The Antioxidant Potential of Vitamins and Their Implication in Metabolic Abnormalities
Abstract
1. Introduction
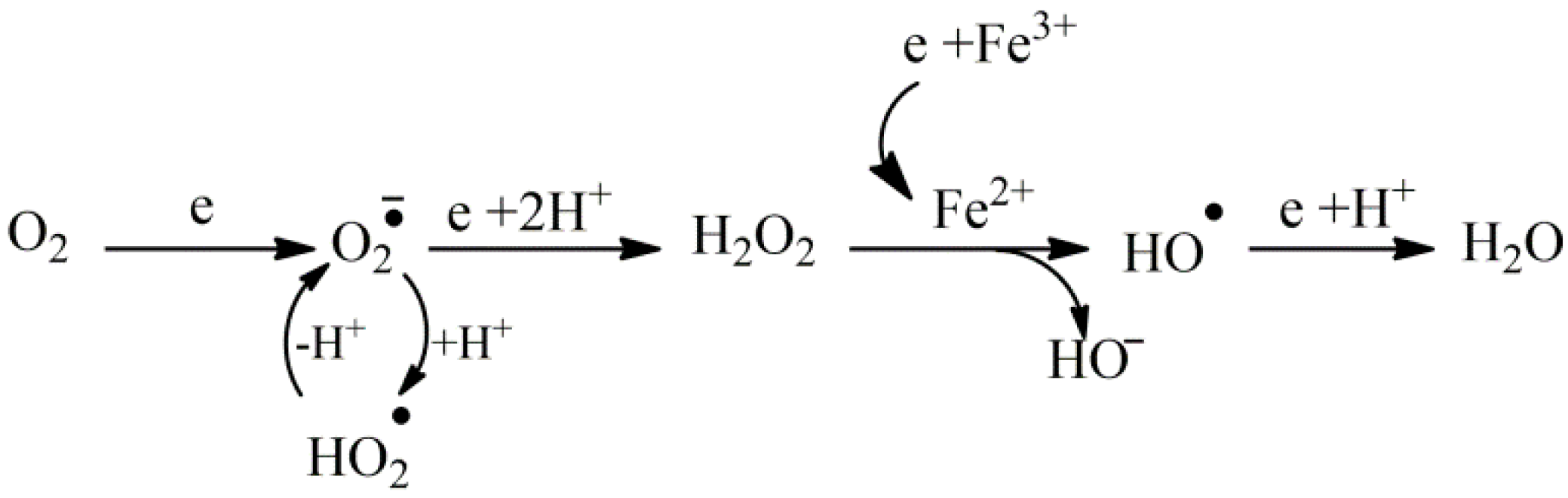
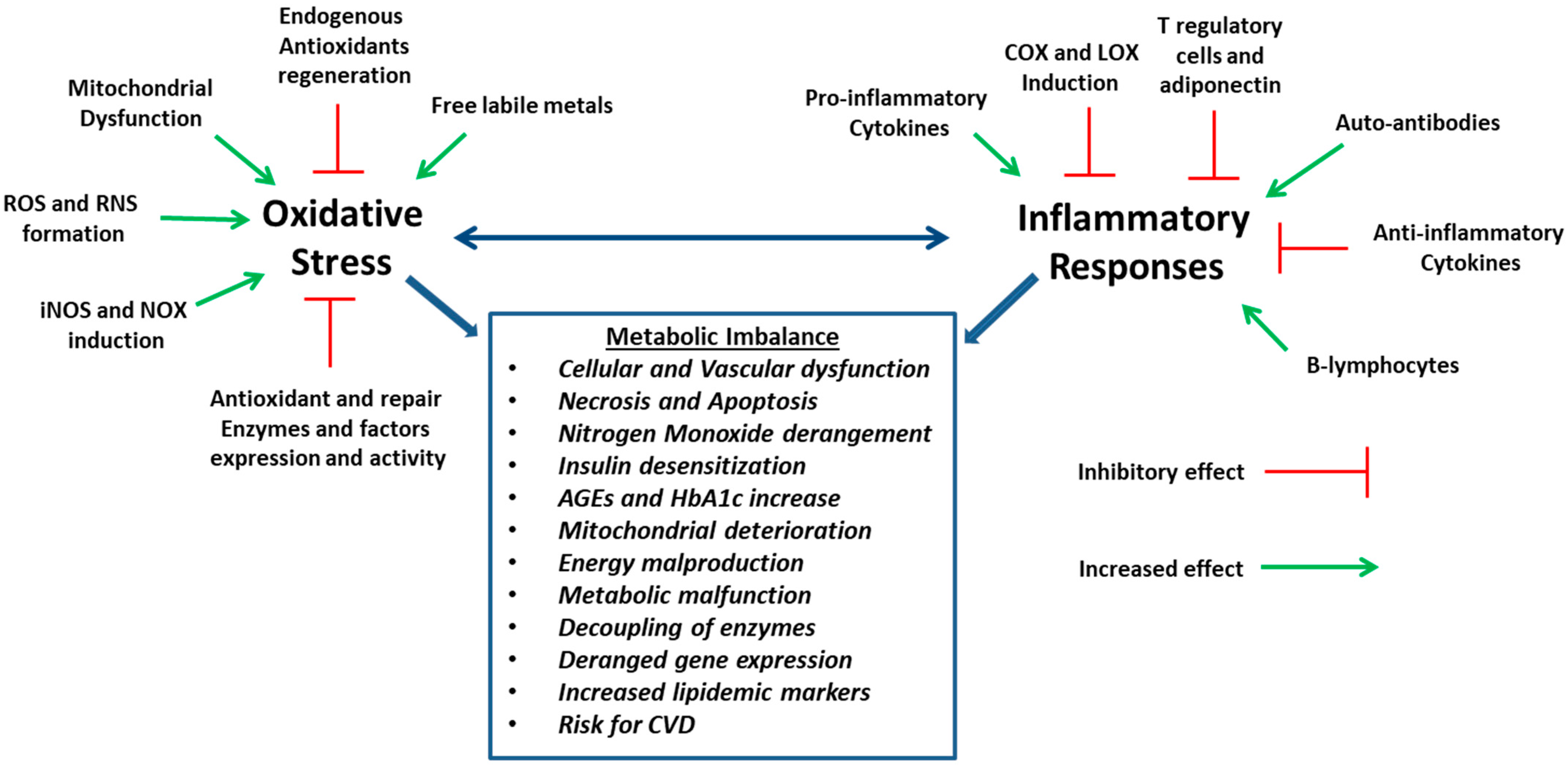
2. Cell Degeneration and Oxidative Stress
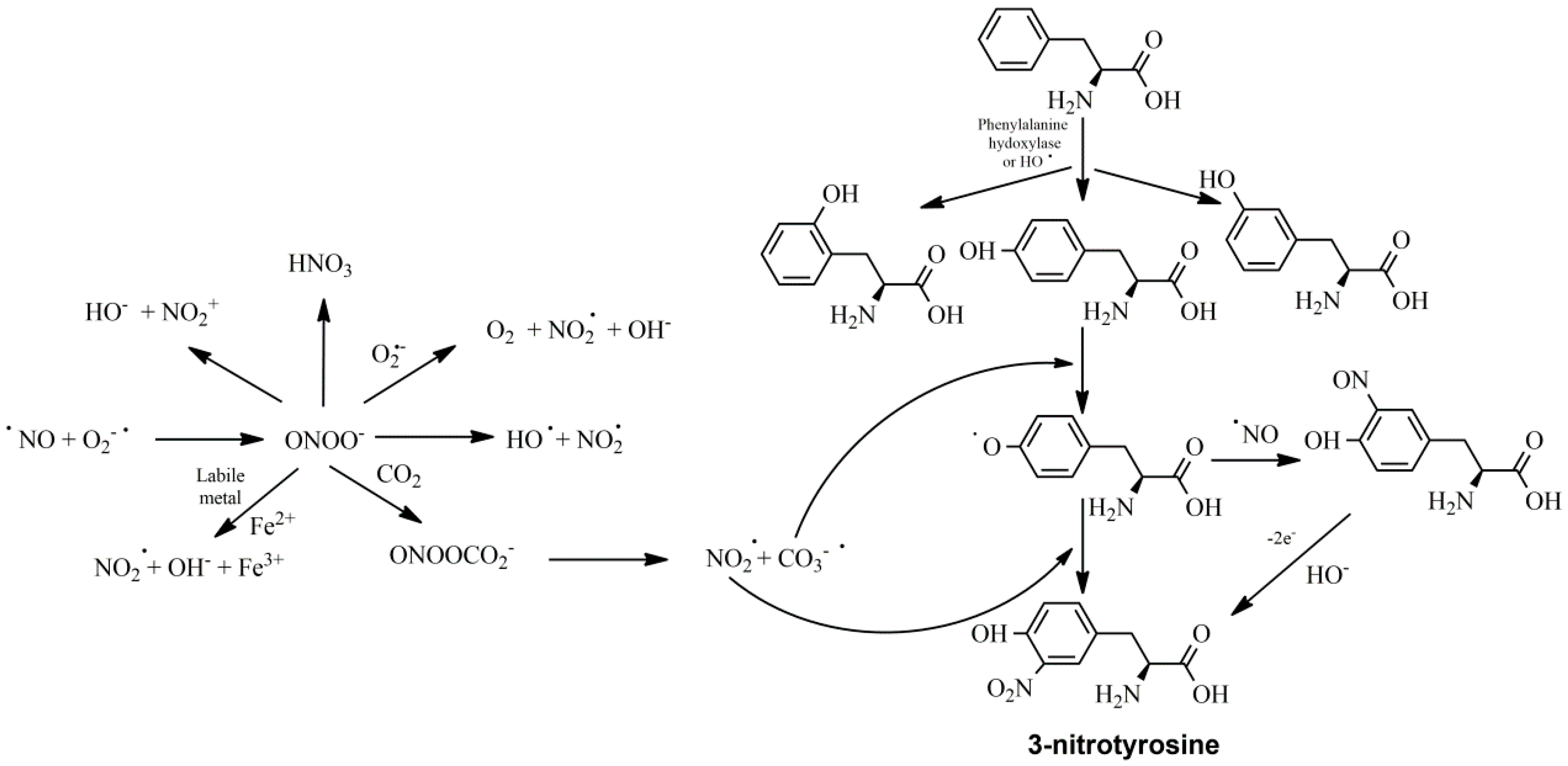
3. Inflammation and Oxidative Stress
4. Oxidative Stress, Metabolic Diseases and Cardiovascular Disorders
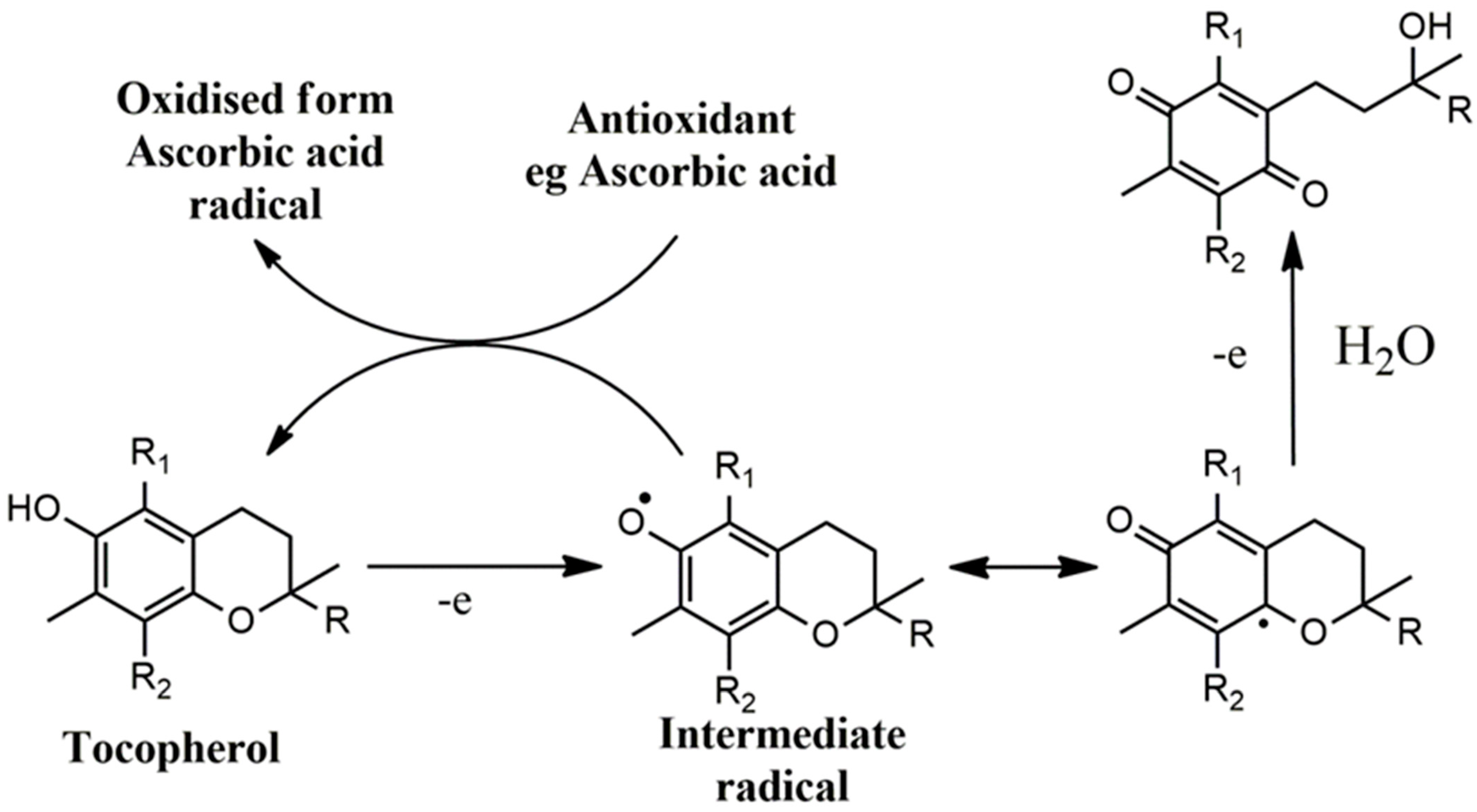
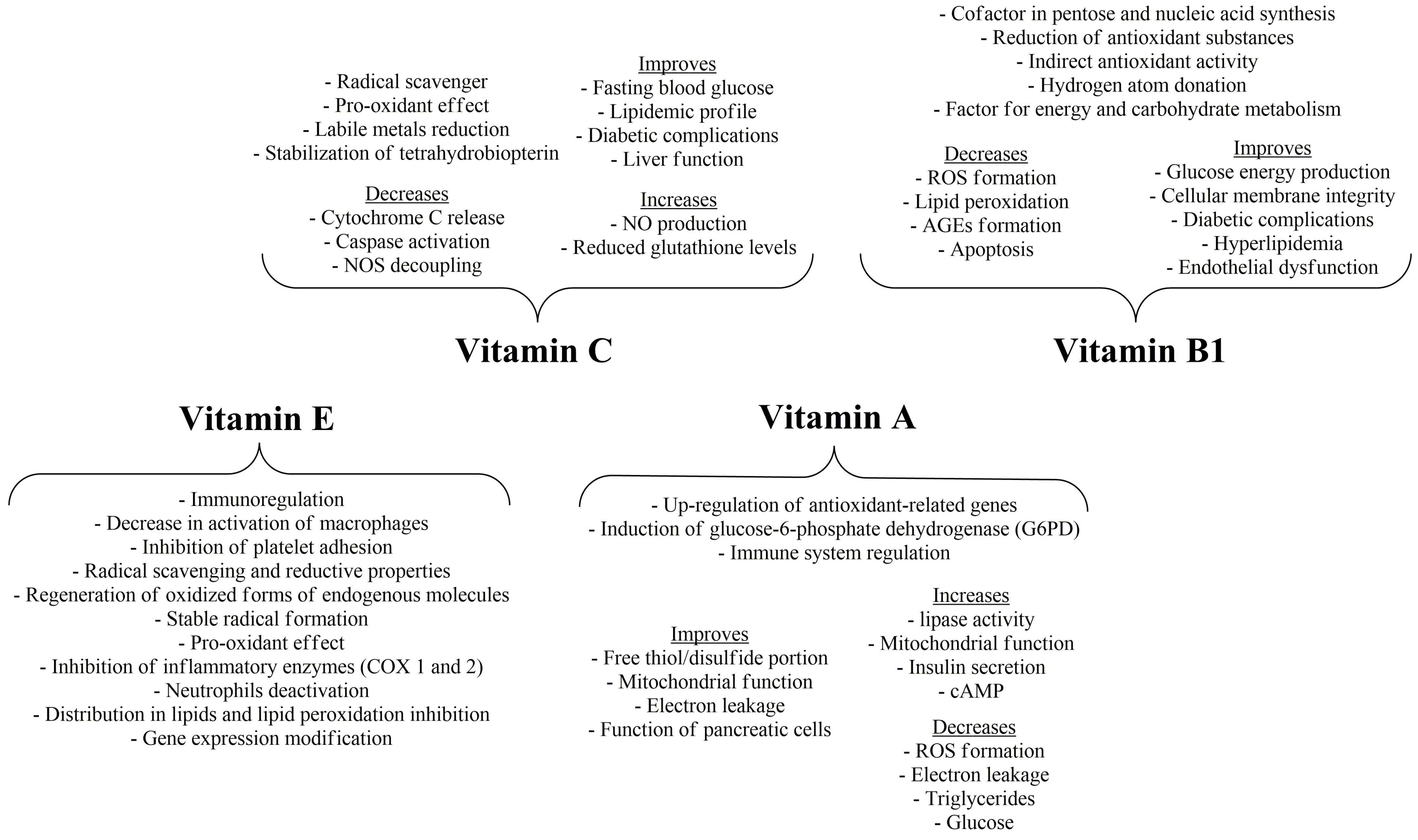
5. Vitamin E and the Potential of Vitamin C
6. Vitamin A
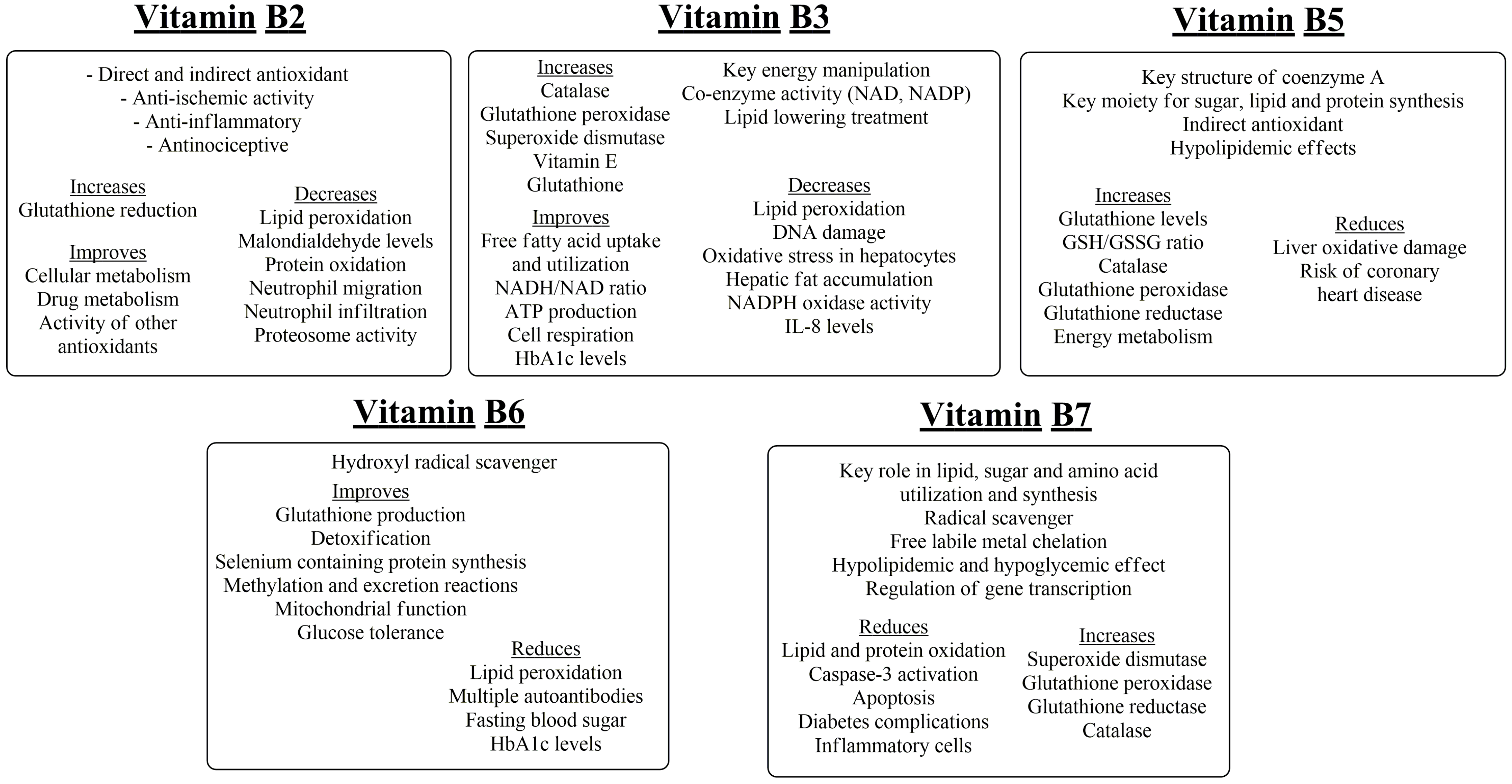
7. Thiamine, Riboflavin
8. Niacin and Pantothenic Acid
