LDL-Cholesterol-Lowering Effects of a Dietary Supplement Containing Onion and Garlic Extract Used in Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics, Approval, and Consent
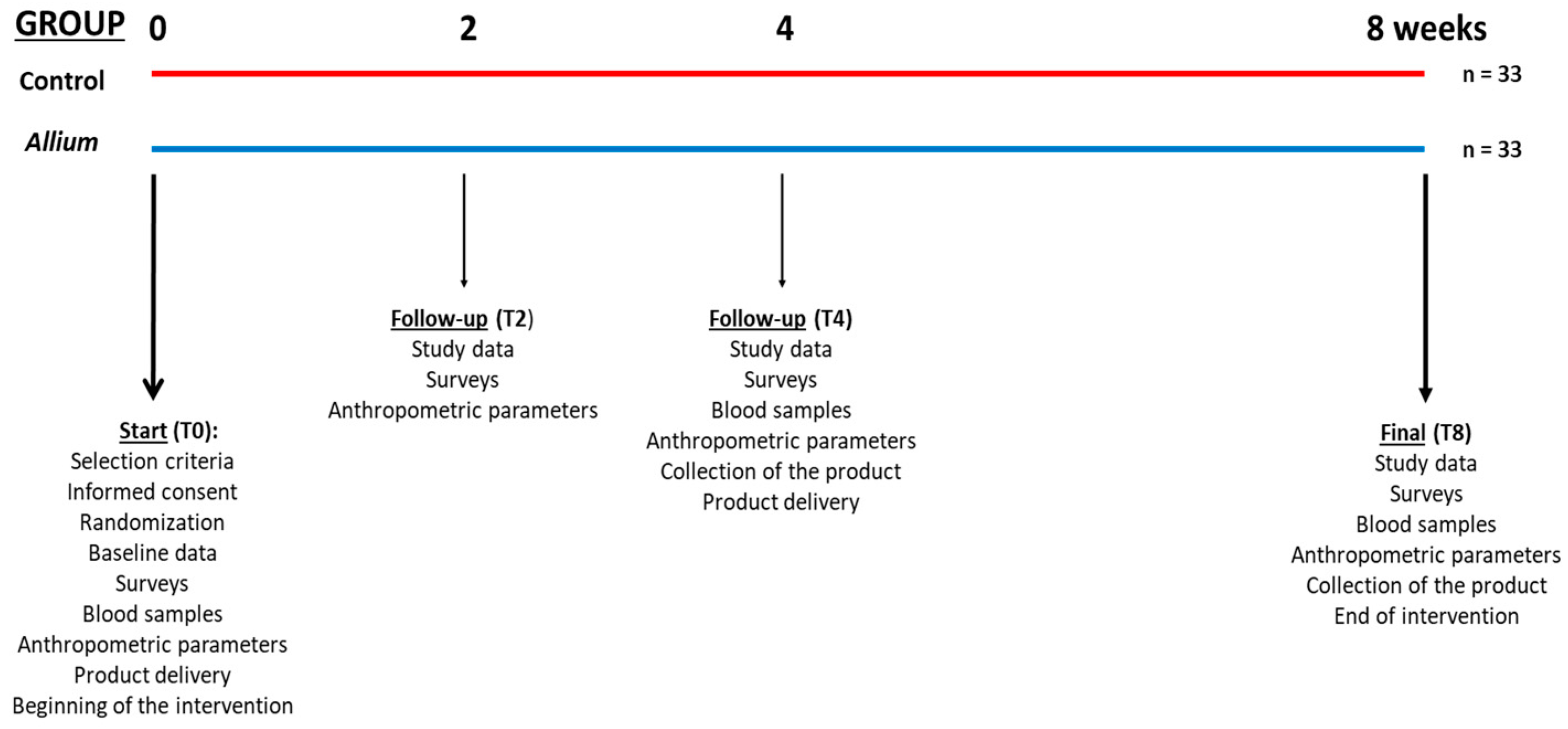
2.2. Subjects and Study Design
2.3. Intervention
2.4. Clinical Parameters
2.5. Statistical Analysis
3. Results
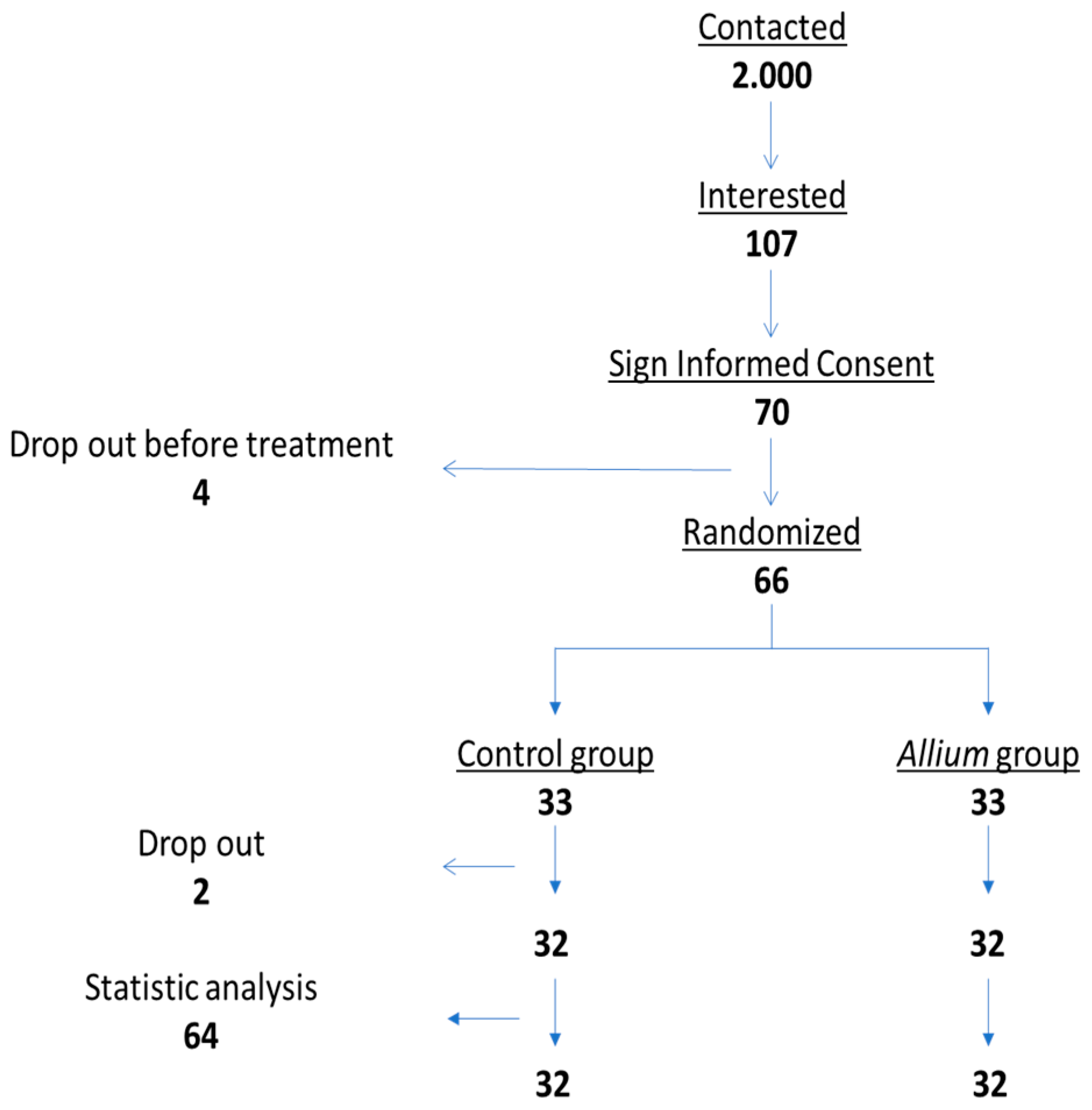
3.1. Study Population
3.2. Safety Assessment
3.3. Biochemical Outcomes
3.4. Habits, Dietary and Anthropometric Changes
3.5. Oxidative and Inflammatory Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badawy, M.; Naing, L.; Johar, S.; Ong, S.; Rahman, H.A.; Tengah, D.; Chong, C.L.; Tuah, N.A.A. Evaluation of cardiovascular diseases risk calculators for CVDs prevention and management: Scoping review. BMC Public Health 2022, 22, 1742. [Google Scholar] [CrossRef]
- Sacramento-Pacheco, J.; Sanchez-Gomez, M.B.; Gomez-Salgado, J.; Novo-Munoz, M.M.; Duarte-Climents, G. Prevalence of Cardiovascular Risk Factors in Spain: A Systematic Review. J. Clin. Med. 2023, 12, 6944. [Google Scholar] [CrossRef]
- Morrison, A.M.; Sullivan, A.E.; Aday, A.W. Atherosclerotic Disease: Pathogenesis and Approaches to Management. Med. Clin. N. Am. 2023, 107, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Tao, T.; Wang, L.Y.; Sun, J.Y.; Wu, C.J.; Zou, W.J. Health benefits of spices in individuals with chemotherapeutic drug-induced cardiotoxicity. Curr. Opin. Pharmacol. 2022, 63, 102187. [Google Scholar] [CrossRef] [PubMed]
- Magrys, A.; Olender, A.; Tchorzewska, D. Antibacterial properties of Allium sativum L. against the most emerging multidrug-resistant bacteria and its synergy with antibiotics. Arch. Microbiol. 2021, 203, 2257–2268. [Google Scholar] [CrossRef]
- Garcia-Garcia, J.; Gracian, C.; Banos, A.; Guillamon, E.; Galvez, J.; Rodriguez-Nogales, A.; Fonolla, J. Beneficial Effects of Daily Consumption of Garlic and Onion Extract Concentrate on Infectious Respiratory Diseases in Elderly Resident Volunteers. Nutrients 2023, 15, 2308. [Google Scholar] [CrossRef]
- Yeh, Y.Y.; Yeh, S.M. Garlic reduces plasma lipids by inhibiting hepatic cholesterol and triacylglycerol synthesis. Lipids 1994, 29, 189–193. [Google Scholar] [CrossRef]
