Cancer and the Microbiome of the Human Body
Abstract
1. Introduction
2. Cancer Background: Prevalence and Physio-Pathological Overview
3. Cancer and the Microbiome
3.1. Carcinogenesis and Oncogenesis Processes Associated with Microbe Metabolism
3.1.1. Metabolic Pathway: Tryptophan-Indoleamine-Arginine-Citrulline
3.1.2. Tumor and Microbial Markers
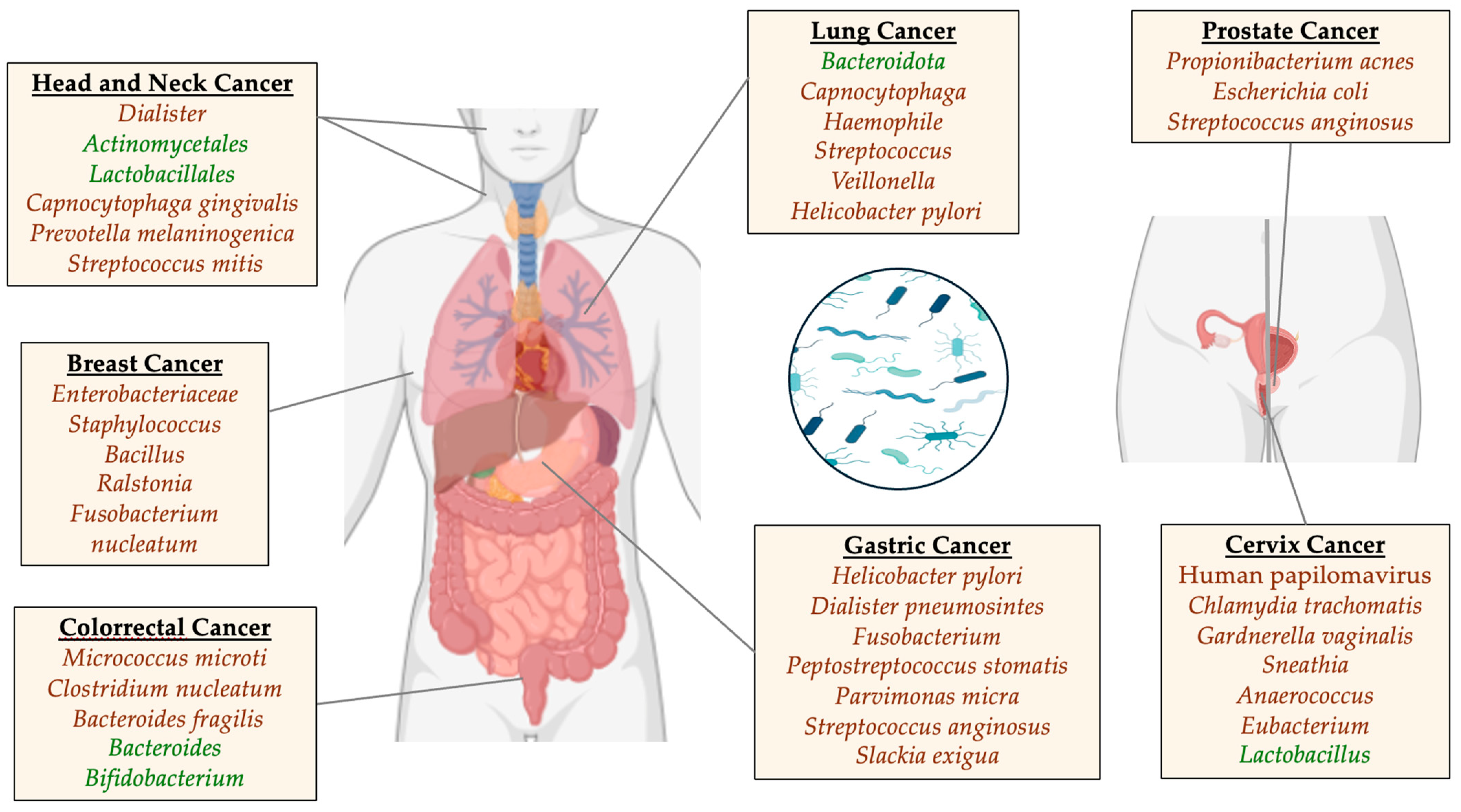
3.2. Lung Cancer
3.3. Head and Neck Cancer
3.4. Breast Cancer
3.5. Gastric Cancer
3.6. Colorectal Cancer
3.7. Prostate Cancer
3.8. Cervix Cancer
4. Diagnostic Methods, Novel Treatments, and Future Perspectives in the Study of the Human Microbiome and Cancer
4.1. Cancer Diagnostic Methods through the Human Microbiome
4.2. Novel Cancer Treatments through the Microbiome
4.3. Outlook and Future Perspectives in the Study of the Human Microbiome and Cancer
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhang, R.; Pu, Y.; Wang, D.; Wang, Y.; Wu, X.; Pan, Y.; Luo, C.; Zhao, G.; Quan, Z.; et al. Sample Collection, DNA Extraction, and Library Construction Protocols of the Human Microbiome Studies in the International Human Phenome Project. Phenomics 2023, 3, 300–308. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Puschhof, J.; Elinav, E. Human microbiome research: Growing pains and future promises. PLoS Biol. 2023, 21, e3002053. [Google Scholar] [CrossRef]
- Ignatiou, A.; Pitsouli, C. Host-diet-microbiota interplay in intestinal nutrition and health. FEBS Lett. 2024. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernandez, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef]
- Shantaram, D.; Hoyd, R.; Blaszczak, A.M.; Antwi, L.; Jalilvand, A.; Wright, V.P.; Liu, J.; Smith, A.J.; Bradley, D.; Lafuse, W.; et al. Obesity-associated microbiomes instigate visceral adipose tissue inflammation by recruitment of distinct neutrophils. Nat. Commun. 2024, 15, 5434. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Miko, E.; Sipos, A.; Toth, E.; Lehoczki, A.; Fekete, M.; Sebo, E.; Kardos, G.; Bai, P. Guideline for designing microbiome studies in neoplastic diseases. Geroscience 2024. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services. 2024. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services#:~:text=In%202022%2C%20there%20were%20an,women%20die%20from%20the%20disease (accessed on 17 July 2024).
