Gluten Is Not Gluten
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.3. Modified Osborne Fractionation
2.4. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
2.5. Gel Permeation High-Performance Liquid Chromatography (GP-HPLC)
2.6. Data Analysis
3. Results and Discussion
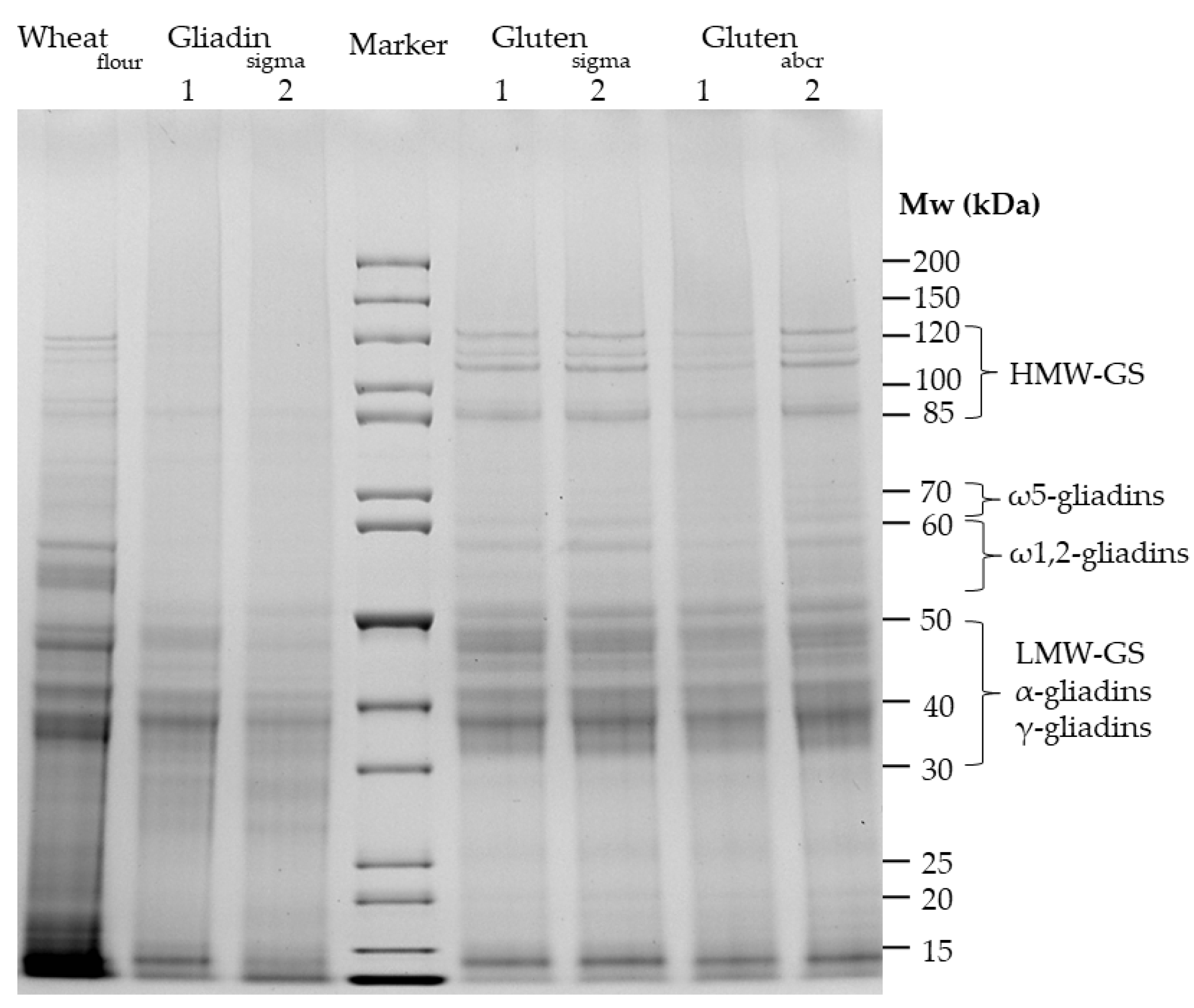
3.1. Qualitative Profile Using SDS-PAGE
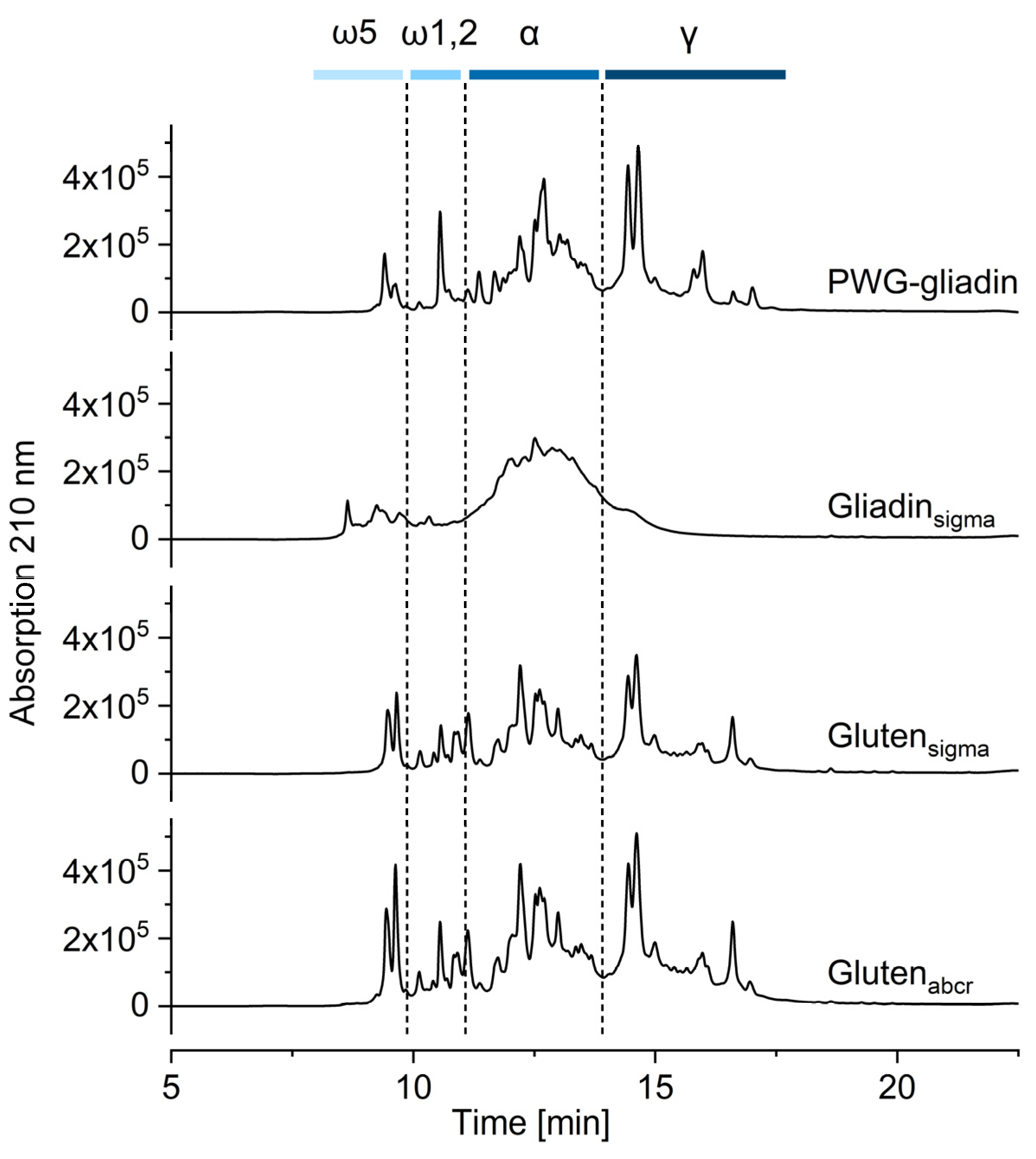
