Healthy Eating beyond Whole Grains—Insight on Associations between Diet Quality and Arterial Stiffness in the Brisighella Heart Study Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Design
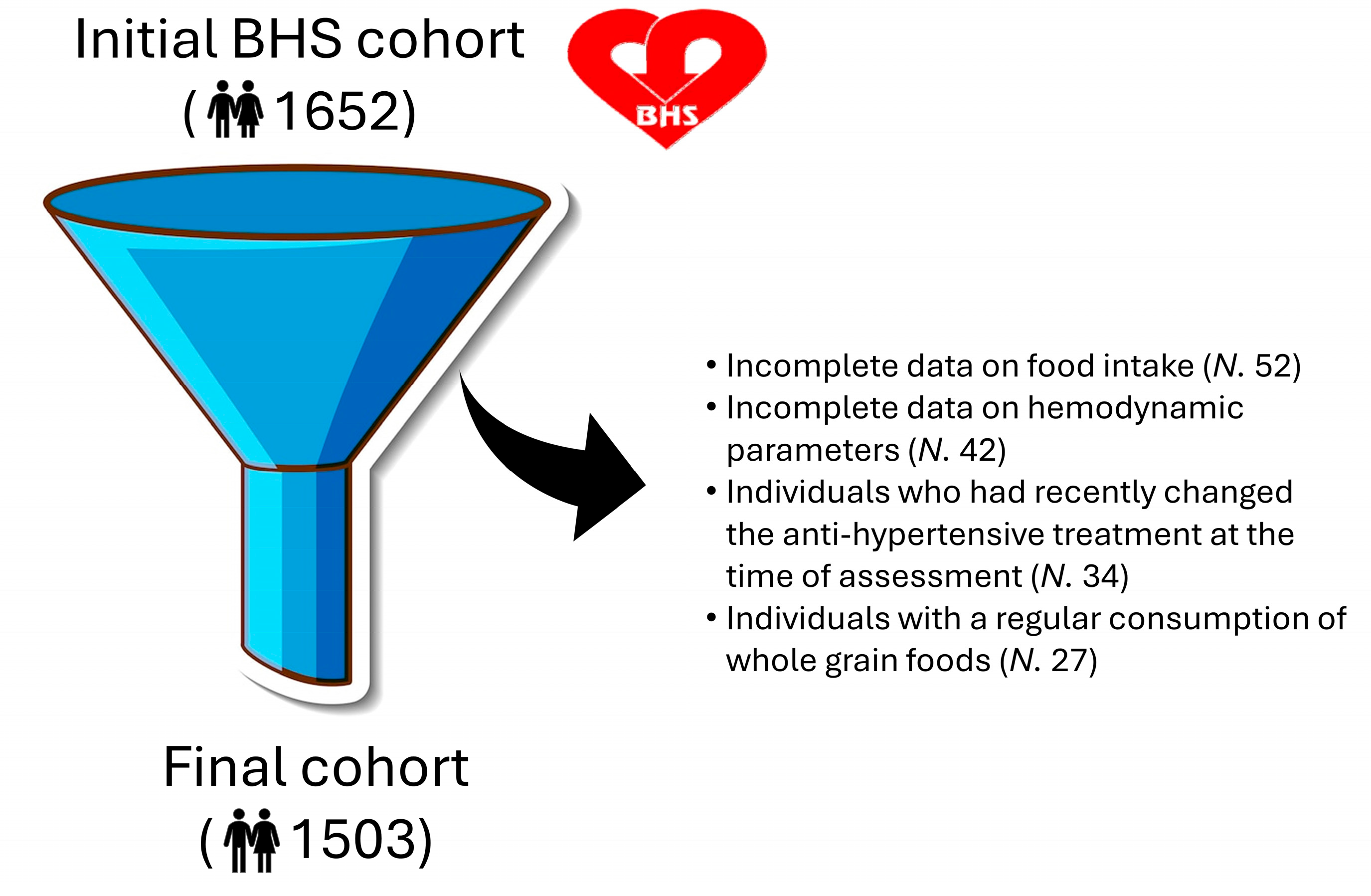
Inclusion and Exclusion Criteria
2.2. Covariate Ascertainment
2.2.1. Clinical Assessment
2.2.2. Hemodynamic Evaluation
2.2.3. Laboratory Assessment
2.3. Statistical Methods
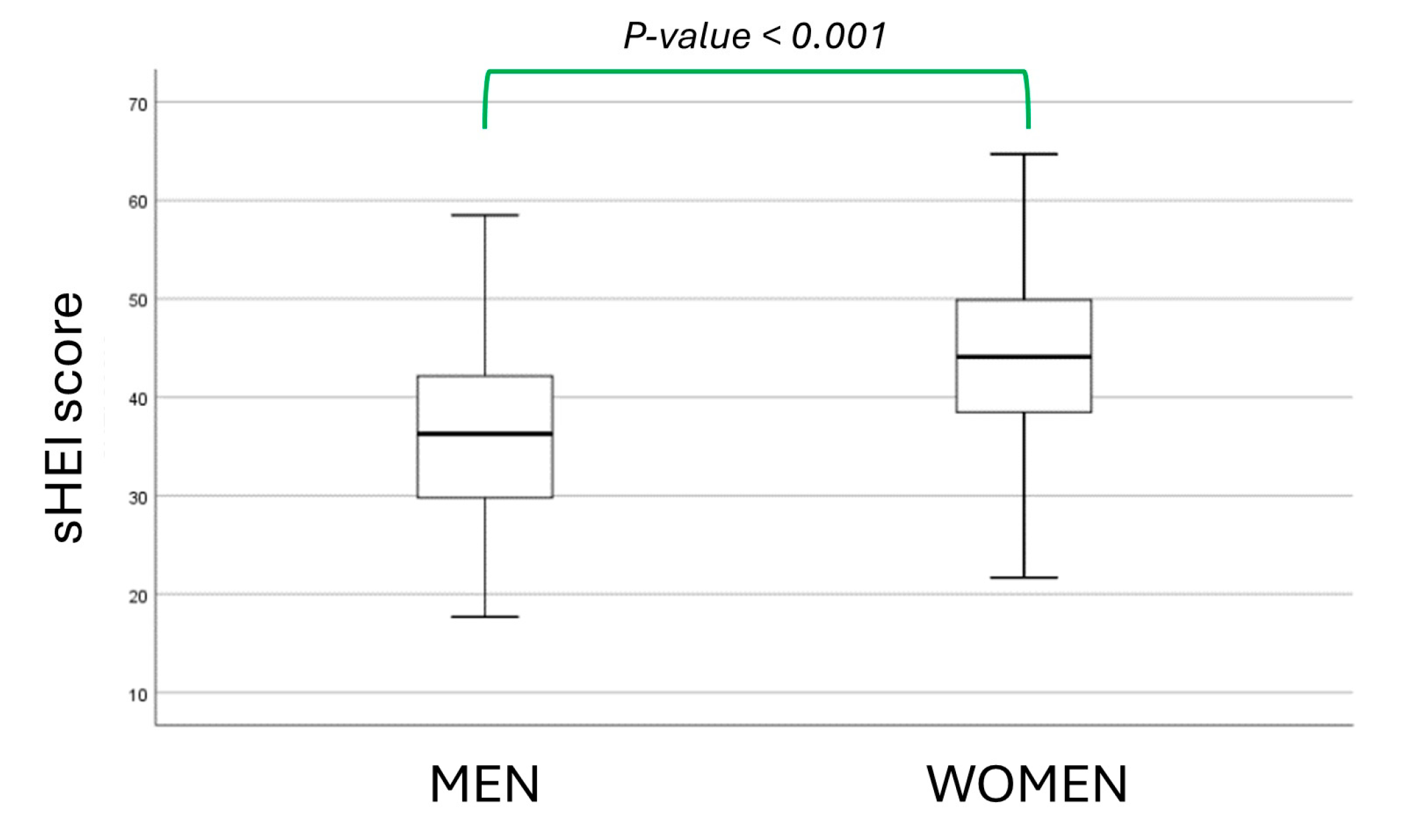
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health Benefits of Cereal Grain- and Pulse-Derived Proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef]
- McKeown, N.M.; Troy, L.M.; Jacques, P.F.; Hoffmann, U.; O’Donnell, C.J.; Fox, C.S. Whole-and refined-grain intakes are differentially associated with abdominal visceral and subcutaneous adiposity in healthy adults: The Framingham Heart Study. Am. J. Clin. Nutr. 2018, 104, 759–769. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; Bhutani, T.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; E Cade, J.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef]
- Kelly, S.A.; Summerbell, C.D.; Brynes, A.; Whittaker, V.; Frost, G.; Td, J.M. Whole grain cereals for the primary or secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2017, 8, CD005051. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972, Erratum in Lancet 2021, 397, 2466. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Derosa, G.; D’Angelo, A.; Ventura, F.; Rizzoli, E.; D’Addato, S.; Borghi, C.; on behalf of the Brisighella Heart Study Group. Lipoprotein(a) Serum Levels Predict Pulse Wave Velocity in Subjects in Primary Prevention for Cardiovascular Disease with Large Apo(a) Isoforms: Data from the Brisighella Heart Study. Biomedicines 2022, 10, 656. [Google Scholar] [CrossRef]
- Parikh, R.M.; Joshi, S.R.; Pandia, K. Index of central obesity is better than waist circumference in defining metabolic syndrome. Metab. Syndr. Relat. Disord. 2009, 7, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Mezzadri, M.; Grandi, E.; Borghi, C.; The Brisighella Heart Study Group. COVID-19-Related Quarantine Effect on Dietary Habits in a Northern Italian Rural Population: Data from the Brisighella Heart Study. Nutrients 2021, 13, 309. [Google Scholar] [CrossRef]