- Cancer Today; International Agency for Research on Cancer; World Health Organization. Data Visualization Tools for Exploring the Global Cancer Burden in 2022. Available online: https://gco.iarc.fr/today/en/dataviz/tables?mode=cancer&group_populations=1&multiple_populations=1 (accessed on 17 July 2024).
- Wilking, N.E.; Hofmarcher, T.; Lindgren, P.; Jönsson, B. The burden and direct cost of cancer in Europe (EU-28). J. Clin. Oncol. 2016, 34, 6618. [Google Scholar] [CrossRef]
- White, M.C.; Peipins, L.A.; Watson, M.; Trivers, K.F.; Holman, D.M.; Rodriguez, J.L. Cancer prevention for the next generation. J. Adolesc. Health 2013, 52, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Kajo, K.; Biringer, K.; Vybohova, D.; Brockmueller, A.; et al. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression-3PM pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Berben, L.; Floris, G.; Wildiers, H.; Hatse, S. Cancer and Aging: Two Tightly Interconnected Biological Processes. Cancers 2021, 13, 1400. [Google Scholar] [CrossRef]
- Ribas, A. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov. 2015, 5, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Assi, M. The differential role of reactive oxygen species in early and late stages of cancer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R646–R653. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R.; Kshitiz. Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef]
- Mohan, V.; Das, A.; Sagi, I. Emerging roles of ECM remodeling processes in cancer. Semin. Cancer Biol. 2020, 62, 192–200. [Google Scholar] [CrossRef]
- Aseervatham, J. Cytoskeletal Remodeling in Cancer. Biology 2020, 9, 385. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Pilaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer-A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the Human Gut: The “Known Unknown” of the Microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. eBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Zuo, T.; Zhan, H.; Zhang, F.; Liu, Q.; Tso, E.Y.K.; Lui, G.C.Y.; Chen, N.; Li, A.; Lu, W.; Chan, F.K.L.; et al. Alterations in Fecal Fungal Microbiome of Patients With COVID-19 During Time of Hospitalization until Discharge. Gastroenterology 2020, 159, 1302–1310.e5. [Google Scholar] [CrossRef]
- van Tilburg Bernardes, E.; Pettersen, V.K.; Gutierrez, M.W.; Laforest-Lapointe, I.; Jendzjowsky, N.G.; Cavin, J.B.; Vicentini, F.A.; Keenan, C.M.; Ramay, H.R.; Samara, J.; et al. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat. Commun. 2020, 11, 2577. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Kandalai, S.; Li, H.; Zhang, N.; Peng, H.; Zheng, Q. The human microbiome and cancer: A diagnostic and therapeutic perspective. Cancer Biol. Ther. 2023, 24, 2240084. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Chu, Q.; Zheng, Q.; Yuan, X.; Su, Y.; Bao, Z.; Lu, J.; Li, L. Current understanding of the intratumoral microbiome in various tumors. Cell Rep. Med. 2023, 4, 100884. [Google Scholar] [CrossRef]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Heymann, C.J.F.; Bard, J.M.; Heymann, M.F.; Heymann, D.; Bobin-Dubigeon, C. The intratumoral microbiome: Characterization methods and functional impact. Cancer Lett. 2021, 522, 63–79. [Google Scholar] [CrossRef]
- Doocey, C.M.; Finn, K.; Murphy, C.; Guinane, C.M. The impact of the human microbiome in tumorigenesis, cancer progression, and biotherapeutic development. BMC Microbiol. 2022, 22, 53. [Google Scholar] [CrossRef]