3.2. Qualitative Profile Using RP-HPLC
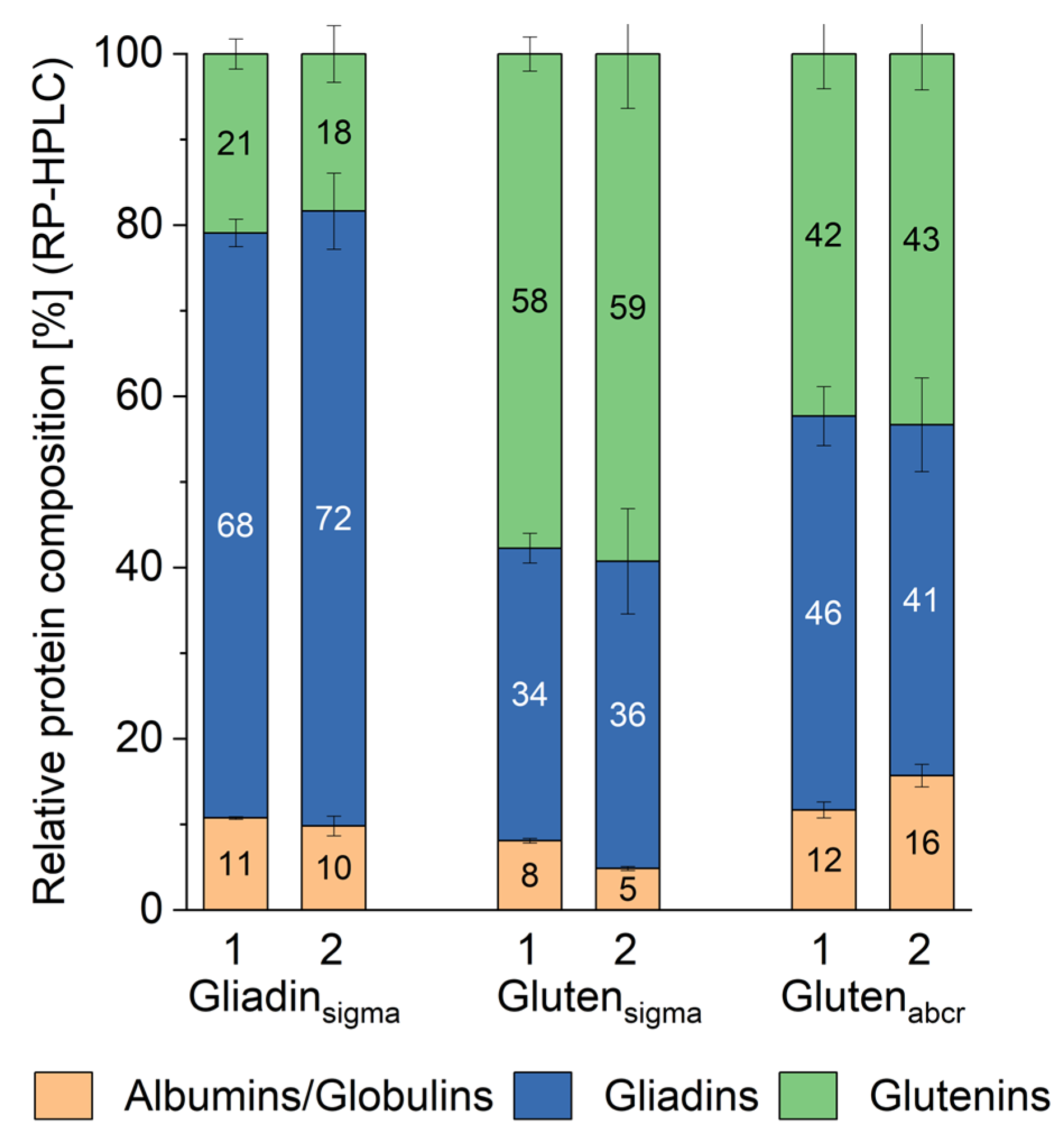
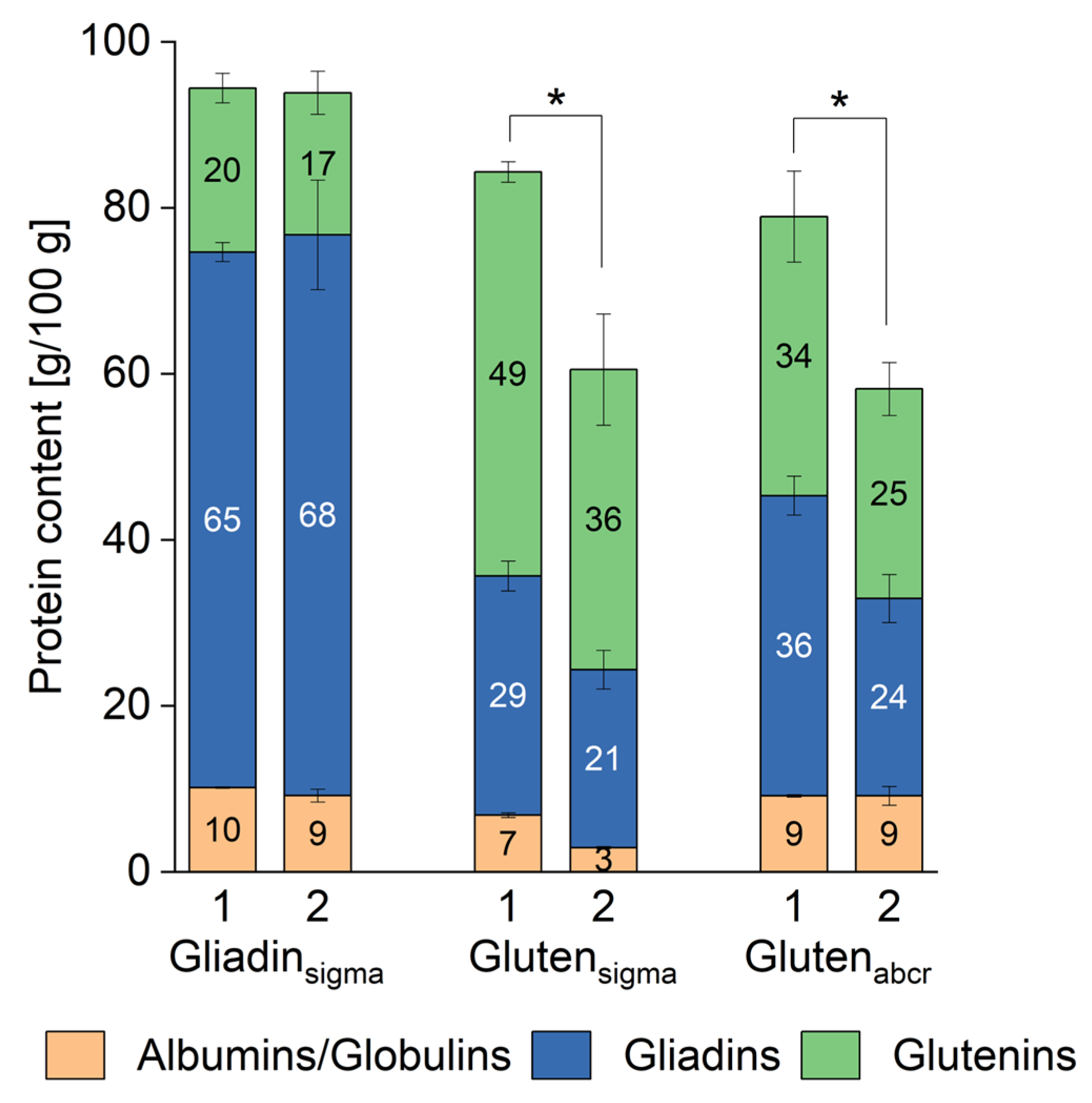
3.3. Quantitative Protein Composition
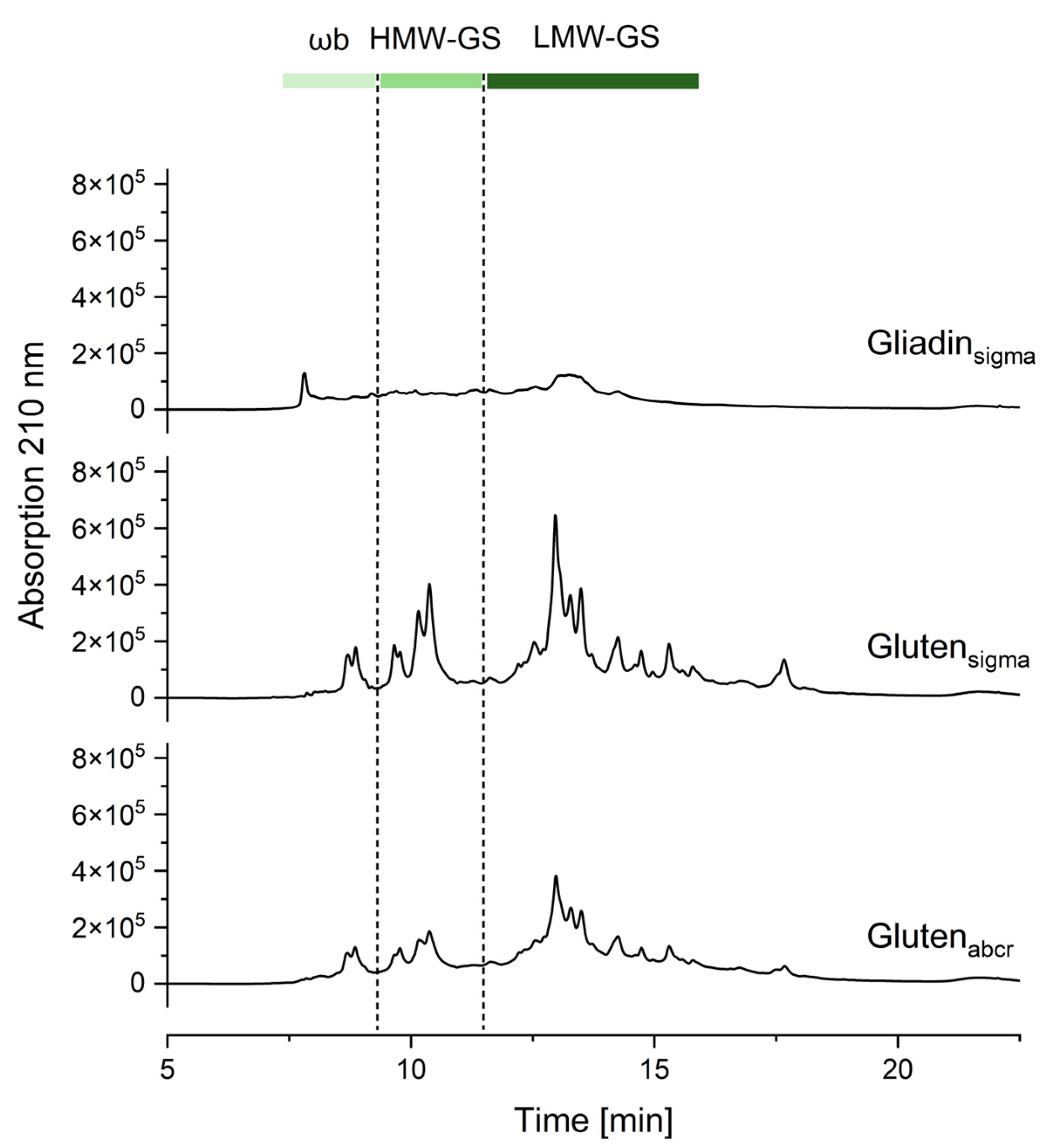