- Schröder, H.; Benitez Arciniega, A.; Soler, C.; Covas, M.I.; Baena-Díez, J.M.; Marrguat, J.; REGICOR investigators; HERMES investigators. Validity of two short screeners for diet quality in time-limited settings. Public Health Nutr. 2012, 15, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Rodríguez, M.L.; García-Cerdán, M.R.; Calonge-Vallejo, A.R.; Tobella-Andreu, L.; Baena-Díez, J.M.; Schröder, H. Feasibility and results of the short Diet Quality Screener in Primary Care: EMAP study. Enferm. Clin. 2016, 26, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Colby, S.; Zhou, W.; Allison, C.; Mathews, A.E.; Olfert, M.D.; Morrell, J.S.; Byrd-Bredbenner, C.; Greene, G.; Brown, O.; Kattelmann, K.; et al. Development and Validation of the Short Healthy Eating Index Survey with a College Population to Assess Dietary Quality and Intake. Nutrients 2020, 12, 2611. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Parsons, T.J.; Sartini, C.; Ellins, E.A.; Halcox, J.; Smith, K.E.; Ash, S.; Lennon, L.T.; Wannamethee, S.G.; Lee, I.M.; Whincup, P.H.; et al. Objectively measured physical activity, sedentary time and subclinical vascular disease: Cross-sectional study in older British men. Prev. Med. 2016, 89, 194–199. [Google Scholar] [CrossRef]
- Müller, J.; Ewert, P.; Hager, A. Increased aortic blood pressure augmentation in patients with congenital heart defects—A cross-sectional study in 1125 patients and 322 controls. Int. J. Cardiol. 2015, 184, 225–229. [Google Scholar] [CrossRef]
- Ageenkova, O.A.; Purygina, M.A. Central aortic blood pressure, augmentation index, and reflected wave transit time: Reproducibility and repeatability of data obtained by oscillometry. Vasc. Health Risk Manag. 2011, 7, 649–656. [Google Scholar] [CrossRef]
- Hickson, S.S.; Butlin, M.; Broad, J.; Avolio, A.P.; Wilkinson, I.B.; McEniery, C.M. Validity and repeatability of the Vicorder apparatus: A comparison with the SphygmoCor device. Hypertens. Res. 2009, 32, 1079–1085. [Google Scholar] [CrossRef]
- Pucci, G.; Cheriyan, J.; Hubsch, A.; Hickson, S.S.; Gajendragadkar, P.R.; Watson, T.; O’Sullivan, M.; Woodcock-Smith, J.; Schillaci, G.; Wilkinson, I.B.; et al. Evaluation of the Vicorder, a novel cuff-based device for the noninvasive estimation of central blood pressure. J. Hypertens. 2013, 31, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.; Fogacci, F.; Punzo, A.; Hrelia, S.; Simoni, P.; Caliceti, C.; Cicero, A.F.G. Treatment with PCSK9 Inhibitor Evolocumab Improves Vascular Oxidative Stress and Arterial Stiffness in Hypercholesterolemic Patients with High Cardiovascular Risk. Antioxidants 2023, 12, 578. [Google Scholar] [CrossRef]