- Nakatsu, G.; Zhou, H.; Wu, W.K.K.; Wong, S.H.; Coker, O.O.; Dai, Z.; Li, X.; Szeto, C.H.; Sugimura, N.; Lam, T.Y.; et al. Alterations in Enteric Virome Are Associated with Colorectal Cancer and Survival Outcomes. Gastroenterology 2018, 155, 529–541.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.; Pletcher, S.D.; Goldberg, A.N.; Barker, B.M.; Cope, E.K. Fungal Microbiota in Chronic Airway Inflammatory Disease and Emerging Relationships with the Host Immune Response. Front. Microbiol. 2017, 8, 2477. [Google Scholar] [CrossRef]
- Fan, D.; Coughlin, L.A.; Neubauer, M.M.; Kim, J.; Kim, M.S.; Zhan, X.; Simms-Waldrip, T.R.; Xie, Y.; Hooper, L.V.; Koh, A.Y. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 2015, 21, 808–814. [Google Scholar] [CrossRef]
- Shatova, O.P.; Zabolotneva, A.A.; Shestopalov, A.V. Molecular Ensembles of Microbiotic Metabolites in Carcinogenesis. Biochemistry 2023, 88, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sharma, N.; Bharmjeet; Das, A. Unraveling the intricate relationship: Influence of microbiome on the host immune system in carcinogenesis. Cancer Rep. 2023, 6, e1892. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, W.; Feng, Y.; Wang, Y.; Sun, T.; Xu, J. Microbial metabolites affect tumor progression, immunity and therapy prediction by reshaping the tumor microenvironment (Review). Int. J. Oncol. 2024, 65, 73. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Sanhueza, E.; McNerney, E.; Onate, S.A.; Garcia, A. Microbiota dysbiosis: A new piece in the understanding of the carcinogenesis puzzle. J. Med. Microbiol. 2016, 65, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, N.; Conacci-Sorrell, M. Kynurenine: An oncometabolite in colon cancer. Cell Stress. 2020, 4, 24–26. [Google Scholar] [CrossRef]
- Wyatt, M.; Greathouse, K.L. Targeting Dietary and Microbial Tryptophan-Indole Metabolism as Therapeutic Approaches to Colon Cancer. Nutrients 2021, 13, 1189. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Larraufie, P.; Jarry, A.; Beguet-Crespel, F.; Marinelli, L.; Ledue, F.; Reimann, F.; Blottiere, H.M.; Lapaque, N. Butyrate Produced by Commensal Bacteria Down-Regulates Indolamine 2,3-Dioxygenase 1 (IDO-1) Expression via a Dual Mechanism in Human Intestinal Epithelial Cells. Front. Immunol. 2018, 9, 2838. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Shestopalov, A.; Shatova, O.; Karbyshev, M.; Gaponov, A.; Moskaleva, N.; Appolonova, S.; Tutelyan, A.; Makarov, V.; Yudin, S.; Roumiantsev, S. “Kynurenine switch” and obesity. Bull. Sib. Med. 2022, 20, 103–111. [Google Scholar]
- Davis, I.; Liu, A. What is the tryptophan kynurenine pathway and why is it important to neurotherapeutics? Expert. Rev. Neurother. 2015, 15, 719–721. [Google Scholar] [CrossRef]
- Marti, I.L.A.A.; Reith, W. Arginine-dependent immune responses. Cell Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Bensalah, K.; Montorsi, F.; Shariat, S.F. Challenges of cancer biomarker profiling. Eur. Urol. 2007, 52, 1601–1609. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Rizk, E.M.; Gartrell, R.D.; Barker, L.W.; Esancy, C.L.; Finkel, G.G.; Bordbar, D.D.; Saenger, Y.M. Prognostic and Predictive Immunohistochemistry-Based Biomarkers in Cancer and Immunotherapy. Hematol. Oncol. Clin. N. Am. 2019, 33, 291–299. [Google Scholar] [CrossRef]
- Schloissnig, S.; Arumugam, M.; Sunagawa, S.; Mitreva, M.; Tap, J.; Zhu, A.; Waller, A.; Mende, D.R.; Kultima, J.R.; Martin, J.; et al. Genomic variation landscape of the human gut microbiome. Nature 2013, 493, 45–50. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, G.; Wang, L.; Zhou, X.; Sun, J.; Li, X.; Zhu, Y.; He, Y.; Kofonikolas, K.; Bogaert, D.; et al. A systematic review of microbial markers for risk prediction of colorectal neoplasia. Br. J. Cancer 2022, 126, 1318–1328. [Google Scholar] [CrossRef]