- Gebhardt, R. Amplification of palmitate-induced inhibition of cholesterol biosynthesis in cultured rat hepatocytes by garlic-derived organosulfur compounds. Phytomedicine 1995, 2, 29–34. [Google Scholar] [CrossRef]
- Gebhardt, R.; Beck, H. Differential inhibitory effects of garlic-derived organosulfur compounds on cholesterol biosynthesis in primary rat hepatocyte cultures. Lipids 1996, 31, 1269–1276. [Google Scholar] [CrossRef]
- Liu, L.; Yeh, Y.Y. Inhibition of cholesterol biosynthesis by organosulfur compounds derived from garlic. Lipids 2000, 35, 197–203. [Google Scholar] [CrossRef]
- Liu, L.; Yeh, Y.Y. S-alk(en)yl cysteines of garlic inhibit cholesterol synthesis by deactivating HMG-CoA reductase in cultured rat hepatocytes. J. Nutr. 2002, 132, 1129–1134. [Google Scholar] [CrossRef]
- Malekpour-Dehkordi, Z.; Javadi, E.; Doosti, M.; Paknejad, M.; Nourbakhsh, M.; Yassa, N.; Gerayesh-Nejad, S.; Heshmat, R. S-Allylcysteine, a garlic compound, increases ABCA1 expression in human THP-1 macrophages. Phytother. Res. 2013, 27, 357–361. [Google Scholar] [CrossRef]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Gonçalves, D.C. Reverse Cholesterol Transport: Molecular Mechanisms and the Non-medical Approach to Enhance HDL Cholesterol. Front. Physiol. 2019, 9, 526. [Google Scholar] [CrossRef]
- Murugavel, P.; Pari, L. Diallyl tetrasulfide protects cadmium-induced alterations in lipids and plasma lipoproteins in rats. Nutr. Res. 2007, 27, 356–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Ding, M.; Su, G.; Zhao, Y. Anti-obesity effect of garlic oil on obese rats via Shenque point administration. J. Ethnopharmacol. 2019, 231, 486–493. [Google Scholar] [CrossRef]
- Yang, C.; Li, L.; Yang, L.; Lu, H.; Wang, S.; Sun, G. Anti-obesity and Hypolipidemic effects of garlic oil and onion oil in rats fed a high-fat diet. Nutr. Metab. 2018, 15, 43. [Google Scholar] [CrossRef]