- Cicero, A.F.; Rosticci, M.; Fogacci, F.; Grandi, E.; D’Addato, S.; Borghi, C.; Brisighella Heart Study Group. High serum uric acid is associated to poorly controlled blood pressure and higher arterial stiffness in hypertensive subjects. Eur. J. Intern. Med. 2017, 37, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, P.; Gowda, V.M. Assessing the Validity of Friedewald’s Formula and Anandraja’s Formula For Serum LDL-Cholesterol Calculation. J. Clin. Diagn. Res. 2015, 9, BC01–BC04. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Delgado, F.; Romero-Cabrera, J.L.; Perez-Martinez, P. Diet and vascular risk. Curr. Opin. Cardiol. 2022, 37, 343–349. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Veronesi, M.; Fogacci, F. Dietary Intervention to Improve Blood Pressure Control: Beyond Salt Restriction. High Blood Press Cardiovasc. Prev. 2021, 28, 547–553. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health. Biofactors 2013, 39, 335–342. [Google Scholar] [CrossRef]
- Hareer, L.W.; Lau, Y.Y.; Mole, F.; Reidlinger, D.P.; O’Neill, H.M.; Mayr, H.L.; Greenwood, H.; Albarqouni, L. The effectiveness of the Mediterranean Diet for primary and secondary prevention of cardiovascular disease: An umbrella review. Nutr. Diet. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- D’Alessandro, A.; De Pergola, G. Mediterranean Diet and Cardiovascular Disease: A Critical Evaluation of A Priori Dietary Indexes. Nutrients 2015, 7, 7863–7888. [Google Scholar] [CrossRef]
- Belderok, B. Developments in bread-making processes. Plant Foods Hum. Nutr. 2000, 55, 1–14. [Google Scholar] [CrossRef]
- Milani, P.; orres-Aguilar, P.; Hamaker, B.; Manary, M.; Abushamma, S.; Laar, A.; Steiner, R.; Ehsani, M.; de la Parra, J.; Skaven-Ruben, D.; et al. The whole grain manifesto: From Green Revolution to Grain Evolution. Glob. Food Secur. 2022, 34, 100649. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Desideri, G.; Grandi, E.; Rizzoli, E.; D’Addato, S.; Borghi, C. Arterial Stiffness, Sugar-Sweetened Beverages and Fruits Intake in a Rural Population Sample: Data from the Brisighella Heart Study. Nutrients 2019, 11, 2674. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; D’Addato, S.; Grandi, E.; Rizzoli, E.; Borghi, C.; on behalf of the Brisighella Heart Study. Self-Reported Coffee Consumption and Central and Peripheral Blood Pressure in the Cohort of the Brisighella Heart Study. Nutrients 2023, 15, 312. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.C.; Gupta, S.C.; Gupta, S.S. An Evidence Base for Heart Disease Prevention using a Mediterranean Diet Comprised Primarily of Vegetarian Food. Recent Adv. Food Nutr. Agric. 2023, 14, 135–143. [Google Scholar] [CrossRef]
- Guarneiri, L.L.; Maki, K.C. An Update on Nutrition Guidance for Cardiovascular Health. Curr. Atheroscler. Rep. 2023, 25, 597–603. [Google Scholar] [CrossRef]
| Parameters | Men (Mean ± SD) | Women (Mean ± SD) | p-Value |
|---|---|---|---|
| Age (years) | 58.2 ± 15.6 | 58.2 ± 15.8 | 0.977 |
| Waist Circumference (cm) | 97.1 ± 11.3 | 87.9 ± 13.5 | <0.001 |
| Hip Circumference (cm) | 100.9 ± 11.8 | 100.4 ± 3.2 | 0.468 |
| BMI (kg/m2) | 27.1 ± 3.9 | 26.3 ± 5.1 | <0.001 |
| Waist/Hip Ratio | 0.95 ± 0.09 | 0.87 ± 0.09 | <0.001 |
| Index of Central Obesity | 0.56 ± 0.07 | 0.56 ± 0.09 | 0.120 |
| Heart Rate (bpm) | 61.5 ± 11.4 | 66.3 ± 11.3 | <0.001 |
| SBP (mmHg) | 140.8 ± 8.6 | 141.2 ± 12.8 | 0.705 |
| DBP (mmHg) | 75.4 ± 4.7 | 71.8 ± 4.5 | <0.001 |
| Pulse Pressure (mmHg) | 65.4 ± 7.3 | 69.3 ± 9.7 | <0.001 |
| MAP (mmHg) | 97.2 ± 11.5 | 94.9 ± 12.5 | <0.001 |
| Pulse Pressure Index | 0.46 ± 0.06 | 0.48 ± 0.06 | <0.001 |
| Aortic BP (mmHg) | 137.5 ± 8.6 | 138.3 ± 12.8 | 0.459 |
| Aortic PP (mmHg) | 62.1 ± 7.2 | 66.5 ± 9.6 | <0.001 |
| Augmentation Index | 24.3 ± 8.8 | 26.7 ± 8.9 | <0.001 |
| Pulse Wave Velocity (m/s) | 9.2 ± 2.2 | 9.1 ± 2.5 | 0.441 |
| Parameters | Men (Mean ± SD) | Women (Mean ± SD) | p-Value |
|---|---|---|---|
| TC (mg/dL) | 210.7 ± 40 | 220.2 ± 39.7 | <0.001 |
| TG (mg/dL) | 126.8 ± 79.2 | 111.1 ± 60.2 | <0.001 |
| HDL-C (mg/dL) | 48 ± 13.9 | 55.1 ± 15.6 | <0.001 |
| LDL-C (mg/dL) | 137.8 ± 36.4 | 143 ± 37.1 | 0.006 |
| Lp(a) (mg/dL) | 20.1 ± 27.9 | 22.9 ± 30 | 0.021 |
| Apo B (mg/dL) | 90.3 ± 18.7 | 92.0 ± 21.9 | 0.099 |
| Apo AI (mg/dL) | 145.6 ± 24.1 | 160.8 ± 29.3 | <0.001 |
| LDL-C/Apo B | 1.53 ± 0.31 | 1.57 ± 0.32 | 0.016 |
| HDL-C/Apo AI | 0.32 ± 0.06 | 0.34 ± 0.06 | 0.062 |