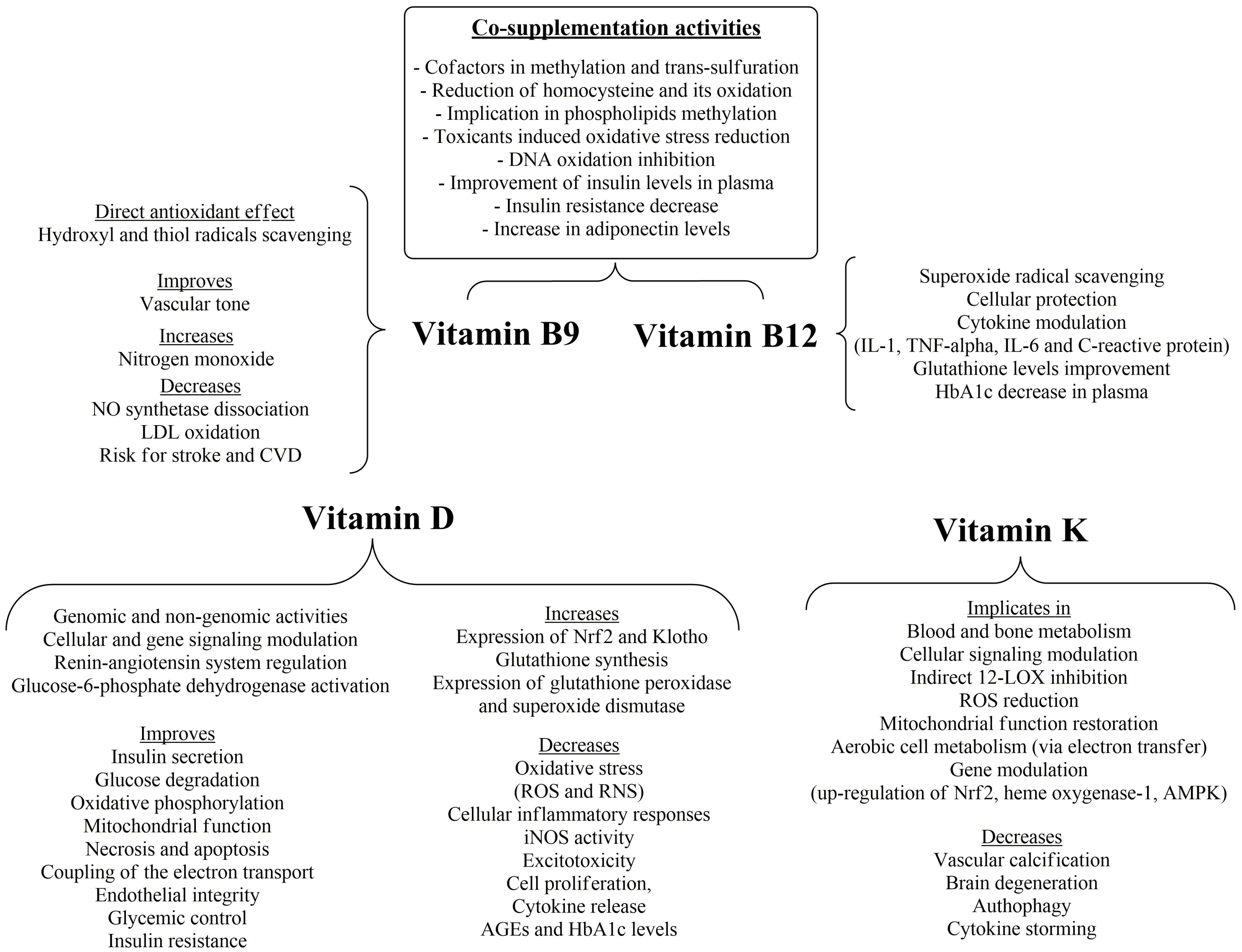
9. Vitamin B6, Biotin, Folic Acid and Vitamin B12
10. Vitamin D
11. Vitamin K
12. Lipoic Acid, Low-Molecular-Weight-Thiol-Containing Compounds, L-Carnitine and Co-Enzyme Q10
13. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Palio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 217, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Shibata, H.; Shimizu, T.; Shibata, S.; Toriumi, H.; Ebine, T. Differential cellular localization of antioxidant enzymes in the trigeminal ganglion. Neuroscience 2013, 248, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Garrel, C.; Faure, P.; Sugino, N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod. Biomed. Online 2012, 25, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, E.; Nobelos, P.; Geronikaki, A.; Rekka, E.A. Selected heterocyclic compounds as antioxidants. Synthesis and biological evaluation. Curr. Top. Med. Chem. 2014, 14, 2462–2477. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Coughlan, M.T.; Cooper, M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 2008, 57, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Moses, G. The safety of commonly used vitamins and minerals. Aust. Prescr. 2021, 44, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense system, and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef]
- Rennen, H.J.J.M.; Bleeker-Rovers, C.P.; Oyen, W.J.G. The pathology of inflammation and infection. In Diagnostic Nuclear Medicine, 2nd ed.; Schiepers, C., Ed.; Springer: Heidelberg, Germany, 2006; p. 114. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Duvall, E.; Wyllie, A.H.; Morris, R.G. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology 1985, 56, 351–358. [Google Scholar]
- Godson, C.; Mitchell, S.; Harvey, K.; Petasis, N.A.; Hogg, N.; Brady, H.R. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 2000, 164, 1663–1667. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Koziol, A.; Plytycz, B.; Arnold, B. Inflammatory macrophages, and not only neutrophils, die by apoptosis during acute peritonitis. Immunobiology 2010, 215, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Bellingan, J.; Caldwell, H.; Howie, E.; Dransfield, I.; Haslett, C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: Inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 1996, 157, 2577–2585. [Google Scholar] [CrossRef]