- Tang, Z.; Liang, D.; Deubler, E.L.; Sarnat, J.A.; Chow, S.S.; Diver, W.R.; Wang, Y. Lung cancer metabolomics: A pooled analysis in the Cancer Prevention Studies. BMC Med. 2024, 22, 262. [Google Scholar] [CrossRef]
- Rodriguez-Canales, J.; Parra-Cuentas, E.; Wistuba, I.I. Diagnosis and Molecular Classification of Lung Cancer. Cancer Treat. Res. 2016, 170, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef]
- Li, X.; Shang, S.; Wu, M.; Song, Q.; Chen, D. Gut microbial metabolites in lung cancer development and immunotherapy: Novel insights into gut-lung axis. Cancer Lett. 2024, 598, 217096. [Google Scholar] [CrossRef]
- Sun, Y.; Wen, M.; Liu, Y.; Wang, Y.; Jing, P.; Gu, Z.; Jiang, T.; Wang, W. The human microbiome: A promising target for lung cancer treatment. Front. Immunol. 2023, 14, 1091165. [Google Scholar] [CrossRef]
- Botticelli, A.; Vernocchi, P.; Marini, F.; Quagliariello, A.; Cerbelli, B.; Reddel, S.; Del Chierico, F.; Di Pietro, F.; Giusti, R.; Tomassini, A.; et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J. Transl. Med. 2020, 18, 49. [Google Scholar] [CrossRef]
- Raza, M.H.; Gul, K.; Arshad, A.; Riaz, N.; Waheed, U.; Rauf, A.; Aldakheel, F.; Alduraywish, S.; Rehman, M.U.; Abdullah, M. Microbiota in cancer development and treatment. J. Cancer Res. Clin. Oncol. 2019, 145, 49–63. [Google Scholar] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar]
- Leng, Q.; Holden, V.K.; Deepak, J.; Todd, N.W.; Jiang, F. Microbiota Biomarkers for Lung Cancer. Diagnostics 2021, 11, 407. [Google Scholar] [CrossRef]
- Zhao, M.; Hou, W.; Pu, D.; Li, Z.; Tu, L.; Ow, C.J.L.; Tian, J.; Li, W. Impact of Pulmonary microbiota on lung cancer treatment-related pneumonia. J. Cancer 2024, 15, 4503–4512. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, W.; Wang, X.; Zhang, J.; Zhou, E.; Tu, Y.; Zou, J.; Su, K.; Yi, H.; Yin, S. Global burden of head and neck cancers from 1990 to 2019. iScience 2024, 27, 109282. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Mathur, R.; Singhavi, H.R.; Malik, A.; Nair, S.; Chaturvedi, P. Role of Poor Oral Hygiene in Causation of Oral Cancer-a Review of Literature. Indian J. Surg. Oncol. 2019, 10, 184–195. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’Addona, A. Oral microbiota in human health and disease: A perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef]
- Bornigen, D.; Ren, B.; Pickard, R.; Li, J.; Ozer, E.; Hartmann, E.M.; Xiao, W.; Tickle, T.; Rider, J.; Gevers, D.; et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci. Rep. 2017, 7, 17686. [Google Scholar] [CrossRef]
- Su Mun, L.; Wye Lum, S.; Kong Yuiin Sze, G.; Hock Yoong, C.; Ching Yung, K.; Kah Lok, L.; Gopinath, D. Association of Microbiome with Oral Squamous Cell Carcinoma: A Systematic Review of the Metagenomic Studies. Int. J. Environ. Res. Public Health 2021, 18, 7224. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome with Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef]
- Banerjee, S.; Tian, T.; Wei, Z.; Peck, K.N.; Shih, N.; Chalian, A.A.; O’Malley, B.W.; Weinstein, G.S.; Feldman, M.D.; Alwine, J.; et al. Microbial Signatures Associated with Oropharyngeal and Oral Squamous Cell Carcinomas. Sci. Rep. 2017, 7, 4036. [Google Scholar] [CrossRef]
- Mager, D.L.; Haffajee, A.D.; Devlin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Varela-Centelles, P. Early Diagnosis and Diagnostic Delay in Oral Cancer. Cancers 2022, 14, 1758. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Aguilar-Ruiz, M.; Ramos-Garcia, P. Challenges in the Early Diagnosis of Oral Cancer, Evidence Gaps and Strategies for Improvement: A Scoping Review of Systematic Reviews. Cancers 2022, 14, 4967. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 27 July 2024).