- Mohammed Basheeruddin Asdaq, S.; Yasmin, F.; Alsalman, A.J.; Al Mohaini, M.; Kamal, M.; Al Hawaj, M.A.; Alsalman, K.J.; Imran, M.; Sreeharsha, N. Obviation of dyslipidemia by garlic oil and its organosulfur compound, diallyl disulphide, in experimental animals. Saudi J. Biol. Sci. 2022, 29, 2520–2525. [Google Scholar] [CrossRef]
- Serrano, J.C.E.; Castro-Boque, E.; Garcia-Carrasco, A.; Moran-Valero, M.I.; Gonzalez-Hedstrom, D.; Bermudez-Lopez, M.; Valdivielso, J.M.; Espinel, A.E.; Portero-Otin, M. Antihypertensive Effects of an Optimized Aged Garlic Extract in Subjects with Grade I Hypertension and Antihypertensive Drug Therapy: A Randomized, Triple-Blind Controlled Trial. Nutrients 2023, 15, 3691. [Google Scholar] [CrossRef]
- Watts, G.F.; Catapano, A.L.; Masana, L.; Zambon, A.; Pirillo, A.; Tokgozoglu, L. Hypercholesterolemia and cardiovascular disease: Focus on high cardiovascular risk patients. Atheroscler. Suppl. 2020, 42, e30–e34. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Pryanishnikov, V.V.; Kunnova, L.M.; Rabinovich, Y.A.; Martirosyan, D.M.; Orekhov, A.N. The effects of time-released garlic powder tablets on multifunctional cardiovascular risk in patients with coronary artery disease. Lipids Health Dis. 2010, 9, 119. [Google Scholar] [CrossRef]
- Anaeigoudari, A.; Safari, H.; Khazdair, M.R. Effects of Nigella sativa, Camellia sinensis, and Allium sativum as food additives on metabolic disorders, a literature review. Front. Pharmacol. 2021, 12, 762182. [Google Scholar] [CrossRef]
- Szulinska, M.; Kregielska-Narozna, M.; Swiatek, J.; Stys, P.; Kuznar-Kaminska, B.; Jakubowski, H.; Walkowiak, J.; Bogdanski, P. Garlic extract favorably modifies markers of endothelial function in obese patients-randomized double blind placebo-controlled nutritional intervention. Biomed. Pharmacother. 2018, 102, 792–797. [Google Scholar] [CrossRef]
- Ried, K.; Toben, C.; Fakler, P. Effect of garlic on serum lipids: An updated meta-analysis. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef]
- Huang, W.; Tang, G.; Zhang, L.; Tao, J.; Wei, Z. Effect of onion on blood lipid profile: A meta-analysis of randomized controlled trials. Food Sci. Nutr. 2021, 9, 3563–3572. [Google Scholar] [CrossRef]