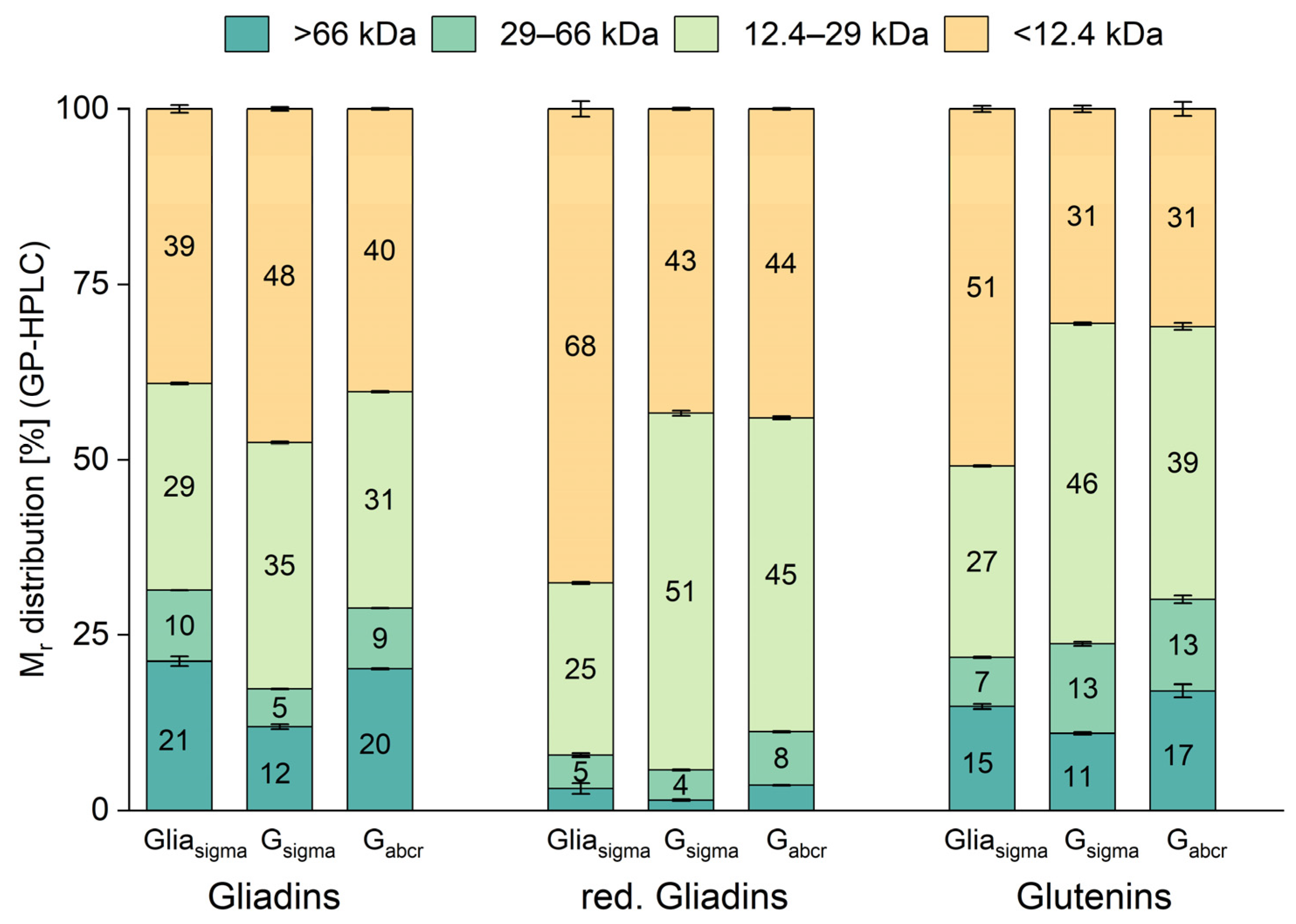
3.4. Relative Molecular Mass Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat gluten functionality as a quality determinant in cereal-based food products. Annu. Rev. Food Sci. Technol. 2012, 3, 469–492. [Google Scholar] [CrossRef]
- CODEX STAN 163-1987; Wheat Protein Products Including Wheat Gluten. FAO: Rome, Italy, 1987; revised in 2001, amended in 2022.
