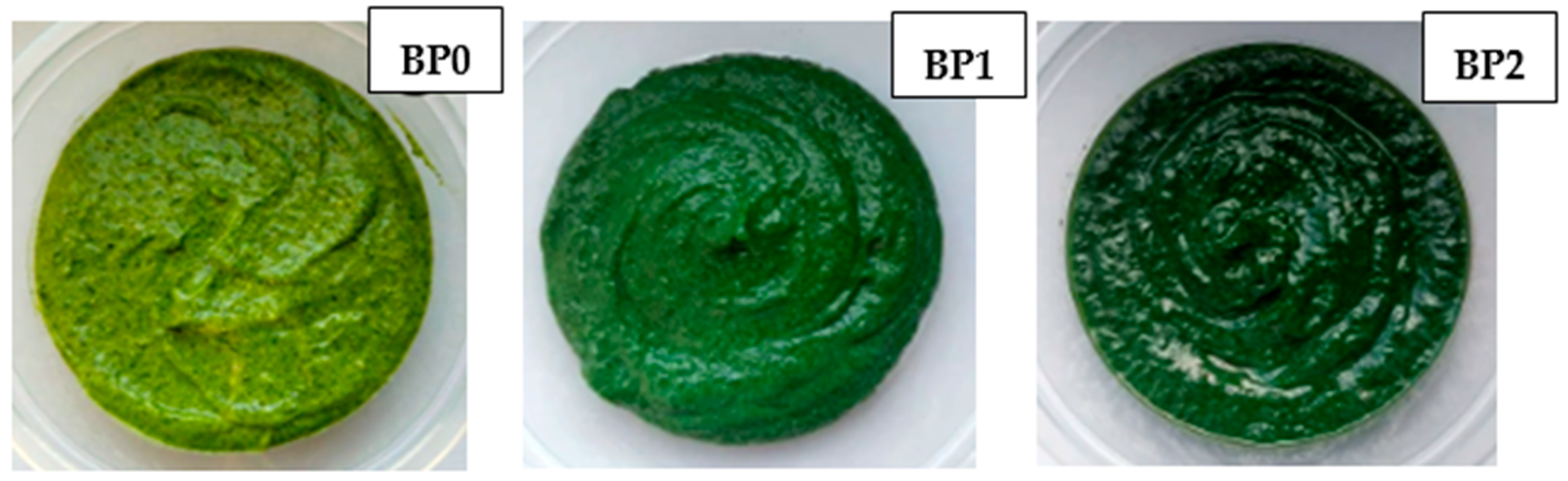
| Sample | Grape Seed Oil | Basil | Cashew Nuts | Yeast Flakes | Lemon Juice | Salt | Spirulina | Granulated Garlic |
|---|---|---|---|---|---|---|---|---|
| [g/100 g] | ||||||||
| BP0 | 35 | 31 | 25 | 5.7 | 2 | 1 | - | 0.3 |
| BP1 | 35 | 30 | 25 | 5.7 | 2 | 1 | 1 | 0.3 |
| BP2 | 35 | 29 | 25 | 5.7 | 2 | 1 | 2 | 0.3 |
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| BP0 | 43.03 ± 0.58 a * | −5.75 ± 0.33 a | 19.57 ± 0.32 a | - |
| BP1 | 36.92 ± 0.39 b | −5.79 ± 0.24 a | 13.19 ± 0.26 b | 8.83 |
| BP2 | 34.77 ± 0.83 c | −5.99 ± 0.24 a | 10.80 ± 0.19 c | 12.05 |
| Sample | Protein | Carbohydrates | Fat | Ash | Moisture | Energy Value [kcal/100 g] |
|---|---|---|---|---|---|---|
| g/100 g Fresh Pesto | ||||||
| BP0 | 6.47 ± 0.25 b * | 9.89 ± 0.47 a | 39.32 ± 0.17 b | 1.83 ± 0.10 a | 43.02 ± 1.14 a | 419.33 ± 4.42 a |
| BP1 | 8.73 ± 0.38 a | 6.67 ± 0.52 b | 40.46 ± 0.13 a | 1.99 ± 0.09 a | 42.21 ± 0.92 a | 425.72 ± 0.03 a |
| BP2 | 9.13 ± 0.20 a | 6.97 ± 0.43 b | 40.43 ± 0.21 a | 1.75 ± 0.02 a | 41.72 ± 0.85 a | 428.26 ± 0.99 a |
| Sample | PD [%] | PA [g/100 g] |
|---|---|---|
| BP0 | 79.89 ± 0.33 a * | 5.17 ± 0.22 b |
| BP1 | 78.75 ± 0.12 a | 6.88 ± 0.29 a |
| BP2 | 77.88 ± 1.12 a | 7.11 ± 0.02 a |
| Sample | Ca | Mg | K | Fe | P |
|---|---|---|---|---|---|
| mg/100 g Fresh Pesto | |||||
| BP0 | 78.66 ± 2.88 b * | 81.14 ± 2.68 b | 342.32 ± 6.18 c | 1.98 ± 0.09 c | 177.71 ± 3.12 b |
| BP1 | 97.64 ± 0.65 a | 87.04 ± 2.63 ab | 382.45 ± 4.85 b | 4.09 ± 0.12 b | 185.51 ± 5.26 b |
| BP2 | 98.47 ± 3.84 a | 91.63 ± 0.44 a | 408.77 ± 2.18 a | 6.15 ± 0.07 a | 234.46 ± 2.62 a |
| Sample | TPC [mg GAE/100 g] | TFC [mg EPI/100 g] | DPPH [μM Trolox/g] | ABTS [μM Trolox/g] |
|---|---|---|---|---|
| BP0 | 172.96 ± 4.6 c * | 21.50 ± 0.13 c | 290.97 ± 14.71 c | 439.61 ± 9.76 c |
| BP1 | 206.32 ± 6.34 b | 24.82 ± 0.28 b | 353.66 ± 5.11 b | 459.00 ± 6.68 b |