- Guan, L.; Chung, H.Y.; Su, Y.; Jiao, R.; Peng, C.; Chen, Z.Y. Hypocholesterolemic activity of onion is mediated by enhancing excretion of fecal sterols in hamsters. Food Funct. 2010, 1, 84–89. [Google Scholar] [CrossRef]
- Ige, S.F.; Akhigbe, R.E. Common onion (Allium cepa) extract reverses cadmium-induced organ toxicity and dyslipidaemia via redox alteration in rats. Pathophysiology 2013, 20, 269–274. [Google Scholar] [CrossRef]
- Campos, K.E.; Diniz, Y.S.; Cataneo, A.C.; Faine, L.A.; Alves, M.J.; Novelli, E.L. Hypoglycaemic and antioxidant effects of onion, Allium cepa: Dietary onion addition, antioxidant activity and hypoglycaemic effects on diabetic rats. Int. J. Food Sci. Nutr. 2003, 54, 241–246. [Google Scholar] [CrossRef]
- Gardner, C.D.; Lawson, L.D.; Block, E.; Chatterjee, L.M.; Kiazand, A.; Balise, R.R.; Kraemer, H.C. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: A randomized clinical trial. Arch. Intern. Med. 2007, 167, 346–353. [Google Scholar] [CrossRef]
- van Doorn, M.B.; Espirito Santo, S.M.; Meijer, P.; Kamerling, I.M.; Schoemaker, R.C.; Dirsch, V.; Vollmar, A.; Haffner, T.; Gebhardt, R.; Cohen, A.F.; et al. Effect of garlic powder on C-reactive protein and plasma lipids in overweight and smoking subjects. Am. J. Clin. Nutr. 2006, 84, 1324–1329. [Google Scholar] [CrossRef]
- Atkin, M.; Laight, D.; Cummings, M.H. The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo controlled trial. J. Diabetes Complicat. 2016, 30, 723–727. [Google Scholar] [CrossRef]
- Wu, Y.R.; Li, L.; Sun, X.C.; Wang, J.; Ma, C.Y.; Zhang, Y.; Qu, H.L.; Xu, R.X.; Li, J.J. Diallyl disulfide improves lipid metabolism by inhibiting PCSK9 expression and increasing LDL uptake via PI3K/Akt-SREBP2 pathway in HepG2 cells. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 322–332. [Google Scholar] [CrossRef]
- Vezza, T.; Garrido-Mesa, J.; Diez-Echave, P.; Hidalgo-Garcia, L.; Ruiz-Malagon, A.J.; Garcia, F.; Sanchez, M.; Toral, M.; Romero, M.; Duarte, J.; et al. Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Attenuates Metabolic Alterations in Mice Fed a High-Fat Diet through Its Anti-Inflammatory and Prebiotic Properties. Nutrients 2021, 13, 2595. [Google Scholar] [CrossRef]