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-gluten uses and industry needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- Schopf, M.; Scherf, K.A. Water absorption capacity determines the functionality of vital gluten related to specific bread volume. Foods 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P. Wheat grain proteins: Past, present, and future. Cereal Chem. 2023, 100, 9–22. [Google Scholar] [CrossRef]
- Iversen, R.; Sollid, L.M. The immunobiology and pathogenesis of celiac disease. Annu. Rev. Pathol. 2023, 18, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Spolidoro, G.C.; Ali, M.M.; Amera, Y.T.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Prevalence estimates of eight big food allergies in Europe: Updated systematic review and meta-analysis. Allergy 2023, 78, 2361–2417. [Google Scholar] [CrossRef] [PubMed]
- CODEX STAN 118-1979; Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten. FAO: Rome, Italy, 1979; revised in 2008, amended in 1983 and 2015.
- Gliadin from Wheat. Available online: https://www.sigmaaldrich.com/DE/en/product/sigma/g3375 (accessed on 5 February 2024).
- Gluten from Wheat. Available online: https://www.sigmaaldrich.com/DE/en/product/sigma/g5004 (accessed on 5 February 2024).
- Amnuaycheewa, P.; Niemann, L.; Goodman, R.E.; Baumert, J.L.; Taylor, S.L. Challenges in gluten analysis: A comparison of four commercial sandwich ELISA kits. Foods 2022, 11, 706. [Google Scholar] [CrossRef]
- Panda, R.; Cho, C.Y.; Ivens, K.O.; Jackson, L.S.; Boyer, M.; Garber, E.A. Multiplex-competitive ELISA for detection and characterization of gluten during yogurt fermentation: Effects of changes in certain fermentation conditions on gluten protein profiles and method reproducibility assessment. J. Agric. Food Chem. 2021, 69, 7742–7754. [Google Scholar] [CrossRef]
- Gregorini, A.; Colomba, M.; Ellis, H.J.; Ciclitira, P.J. Immunogenicity characterization of two ancient wheat α-gliadin peptides related to coeliac disease. Nutrients 2009, 1, 276–290. [Google Scholar] [CrossRef]
- Wei, G.; Tian, N.; Siezen, R.; Schuppan, D.; Helmerhorst, E.J. Identification of food-grade subtilisins as gluten-degrading enzymes to treat celiac disease. Am. J. Physiol. 2016, 311, G571–G580. [Google Scholar] [CrossRef]
- Jayawardana, I.A.; Boland, M.J.; Higgs, K.; Zou, M.; Loo, T.; Mcnabb, W.C.; Montoya, C.A. The kiwifruit enzyme actinidin enhances the hydrolysis of gluten proteins during simulated gastrointestinal digestion. Food Chem. 2021, 341, 128239. [Google Scholar] [CrossRef]
- Gnodi, E.; Mancuso, C.; Elli, L.; Ballarini, E.; Meneveri, R.; Beaulieu, J.F.; Barisani, D. Gliadin, through the activation of innate immunity, triggers lncRNA NEAT1 expression in celiac disease duodenal mucosa. Int. J. Mol. Sci. 2021, 22, 1289. [Google Scholar] [CrossRef] [PubMed]
- Gianfrani, C.; Siciliano, R.A.; Facchiano, A.M.; Camarca, A.; Mazzeo, M.F.; Costantini, S.; Salvati, V.M.; Maurano, F.; Mazzarella, G.; Iaquinto, G.; et al. Transamidation of wheat flour inhibits the response to gliadin of intestinal T cells in celiac disease. Gastroenterology 2007, 133, 780–789. [Google Scholar] [CrossRef]
- Catassi, C.; Fabiani, E.; Iacono, G.; D’Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; De Vitis, I.; et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr. 2007, 85, 160–166. [Google Scholar] [CrossRef]