- Alkabban, F.; Ferguson, T. Breast Cancer. [Updated 2020 Nov 10]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of breast cancer. In Breast Cancer [Internet]; Exon Publications: Brisbane City, QLD, Canada, 2022. [Google Scholar]
- Braun, L.; Mietzsch, F.; Seibold, P.; Schneeweiss, A.; Schirmacher, P.; Chang-Claude, J.; Peter Sinn, H.; Aulmann, S. Intrinsic breast cancer subtypes defined by estrogen receptor signalling-prognostic relevance of progesterone receptor loss. Mod. Pathol. 2013, 26, 1161–1171. [Google Scholar] [CrossRef]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar]
- Costantini, L.; Magno, S.; Albanese, D.; Donati, C.; Molinari, R.; Filippone, A.; Masetti, R.; Merendino, N. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci. Rep. 2018, 8, 16893. [Google Scholar]
- Thompson, K.J.; Ingle, J.N.; Tang, X.; Chia, N.; Jeraldo, P.R.; Walther-Antonio, M.R.; Kandimalla, K.K.; Johnson, S.; Yao, J.Z.; Harrington, S.C. A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS ONE 2017, 12, e0188873. [Google Scholar]
- Herrera-Quintana, L.; Vazquez-Lorente, H.; Plaza-Diaz, J. Breast Cancer: Extracellular Matrix and Microbiome Interactions. Int. J. Mol. Sci. 2024, 25, 7226. [Google Scholar] [CrossRef]
- Alvarez-Mercado, A.I.; Del Valle Cano, A.; Fernandez, M.F.; Fontana, L. Gut Microbiota and Breast Cancer: The Dual Role of Microbes. Cancers 2023, 15, 443. [Google Scholar] [CrossRef]
- Mathebela, P.; Damane, B.P.; Mulaudzi, T.V.; Mkhize-Khwitshana, Z.L.; Gaudji, G.R.; Dlamini, Z. Influence of the Microbiome Metagenomics and Epigenomics on Gastric Cancer. Int. J. Mol. Sci. 2022, 23, 13750. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Seeneevassen, L.; Bessede, E.; Megraud, F.; Lehours, P.; Dubus, P.; Varon, C. Gastric Cancer: Advances in Carcinogenesis Research and New Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 3418. [Google Scholar] [CrossRef]
- Lopez, M.J.; Carbajal, J.; Alfaro, A.L.; Saravia, L.G.; Zanabria, D.; Araujo, J.M.; Quispe, L.; Zevallos, A.; Buleje, J.L.; Cho, C.E.; et al. Characteristics of gastric cancer around the world. Crit. Rev. Oncol. Hematol. 2023, 181, 103841. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Gundamaraju, R.; Rajeev, A. Diversification and deleterious role of microbiome in gastric cancer. Cancer Rep. 2023, 6, e1878. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, J.; Rai, R.P.; Prasad, K.N. Role of Helicobacter pylori in gastric cancer: Updates. World J. Gastrointest. Oncol. 2016, 8, 147–158. [Google Scholar] [CrossRef]
- Zilberstein, B.; Quintanilha, A.G.; Santos, M.A.; Pajecki, D.; Moura, E.G.; Alves, P.R.; Maluf Filho, F.; de Souza, J.A.; Gama-Rodrigues, J. Digestive tract microbiota in healthy volunteers. Clinics 2007, 62, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, L.; Graham, D.Y. Microbiome and Gastric Cancer. Dig. Dis. Sci. 2020, 65, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Wang, A.; Chu, A.N.; Gong, Y.H.; Yuan, Y. Mucosa-Associated Microbiota in Gastric Cancer Tissues Compared with Non-cancer Tissues. Front. Microbiol. 2019, 10, 1261. [Google Scholar] [CrossRef]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genom. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Zhan, Z.S.; Zheng, Z.S.; Shi, J.; Chen, J.; Wu, S.Y.; Zhang, S.Y. Unraveling colorectal cancer prevention: The vitamin D–gut flora–immune system nexus. World J. Gastrointest. Oncol. 2024, 16, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Salim, F.; Mizutani, S.; Shiba, S.; Takamaru, H.; Yamada, M.; Nakajima, T.; Yachida, T.; Soga, T.; Saito, Y.; Fukuda, S.; et al. Fusobacterium species are distinctly associated with patients with Lynch syndrome colorectal cancer. iScience 2024, 27, 110181. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar]
- Ternes, D.; Karta, J.; Tsenkova, M.; Wilmes, P.; Haan, S.; Letellier, E. Microbiome in colorectal cancer: How to get from meta-omics to mechanism? Trends Microbiol. 2020, 28, 401–423. [Google Scholar]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [PubMed]
- McQuade, J.L.; Daniel, C.R.; Helmink, B.A.; Wargo, J.A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019, 20, e77–e91. [Google Scholar] [PubMed]