- Ried, K. Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: A review and meta-analysis. Exp. Ther. Med. 2020, 19, 1472–1478. [Google Scholar] [CrossRef]
- Wang, H.P.; Yang, J.; Qin, L.Q.; Yang, X.J. Effect of garlic on blood pressure: A meta-analysis. J. Clin. Hypertens. 2015, 17, 223–231. [Google Scholar] [CrossRef]
- Castro, C.; Lorenzo, A.G.; Gonzalez, A.; Cruzado, M. Garlic components inhibit angiotensin II-induced cell-cycle progression and migration: Involvement of cell-cycle inhibitor p27(Kip1) and mitogen-activated protein kinase. Mol. Nutr. Food Res. 2010, 54, 781–787. [Google Scholar] [CrossRef]
- Al-Qattan, K.K.; Khan, I.; Alnaqeeb, M.A.; Ali, M. Thromboxane-B2, prostaglandin-E2 and hypertension in the rat 2-kidney 1-clip model: A possible mechanism of the garlic induced hypotension. Prostaglandins Leukot. Essent. Fatty Acids 2001, 64, 5–10. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Challa, O.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Almutiri, A.H.; Alshammari, M.S. Cytoprotective Potential of Aged Garlic Extract (AGE) and Its Active Constituent, S-allyl-l-cysteine, in Presence of Carvedilol during Isoproterenol-Induced Myocardial Disturbance and Metabolic Derangements in Rats. Molecules 2021, 26, 3203. [Google Scholar] [CrossRef]
- Sharifi, A.M.; Darabi, R.; Akbarloo, N. Investigation of antihypertensive mechanism of garlic in 2K1C hypertensive rat. J. Ethnopharmacol. 2003, 86, 219–224. [Google Scholar] [CrossRef]
- Al-Qattan, K.K.; Thomson, M.; Al-Mutawa’a, S.; Al-Hajeri, D.; Drobiova, H.; Ali, M. Nitric oxide mediates the blood-pressure lowering effect of garlic in the rat two-kidney, one-clip model of hypertension. J. Nutr. 2006, 136, 774S–776S. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Ho, X.L.; Tsen, S.Y.; Ng, M.Y.; Lee, W.N.; Low, A.; Loke, W.M. Aged Garlic Supplement Protects Against Lipid Peroxidation in Hypercholesterolemic Individuals. J. Med. Food 2016, 19, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Ide, N.; Lau, B.H. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J. Nutr. 2001, 131, 1020S–1026S. [Google Scholar] [CrossRef] [PubMed]