- Schuppan, D.; Mäki, M.; Lundin, K.E.; Isola, J.; Friesing-Sosnik, T.; Taavela, J.; Popp, A.; Koskenpato, J.; Langhorst, J.; Hovde, Ø.; et al. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N. Engl. J. Med. 2021, 385, 35–45. [Google Scholar] [CrossRef]
- Dotsenko, V.; Oittinen, M.; Taavela, J.; Popp, A.; Peräaho, M.; Staff, S.; Sarin, J.; Leon, F.; Isola, J.; Mäki, M.; et al. Genome-wide transcriptomic analysis of intestinal mucosa in celiac disease patients on a gluten-free diet and postgluten challenge. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 13–32. [Google Scholar] [CrossRef]
- Murray, J.A.; Syage, J.A.; Wu, T.T.; Dickason, M.A.; Ramos, A.G.; Van Dyke, C.; Horwath, I.; Lavin, P.T.; Mäki, M.; Hujoel, I.; et al. Latiglutenase protects the mucosa and attenuates symptom severity in patients with celiac disease exposed to a gluten challenge. Gastroenterology 2022, 163, 1510–1521. [Google Scholar] [CrossRef]
- Singh, A.; Kleinhenz, J.; Brill, H.; Fahey, L.; Silvester, J.A.; Sparks, B.; Verma, R.; Lee, D.; Mallon, D.; Leonard, M.M. A clinician’s guide to gluten challenge. J. Ped. Gastroenterol. Nutr. 2023, 77, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Hajas, L.; Scherf, K.A.; Török, K.; Bugyi, Z.; Schall, E.; Poms, R.E.; Koehler, P.; Tömösközi, S. Variation in protein composition among wheat (Triticum aestivum L.) cultivars to identify cultivars suitable as reference material for wheat gluten analysis. Food Chem. 2018, 267, 387–394. [Google Scholar] [CrossRef]
- Schopf, M.; Wehrli, M.C.; Becker, T.; Jekle, M.; Scherf, K.A. Fundamental characterization of wheat gluten. Eur. Food Res. Technol. 2021, 247, 985–997. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Daveson, A.J.M.; Ee, H.C.; Goel, G.; MacDougall, J.; Acaster, S.; Goldstein, K.E.; Dzuris, J.L.; Neff, K.M.; Truitt, K.E.; et al. Elevated serum interleukin-2 after gluten correlates with symptoms and is a potential diagnostic biomarker for coeliac disease. Aliment. Pharmacol. Ther. 2019, 50, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Schwalb, T.; Wieser, H.; Koehler, P. Comparison of different protein references and ELISA kits for the detection of gluten in foods. In Proceedings of the 24th Meeting of the Working Group on Prolamin Analysis and Toxicity, Ancona, Italy, 30 September–2 October 2010; Stern, M., Ed.; pp. 23–29. Available online: https://www.wgpat.com/proceeding_24th.pdf (accessed on 5 February 2024).
- van Eckert, R.; Berghofer, E.; Ciclitira, P.J.; Chirdo, F.; Denery-Papini, S.; Ellis, H.J.; Ferranti, P.; Goodwin, P.; Immer, U.; Mamone, G.; et al. Towards a new gliadin reference material–isolation and characterisation. J. Cereal Sci. 2006, 43, 331–341. [Google Scholar] [CrossRef]
- Lagrain, B.; Rombouts, I.; Wieser, H.; Delcour, J.A.; Koehler, P. A reassessment of the electrophoretic mobility of high molecular weight glutenin subunits of wheat. J. Cereal Sci. 2012, 56, 726–732. [Google Scholar] [CrossRef]
- Xhaferaj, M.; Muskovics, G.; Schall, E.; Bugyi, Z.; Tömösközi, S.; Scherf, K.A. Characterization of rye flours and their potential as reference material for gluten analysis. Food Chem. 2023, 408, 135148. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Antes, S.; Seilmeier, W. Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. J. Cereal Sci. 1998, 75, 644–650. [Google Scholar] [CrossRef]