- Scott, A.J.; Alexander, J.L.; Merrifield, C.A.; Cunningham, D.; Jobin, C.; Brown, R.; Alverdy, J.; O’Keefe, S.J.; Gaskins, H.R.; Teare, J. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 2019, 68, 1624–1632. [Google Scholar]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar]
- Montalban-Arques, A.; Scharl, M. Intestinal microbiota and colorectal carcinoma: Implications for pathogenesis, diagnosis, and therapy. eBioMedicine 2019, 48, 648–655. [Google Scholar] [PubMed]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Oliero, M.; Alaoui, A.A.; McCartney, C.; Santos, M.M. Colorectal cancer and inulin supplementation: The good, the bad, and the unhelpful. Gastroenterol. Rep. 2024, 12, goae058. [Google Scholar] [CrossRef]
- Lopez, L.R.; Bleich, R.M.; Arthur, J.C. Microbiota Effects on Carcinogenesis: Initiation, Promotion, and Progression. Annu. Rev. Med. 2021, 72, 243–261. [Google Scholar] [CrossRef]
- Al-Rashidi, R.R.; Noraldeen, S.A.M.; Kareem, A.K.; Mahmoud, A.K.; Kadhum, W.R.; Ramirez-Coronel, A.A.; Iswanto, A.H.; Obaid, R.F.; Jalil, A.T.; Mustafa, Y.F.; et al. Malignant function of nuclear factor-kappaB axis in prostate cancer: Molecular interactions and regulation by non-coding RNAs. Pharmacol. Res. 2023, 194, 106775. [Google Scholar] [CrossRef]
- Xia, B.; Wang, J.; Zhang, D.; Hu, X. The human microbiome links to prostate cancer risk and treatment (Review). Oncol. Rep. 2023, 49, 123. [Google Scholar] [CrossRef] [PubMed]
- Vynckier, P.; Annemans, L.; Raes, S.; Amrouch, C.; Lindgren, P.; Majek, O.; Beyer, K.; Leenen, R.C.A.; Venderbos, L.D.F.; Denijs, F.; et al. Systematic Review on the Cost Effectiveness of Prostate Cancer Screening in Europe. Eur. Urol. 2024; in press. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Bombelli, R.; Baci, D.; Mortara, L. Microbiome and Prostate Cancer: A Novel Target for Prevention and Treatment. Int. J. Mol. Sci. 2023, 24, 1511. [Google Scholar] [CrossRef]
- Fujita, K.; Matsushita, M.; Banno, E.; De Velasco, M.A.; Hatano, K.; Nonomura, N.; Uemura, H. Gut microbiome and prostate cancer. Int. J. Urol. 2022, 29, 793–798. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, K.; Hatano, K.; De Velasco, M.A.; Tsujimura, A.; Uemura, H.; Nonomura, N. Emerging Relationship between the Gut Microbiome and Prostate Cancer. World J. Mens. Health 2023, 41, 759–768. [Google Scholar] [CrossRef]
- Javier-DesLoges, J.; McKay, R.R.; Swafford, A.D.; Sepich-Poore, G.D.; Knight, R.; Parsons, J.K. The microbiome and prostate cancer. Prostate Cancer Prostatic Dis. 2022, 25, 159–164. [Google Scholar] [CrossRef]
- Rizzo, A.; Santoni, M.; Mollica, V.; Fiorentino, M.; Brandi, G.; Massari, F. Microbiota and prostate cancer. Semin. Cancer Biol. 2022, 86, 1058–1065. [Google Scholar] [CrossRef]
- Porter, C.M.; Shrestha, E.; Peiffer, L.B.; Sfanos, K.S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 345–354. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, K.; Nonomura, N. Influence of Diet and Nutrition on Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 1447. [Google Scholar] [CrossRef] [PubMed]
- Pimple, S.; Mishra, G. Cancer cervix: Epidemiology and disease burden. Cytojournal 2022, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Tekalign, T.; Teshome, M. Prevalence and determinants of late-stage presentation among cervical cancer patients, a systematic review and meta-analysis. PLoS ONE 2022, 17, e0267571. [Google Scholar] [CrossRef]
- Gargiulo Isacco, C.; Balzanelli, M.G.; Garzone, S.; Lorusso, M.; Inchingolo, F.; Nguyen, K.C.D.; Santacroce, L.; Mosca, A.; Del Prete, R. Alterations of Vaginal Microbiota and Chlamydia trachomatis as Crucial Co-Causative Factors in Cervical Cancer Genesis Procured by HPV. Microorganisms 2023, 11, 662. [Google Scholar] [CrossRef]
- Plisko, O.; Zodzika, J.; Jermakova, I.; Pcolkina, K.; Prusakevica, A.; Liepniece-Karele, I.; Donders, G.G.G.; Rezeberga, D. Aerobic Vaginitis-Underestimated Risk Factor for Cervical Intraepithelial Neoplasia. Diagnostics 2021, 11, 97. [Google Scholar] [CrossRef]
- Nunes, S.C.; Serpa, J. Recycling the Interspecific Relations with Epithelial Cells: Bacteria and Cancer Metabolic Symbiosis. Adv. Exp. Med. Biol. 2020, 1219, 77–91. [Google Scholar] [CrossRef]