- Morihara, N.; Hino, A.; Miki, S.; Takashima, M.; Suzuki, J.I. Aged garlic extract suppresses inflammation in apolipoprotein E-knockout mice. Mol. Nutr. Food Res. 2017, 61, 1700308. [Google Scholar] [CrossRef]
- Kim, J.; Cha, Y.J.; Lee, K.H.; Park, E. Effect of onion peel extract supplementation on the lipid profile and antioxidative status of healthy young women: A randomized, placebo-controlled, double-blind, crossover trial. Nutr. Res. Pract. 2013, 7, 373–379. [Google Scholar] [CrossRef] [PubMed]
| Control Group (n = 33) | Treatment Group (n = 33) | |
|---|---|---|
| Age (years) | 39.3 (±11.7) | 41.5 (±13.1) |
| Gender | ||
| Male | 18 (54.5%) | 16 (48.5%) |
| Female | 15 (45.5%) | 17 (51.5%) |
| Tobacco | ||
| Non-smoker | 19 (57.6%) | 19 (57.6%) |
| Smoker | 5 (15.2%) | 7 (21.2%) |
| Former smoker | 9 (27.3%) | 7 (21.2%) |
| Alcohol | ||
| No drinker | 8 (24.2%) | 9 (27.3%) |
| Drinker | 25 (75.8%) | 24 (72.7%) |
| Physical activity | ||
| Sedentary | 1 (3%) | 1 (3%) |
| Daily homework | 10 (30.3%) | 13 (39.4%) |
| Regular activity | 14 (42.4%) | 15 (45.5%) |
| Gymnasium | 8 (24.2%) | 4 (12.1%) |
| Weight (kg) | 76.4 (±18.3) | 73.3 (±16.2) |
| BMI (kg/m2) | 25.5 (±4.5) | 26.4 (±4.4) |
| Abdominal perimeter (cm) | 80.2 (±13.2) | 83.2 (±15.0) |
| Systolic pressure (mmHg) | 107.5(±12.8) | 110.0 (±13.1) |
| Diastolic pressure (mmHg) | 75.8 (±9.6) | 77.1 (±9.5) |
| LDL cholesterol (mg/dL) | 115.4 (±26.2) | 122.9 (±29.1) |
| Total cholesterol (mg/dL) | 213.2 (±36.1) | 228.9 (±37.5) |
| HDL cholesterol (mg/dL) | 57.8 (±18.2) | 59.5 (±15.3) |
| Triglycerides (mg/dL) | 94.5 (±52.7) | 106.3 (±59.1) |
| Glucose (mg/dL) | 95.2 (±13.9) | 92.5 (±10.9) |
| Group | T0 | T2 | T4 | T8 | p Treatment:Time | |
|---|---|---|---|---|---|---|
| LDL cholesterol (mg/dL) | Control | 115.4 ± 26.7 | 115.9 ± 24.3 | 118.7 ± 30.9 | 118.5 ± 31.4 | 0.006 |
| Treatment | 125.0 ± 27.1 | 123.0 ± 26.5 | 120.4 ± 27.6 * | 117.0 ± 27.0 **,† | ||
| Total cholesterol (mg/dL) | Control | 213.7 ± 36.6 | 215.7 ± 34.9 | 218.1 ± 43.6 | 217.5 ± 42.2 | 0.009 |
| Treatment | 231.9 ± 33.8 a | 225.9 ± 34.7 | 220.4 ± 36.3 ** | 220.2 ± 34.4 ** | ||