| Fasting glucose (mg/dL) | 98.2 ± 11 | 93.0 ± 17.9 | <0.001 |
| AST (U/L) | 24.9 ± 8.7 | 22.2 ± 12 | 0.002 |
| ALT (U/L) | 28.6 ± 6.7 | 20.8 ± 11 | <0.001 |
| Gamma-GT (U/L) | 33.6 ± 11.1 | 21.3 ± 12.8 | <0.001 |
| SUA (mg/dL) | 5.9 ± 1.1 | 4.6 ± 1.2 | <0.001 |
| Creatinine (mg/dL) | 1.14 ± 0.17 | 0.95 ± 0.16 | <0.001 |
| eGFR (mL/min) | 72.7 ± 15.1 | 69 ± 15.7 | <0.001 |
| CPK (U/L) | 147.1 ± 87.1 | 113.3 ± 72.8 | <0.001 |
| Sex | Predictors | Direction of the Prediction | B | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| Men | eGFR |  | −0.148 | −0.259 | −0.038 | <0.001 |
| SUA |  | 0.220 | 0.095 | 0.320 | 0.001 | |
| sHEI |  | −0.231 | −0.327 | −0.089 | <0.001 | |
| Women | BMI |  | 0.174 | 0.111 | 0.331 | 0.002 |
| eGFR |  | −0.152 | −0.266 | −0.052 | <0.001 | |
| SUA |  | 0.278 | 0.158 | 0.354 | <0.001 | |
| sHEI |  | −0.218 | −0.308 | −0.115 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, M.; Fogacci, F.; D’Addato, S.; Grandi, E.; Borghi, C.; Cicero, A.F.G., on behalf of the Brisighella Heart Study Group. Healthy Eating beyond Whole Grains—Insight on Associations between Diet Quality and Arterial Stiffness in the Brisighella Heart Study Cohort. Nutrients 2024, 16, 2792. https://doi.org/10.3390/nu16162792
Giovannini M, Fogacci F, D’Addato S, Grandi E, Borghi C, Cicero AFG on behalf of the Brisighella Heart Study Group. Healthy Eating beyond Whole Grains—Insight on Associations between Diet Quality and Arterial Stiffness in the Brisighella Heart Study Cohort. Nutrients. 2024; 16(16):2792. https://doi.org/10.3390/nu16162792
Chicago/Turabian StyleGiovannini, Marina, Federica Fogacci, Sergio D’Addato, Elisa Grandi, Claudio Borghi, and Arrigo F. G. Cicero on behalf of the Brisighella Heart Study Group. 2024. "Healthy Eating beyond Whole Grains—Insight on Associations between Diet Quality and Arterial Stiffness in the Brisighella Heart Study Cohort" Nutrients 16, no. 16: 2792. https://doi.org/10.3390/nu16162792
APA StyleGiovannini, M., Fogacci, F., D’Addato, S., Grandi, E., Borghi, C., & Cicero, A. F. G., on behalf of the Brisighella Heart Study Group. (2024). Healthy Eating beyond Whole Grains—Insight on Associations between Diet Quality and Arterial Stiffness in the Brisighella Heart Study Cohort. Nutrients, 16(16), 2792. https://doi.org/10.3390/nu16162792