| BP2 | 259.24 ± 9.30 a | 29.88 ± 0.8 a | 392.41 ± 13.58 a | 484.56 ± 2.16 a |
| Sample | Hardness [N] | Adhesiveness | Cohesion | Springiness |
|---|---|---|---|---|
| BP0 | 70.46 ± 1.50 c * | −47.92 ± 1.28 a | 0.08 ± 0.01 a | 13.28 ± 0.42 a |
| BP1 | 76.31 ± 2.25 b | −53.87 ± 2.87 a | 0.08 ± 0.01 a | 12.90 ± 0.49 a |
| BP2 | 83.62 ± 1.14 a | −51.03 ± 5.24 a | 0.08 ± 0.01 a | 13.04 ± 0.40 a |
| Sample | Evaluation Parameters | Overall Rating | ||||
|---|---|---|---|---|---|---|
| Overall Appearance | Color | Taste | Aroma | Consistency | ||
| BP0 | 4.26 ± 0.70 b * | 4.09 ± 0.78 b | 4.17 ± 0.71 b | 4.23 ± 0.60 a | 4.34 ± 0.76 ab | 4.22 |
| BP1 | 4.77 ± 0.43 a | 4.49 ± 0.61 a | 4.57 ± 0.50 a | 4.26 ± 0.61 a | 4.49 ± 0.66 a | 4.56 |
| BP2 | 4.00 ± 0.64 b | 3.97 ± 0.62 b | 4.00 ± 0.69 b | 4.09 ± 0.61 a | 4.03 ± 0.66 b | 4.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgórska-Kryszczuk, I. Effect of Arthrospira platensis (Spirulina) Fortification on Physicochemical, Nutritional, Bioactive, Textural, and Sensory Properties of Vegan Basil Pesto. Nutrients 2024, 16, 2825. https://doi.org/10.3390/nu16172825
Podgórska-Kryszczuk I. Effect of Arthrospira platensis (Spirulina) Fortification on Physicochemical, Nutritional, Bioactive, Textural, and Sensory Properties of Vegan Basil Pesto. Nutrients. 2024; 16(17):2825. https://doi.org/10.3390/nu16172825
Chicago/Turabian StylePodgórska-Kryszczuk, Izabela. 2024. "Effect of Arthrospira platensis (Spirulina) Fortification on Physicochemical, Nutritional, Bioactive, Textural, and Sensory Properties of Vegan Basil Pesto" Nutrients 16, no. 17: 2825. https://doi.org/10.3390/nu16172825
APA StylePodgórska-Kryszczuk, I. (2024). Effect of Arthrospira platensis (Spirulina) Fortification on Physicochemical, Nutritional, Bioactive, Textural, and Sensory Properties of Vegan Basil Pesto. Nutrients, 16(17), 2825. https://doi.org/10.3390/nu16172825