| HDL cholesterol (mg/dL) | Control | 58.3 ± 18.3 | 60.1 ± 17.3 * | 60.8 ± 17.6 * | 60.9 ± 18.7 * | 0.227 |
| Treatment | 60.3 ± 14.7 | 60.3 ± 14.6 | 60.0 ± 14.4 | 59.7 ± 16.3 | ||
| Triglycerides (mg/dL) | Control | 88.7 ± 31.2 | 92.6 ± 31.9 | 94.6 ± 43.0 † | 84.7 ± 32.8 | 0.071 |
| Treatment | 99.5 ± 54.5 | 92.1 ± 37.7 | 86.5 ± 36.1 | 90.9 ± 38.7 | ||
| Glucose (mg/dL) | Control | 94.2 ± 12.8 | 93.3 ± 11.6 | 93.4 ± 9.7 | 92.1 ± 11.1 | 0.962 |
| Treatment | 93.2 ± 10.4 | 92.7 ± 8.8 | 92.7 ± 8.3 | 92.1 ± 10.1 | ||
| ALT (U/L) | Control | 17.6 ± 8.7 | 17.5 ± 8.3 | 18.1 ± 10.1 | 17.9 ± 10.7 | 0.220 |
| Treatment | 17.8 ± 8.5 | 17.2 ± 8.0 | 15.0 ± 7.5 * | 15.7 ± 6.9 | ||
| AST (U/L) | Control | 21.2 ± 4.7 | 23.1 ± 14.2 | 22.9 ± 10.1 | 23.1 ± 8.9 | 0.071 |
| Treatment | 25.3 ± 13.5 | 22.4 ± 6.9 | 20.1 ± 6.3 *,† | 23.2 ± 7.6 | ||
| Gamma-GT (U/L) | Control | 21.3 ± 13.2 | 21.7 ± 14.4 | 21.8 ± 13.2 | 23.4 ± 14.8 ^ | 0.050 |
| Treatment | 22.4 ± 13.8 | 21.5 ± 13.8 | 20.8 ± 12.8 * | 20.8 ± 13.2 | ||
| Creatinine (mg/dL) | Control | 0.96 ± 0.14 | 0.89 ± 0.22 ** | 0.92 ± 0.18 * | 0.95 ± 0.17 †† | 0.505 |
| Treatment | 0.95 ± 0.20 | 0.92 ± 0.24 | 0.93 ± 0.24 | 0.93 ± 0.22 |
| Group | T0 | T2 | T4 | T8 | p Treatment:Time | |
|---|---|---|---|---|---|---|
| LDL cholesterol (mg/dL) | Control | 133.2 ± 21.8 | 129.6 ± 23.4 | 137.0 ± 27.5 † | 138.2 ± 27.6 †† | 0.004 |
| Treatment | 136.5 ± 22.6 | 133.6 ± 22.8 | 130.3 ± 24.9 * | 126.6 ± 25.5 **,† |
| Group | T0 | T4 | T8 | p Treatment:Time | |
|---|---|---|---|---|---|
| Weight (kg) | Control | 73.3 ± 16.2 | 72.4 ± 17.4 | 73.6 ± 16.5 | 0.672 |
| Treatment | 76.4 ±18.4 | 76.4 ± 18.2 | 76.8 ± 18.6 *,^ | ||
| Systolic pressure (mmHg) | Control | 107.5 ± 12.8 | 104.9 ± 11.5 | 103.2 ± 12.7 * | 0.858 |
| Treatment | 110.0 ±13.1 | 108.0 ± 13.5 | 105.1 ± 13.4 ***,^ | ||
| Diastolic pressure (mmHg) | Control | 75.8 ± 9.6 | 75.3 ± 8.4 | 73.7 ± 9.1 * | 0.518 |
| Treatment | 77.1 ± 9.5 | 75.0 ± 8.6 | 73.4 ± 8.0 ** |
| Group | T0 | T2 | T4 | T8 | p Treatment:Time | |
|---|---|---|---|---|---|---|
| Oxidized LDL (U/L) | Control | 115.4 ± 45.8 | 118.4 ± 48.6 | 121.5 ± 47.7 | 116.1 ± 47.6 | ≤0.001 |