- Wakabayashi, R.; Nakahama, Y.; Nguyen, V.; Espinoza, J.L. The Host-Microbe Interplay in Human Papillomavirus-Induced Carcinogenesis. Microorganisms 2019, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gao, Y.; Zheng, Y.; Shang, X. KF-finder: Identification of key factors from host-microbial networks in cervical cancer. BMC Syst. Biol. 2018, 12, 54. [Google Scholar] [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef]
- Cullin, N.; Azevedo Antunes, C.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Yan, X.; Yang, M.; Liu, J.; Gao, R.; Hu, J.; Li, J.; Zhang, L.; Shi, Y.; Guo, H.; Cheng, J.; et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am. J. Cancer Res. 2015, 5, 3111–3122. [Google Scholar] [PubMed]
- Yu, J.; Feng, Q.; Wong, S.H.; Zhang, D.; Liang, Q.Y.; Qin, Y.; Tang, L.; Zhao, H.; Stenvang, J.; Li, Y.; et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 2017, 66, 70–78. [Google Scholar] [CrossRef]
- Xie, Y.H.; Gao, Q.Y.; Cai, G.X.; Sun, X.M.; Sun, X.M.; Zou, T.H.; Chen, H.M.; Yu, S.Y.; Qiu, Y.W.; Gu, W.Q.; et al. Fecal Clostridium symbiosum for Noninvasive Detection of Early and Advanced Colorectal Cancer: Test and Validation Studies. eBioMedicine 2017, 25, 32–40. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, Z.; Xue, Y.; Zhang, J.; Zhu, J.; Gao, R.; Yao, S.; Ye, Y.; Wang, S.; Lin, C.; et al. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes 2020, 11, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Q.; Li, T.; Nakatsu, G.; Chen, Y.X.; Yau, T.O.; Chu, E.; Wong, S.; Szeto, C.H.; Ng, S.C.; Chan, F.K.L.; et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut 2020, 69, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ni, H.; Wang, X.; Xu, Q.; Zhang, J.; Jiang, L.; Wang, B.; Song, S.; Zhu, X. A New Biomarker of Fecal Bacteria for Non-Invasive Diagnosis of Colorectal Cancer. Front. Cell Infect. Microbiol. 2021, 11, 744049. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Shi, X.; Du, Y.; Niu, Y.; Jin, G.; Wang, Z.; Lyu, J. Gut microbiome analysis as a predictive marker for the gastric cancer patients. Appl. Microbiol. Biotechnol. 2021, 105, 803–814. [Google Scholar] [CrossRef]
- Narunsky-Haziza, L.; Sepich-Poore, G.D.; Livyatan, I.; Asraf, O.; Martino, C.; Nejman, D.; Gavert, N.; Stajich, J.E.; Amit, G.; Gonzalez, A.; et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 2022, 185, 3789–3806.e17. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, L.; Xu, F.; Liu, M.; Song, Y.; Chen, J.; Zhan, H.; Zhang, Y.; Xu, D.; Chen, Y.; et al. Microbiome-metabolome analysis reveals cervical lesion alterations. Acta Biochim. Biophys. Sin. 2022, 54, 1552–1560. [Google Scholar] [CrossRef]
- Negrut, R.L.; Cote, A.; Maghiar, A.M. Exploring the Potential of Oral Microbiome Biomarkers for Colorectal Cancer Diagnosis and Prognosis: A Systematic Review. Microorganisms 2023, 11, 1586. [Google Scholar] [CrossRef]
- Tito, R.Y.; Verbandt, S.; Aguirre Vazquez, M.; Lahti, L.; Verspecht, C.; Llorens-Rico, V.; Vieira-Silva, S.; Arts, J.; Falony, G.; Dekker, E.; et al. Microbiome confounders and quantitative profiling challenge predicted microbial targets in colorectal cancer development. Nat. Med. 2024, 30, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yin, Y.; Jiang, Y.; Yang, Y.; Wang, W.; Wang, X.; Ge, Y.; Liu, B.; Yao, L. Relationship between vaginal and oral microbiome in patients of human papillomavirus (HPV) infection and cervical cancer. J. Transl. Med. 2024, 22, 396. [Google Scholar] [CrossRef]
- Chen, F.; Dai, X.; Zhou, C.C.; Li, K.X.; Zhang, Y.J.; Lou, X.Y.; Zhu, Y.M.; Sun, Y.L.; Peng, B.X.; Cui, W. Integrated analysis of the faecal metagenome and serum metabolome reveals the role of gut microbiome-associated metabolites in the detection of colorectal cancer and adenoma. Gut 2022, 71, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Irajizad, E.; Kenney, A.; Tang, T.; Vykoukal, J.; Wu, R.; Murage, E.; Dennison, J.B.; Sans, M.; Long, J.P.; Loftus, M.; et al. A blood-based metabolomic signature predictive of risk for pancreatic cancer. Cell Rep. Med. 2023, 4, 101194. [Google Scholar] [CrossRef]
- Kong, C.; Liang, L.; Liu, G.; Du, L.; Yang, Y.; Liu, J.; Shi, D.; Li, X.; Ma, Y. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut 2023, 72, 1129–1142. [Google Scholar] [CrossRef]