- Gabler, A.M.; Scherf, K.A. Comparative characterization of gluten and hydrolyzed wheat proteins. Biomolecules 2020, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, G.; Walker, M.J.; Singh, M.; Gray, K.; Cowen, S.; Ellison, S.L.; Rogers, A.; Balasundaram, A.; Burns, M.; Mills, E.C. Clinically and industrially relevant incurred reference materials to improve analysis of food allergens, milk, egg, almond, hazelnut and walnut. Food Chem. 2024, 434, 137391. [Google Scholar] [CrossRef]
- Schalk, K.; Lexhaller, B.; Koehler, P.; Scherf, K.A. Isolation and characterization of gluten protein types from wheat, rye, barley and oats for use as reference materials. PLoS ONE 2017, 12, e0172819. [Google Scholar] [CrossRef]
- Gabler, A.M.; Gebhard, J.; Norwig, M.-C.; Eberlein, B.; Biedermann, T.; Brockow, K.; Scherf, K.A. Basophil activation to gluten and non-gluten proteins in wheat-dependent exercise-induced anaphylaxis. Front. Allergy 2022, 3, 822554. [Google Scholar] [CrossRef]
- Blish, M.J.; Sandstedt, R.M. An improved method for the preparation of wheat gliadin. Cereal Chem. 1926, 3, 144–149. [Google Scholar]
- Kaushik, R.; Kumar, N.; Sihag, M.K.; Ray, A. Isolation, characterization of wheat gluten and its regeneration properties. J. Food Sci. Technol. 2015, 52, 5930–5937. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Wieser, H.; Koehler, P. Isolation and characterization of high-molecular-weight (HMW) gliadins from wheat flour. Cereal Chem. 2016, 93, 536–542. [Google Scholar] [CrossRef]
- Branlard, G.; Faye, A.; Rhazi, L.; Tahir, A.; Lesage, V.; Aussenac, T. Genetic and environmental factors associated to glutenin polymer characteristics of wheat. Foods 2020, 9, 683. [Google Scholar] [CrossRef] [PubMed]
| Sample | Batch | ω5-Gliadins | ω1,2-Gliadins | α-Gliadins | γ-Gliadins |
|---|---|---|---|---|---|
| [%] 1 | |||||
| Gliadinsigma | 1 | 10.1 ± 0.1 B | 4.9 ± 0.1 C | 73.5 ± 0.2 A | 11.5 ± 0.2 F |
| 2 | 11.2 ± 0.2 A | 5.3 ± 0.6 C | 54.6 ± 0.9 B | 28.9 ± 0.1 E | |
| Glutensigma | 1 | 8.3 ± 0.1 D | 5.0 ± 0.1 C | 46.2 ± 0.5 D | 40.5 ± 0.8 BC |
| 2 | 9.5 ± 0.2 C | 13.4 ± 0.3 A | 41.8 ± 0.1 E | 35.3 ± 0.4 D | |
| Glutenabcr | 1 | 8.7 ± 0.1 D | 5.3 ± 0.1 C | 46.0 ± 0.8 D | 40.0 ± 0.7 C |
| 2 | 8.6 ± 0.1 D | 4.9 ± 0.3 C | 43.2 ± 0.2 E | 43.3 ± 0.5 A | |
| PWG-Gliadin | 4.1 ± 0.2 E | 6.3 ± 0.1 B | 47.9 ± 0.4 C | 41.7 ± 0.3 B | |
| Sample | Batch | ωb-Gliadins | HMW-GS | LMW-GS |
|---|---|---|---|---|
| [%] 1 | ||||
| Gliadinsigma | 1 | 13.2 ± 0.5 A | 30.9 ± 3.5 A | 55.9 ± 0.5 C |
| 2 | 8.7 ± 1.0 B | 26.7 ± 0.5 A | 64.5 ± 0.3 B | |
| Glutensigma | 1 | 6.8 ± 0.2 CD | 23.4 ± 0.6 A | 69.8 ± 0.1 A |
| 2 | 5.0 ± 1.1 D | 26.1 ± 2.1 A | 68.9 ± 0.9 A | |
| Glutenabcr | 1 | 7.2 ± 0.4 BC | 23.9 ± 5.2 A | 68.9 ± 0.1 A |
| 2 | 5.0 ± 0.9 D | 28.6 ± 2.4 A | 66.5 ± 1.4 B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xhaferaj, M.; Scherf, K.A. Gluten Is Not Gluten. Nutrients 2024, 16, 2745. https://doi.org/10.3390/nu16162745
Xhaferaj M, Scherf KA. Gluten Is Not Gluten. Nutrients. 2024; 16(16):2745. https://doi.org/10.3390/nu16162745
Chicago/Turabian StyleXhaferaj, Majlinda, and Katharina Anne Scherf. 2024. "Gluten Is Not Gluten" Nutrients 16, no. 16: 2745. https://doi.org/10.3390/nu16162745
APA StyleXhaferaj, M., & Scherf, K. A. (2024). Gluten Is Not Gluten. Nutrients, 16(16), 2745. https://doi.org/10.3390/nu16162745