| Treatment | 143.9 ± 54.9 aa | 130.2 ± 56.9 *** | 119.4 ± 43.4 **,†† | 98.6 ± 40.8 ***,††,^^,a | ||
| MDA (ng/mL) | Control | 63.2 ± 49.5 | 62.1 ± 53.3 | 69.2 ± 51.3 | 63.5 ± 44.9 | 0.247 |
| Treatment | 67.8 ± 60.8 | 58.4 ± 39.9 | 59.1 ± 36.9 | 57.5 ± 35.2 | ||
| IL-6 (pg/mL) | Control | 4.4 ± 5.7 | 5.2 ± 7.3 | 6.9 ± 13.0 | 6.6 ± 7.9 ** | ≤0.001 |
| Treatment | 18.4 ± 49.7 a | 12.7 ± 31.1 | 11.3 ± 26.5 | 8.7 ± 19.6 | ||
| Nitric oxide (µM) | Control | 33.3 ± 6.1 | 34.4 ± 4.0 | 35.2 ± 5.0 * | 35.2 ± 5.7 | 0.646 |
| Treatment | 33.2 ± 4.9 | 34.4 ± 3.6 | 34.1 ± 4.3 | 33.9 ± 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vezza, T.; Guillamón, E.; García-García, J.; Baños, A.; Mut-Salud, N.; García-López, J.D.; Gómez-Fernández, G.O.; Rodriguez-Nogales, A.; Gálvez, J.; Fonollá, J. LDL-Cholesterol-Lowering Effects of a Dietary Supplement Containing Onion and Garlic Extract Used in Healthy Volunteers. Nutrients 2024, 16, 2811. https://doi.org/10.3390/nu16162811
Vezza T, Guillamón E, García-García J, Baños A, Mut-Salud N, García-López JD, Gómez-Fernández GO, Rodriguez-Nogales A, Gálvez J, Fonollá J. LDL-Cholesterol-Lowering Effects of a Dietary Supplement Containing Onion and Garlic Extract Used in Healthy Volunteers. Nutrients. 2024; 16(16):2811. https://doi.org/10.3390/nu16162811
Chicago/Turabian StyleVezza, Teresa, Enrique Guillamón, Jorge García-García, Alberto Baños, Nuria Mut-Salud, Jose David García-López, Germán O. Gómez-Fernández, Alba Rodriguez-Nogales, Julio Gálvez, and Juristo Fonollá. 2024. "LDL-Cholesterol-Lowering Effects of a Dietary Supplement Containing Onion and Garlic Extract Used in Healthy Volunteers" Nutrients 16, no. 16: 2811. https://doi.org/10.3390/nu16162811
APA StyleVezza, T., Guillamón, E., García-García, J., Baños, A., Mut-Salud, N., García-López, J. D., Gómez-Fernández, G. O., Rodriguez-Nogales, A., Gálvez, J., & Fonollá, J. (2024). LDL-Cholesterol-Lowering Effects of a Dietary Supplement Containing Onion and Garlic Extract Used in Healthy Volunteers. Nutrients, 16(16), 2811. https://doi.org/10.3390/nu16162811