- Tang, W.; Putluri, V.; Ambati, C.R.; Dorsey, T.H.; Putluri, N.; Ambs, S. Liver- and Microbiome-derived Bile Acids Accumulate in Human Breast Tumors and Inhibit Growth and Improve Patient Survival. Clin. Cancer Res. 2019, 25, 5972–5983. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Manghi, P.; Asnicar, F.; Pasolli, E.; Armanini, F.; Zolfo, M.; Beghini, F.; Manara, S.; Karcher, N.; Pozzi, C.; et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 2019, 25, 667–678. [Google Scholar] [CrossRef]
- Gihawi, A.; Ge, Y.; Lu, J.; Puiu, D.; Xu, A.; Cooper, C.S.; Brewer, D.S.; Pertea, M.; Salzberg, S.L. Major data analysis errors invalidate cancer microbiome findings. mBio 2023, 14, e0160723. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zheng, C.C.; Huang, Y.N.; He, M.L.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Chen, S.; Qian, K.; Zhang, G.; Zhang, M. Akkermansia muciniphila and its outer membrane protein Amuc_1100 prophylactically attenuate 5-fluorouracil-induced intestinal mucositis. Biochem. Biophys. Res. Commun. 2022, 614, 34–40. [Google Scholar] [CrossRef]
- Coelho-Rocha, N.D.; de Jesus, L.C.L.; Barroso, F.A.L.; da Silva, T.F.; Ferreira, E.; Goncalves, J.E.; Dos Santos Martins, F.; de Oliveira Carvalho, R.D.; Barh, D.; Azevedo, V.A.C. Evaluation of Probiotic Properties of Novel Brazilian Lactiplantibacillus plantarum Strains. Probiotics Antimicrob. Proteins 2023, 15, 160–174. [Google Scholar] [CrossRef]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Lu, W.; Shen, L.; Wu, Y.; Zhang, Z. The gut microbiota as a booster for radiotherapy: Novel insights into radio-protection and radiation injury. Exp. Hematol. Oncol. 2023, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Herranz, M.; Rafail, S.; Beghi, S.; Gil-de-Gomez, L.; Verginadis, I.; Bittinger, K.; Pustylnikov, S.; Pierini, S.; Perales-Linares, R.; Blair, I.A.; et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Investig. 2020, 130, 466–479. [Google Scholar] [CrossRef]
- Shiao, S.L.; Kershaw, K.M.; Limon, J.J.; You, S.; Yoon, J.; Ko, E.Y.; Guarnerio, J.; Potdar, A.A.; McGovern, D.P.B.; Bose, S.; et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 2021, 39, 1202–1213.e6. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Gazzaniga, F.S.; Wu, M.; Luthens, A.K.; Gillis, J.; Zheng, W.; LaFleur, M.W.; Johnson, S.B.; Morad, G.; Park, E.M.; et al. Targeting PD-L2-RGMb overcomes microbiome-related immunotherapy resistance. Nature 2023, 617, 377–385. [Google Scholar] [CrossRef]
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Thi-Thu Ngo, H.; Son, J.; Hong, Y.; Min, J.J. Exploiting bacteria for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2024, 21, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, L.; Rong, H.; Liu, L.; Yin, J. Engineering Microorganisms for Cancer Immunotherapy. Adv. Healthc. Mater. 2024, 13, e2304649. [Google Scholar] [CrossRef] [PubMed]

| Cancer Type | Number of Cases | ASR | Crude Rate | Cumulative Risk 74 |
|---|---|---|---|---|
| Trachea, bronchus and lung | 2,480,675 | 23.6 | 31.5 | 2.9 |
| Breast | 2,296,840 | 46.8 | 58.7 | 5.1 |
| Colorectum | 1,926,425 | 18.4 | 24.4 | 2.1 |
| Prostate | 1,467,854 | 29.4 | 37.0 | 3.7 |
| Stomach | 968,784 | 9.2 | 12.3 | 1.1 |
| Liver and intrahepatic bile ducts | 866,136 | 8.6 | 11.0 | 1.0 |
| Thyroid | 821,214 | 9.1 | 10.4 | 0.91 |
| Cervix uteri | 662,301 | 14.1 | 16.9 | 1.5 |
| Bladder | 614,298 | 5.6 | 7.8 | 0.64 |
| Non-Hodgkin lymphoma | 553,389 | 5.6 | 7.0 | 0.60 |
| All cancers excl. non-melanoma skin cancer | 18,741,966 | 186.5 | 237.7 | 19.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Quintana, L.; Vázquez-Lorente, H.; Lopez-Garzon, M.; Cortés-Martín, A.; Plaza-Diaz, J. Cancer and the Microbiome of the Human Body. Nutrients 2024, 16, 2790. https://doi.org/10.3390/nu16162790
Herrera-Quintana L, Vázquez-Lorente H, Lopez-Garzon M, Cortés-Martín A, Plaza-Diaz J. Cancer and the Microbiome of the Human Body. Nutrients. 2024; 16(16):2790. https://doi.org/10.3390/nu16162790
Chicago/Turabian StyleHerrera-Quintana, Lourdes, Héctor Vázquez-Lorente, Maria Lopez-Garzon, Adrián Cortés-Martín, and Julio Plaza-Diaz. 2024. "Cancer and the Microbiome of the Human Body" Nutrients 16, no. 16: 2790. https://doi.org/10.3390/nu16162790
APA StyleHerrera-Quintana, L., Vázquez-Lorente, H., Lopez-Garzon, M., Cortés-Martín, A., & Plaza-Diaz, J. (2024). Cancer and the Microbiome of the Human Body. Nutrients, 16(16), 2790. https://doi.org/10.3390/nu16162790