- Smith, W.L.; Malkowski, M.G. Interactions of fatty acids, nonsteroidal anti-inflammatory drugs, and coxibs with the catalytic and allosteric subunits of cyclooxygenases-1 and -2. J. Biol. Chem. 2019, 294, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Samuelsson, B. On the mechanism of the biosynthesis of prostaglandins E-1 and F-1-alpha. J. Biol. Chem. 1967, 242, 5336–5343. [Google Scholar] [CrossRef]
- Prigge, S.T.; Boyington, J.C.; Faig, M.; Doctor, K.S.; Gaffney, B.J.; Amzel, L.M. Structure and mechanism of lipoxygenases. Biochimie 1997, 79, 629–636. [Google Scholar] [CrossRef]
- Erba, F.; Mei, G.; Minicozzi, V.; Sabatucci, A.; Di Venere, A.; Maccarrone, M. Conformational Dynamics of Lipoxygenases and Their Interaction with Biological Membranes. Int. J. Mol. Sci. 2024, 25, 2241. [Google Scholar] [CrossRef]
- Forman, H.J.; Torres, M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002, 166, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Bayraktutan, U.; Blayney, L.; Shah, A.M. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Harris, C.C. Inflammation and cancer: An ancient link with novel potentials. Int. J. Cancer 2007, 121, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Sanlioglu, S.; Williams, C.M.; Samavati, L.; Butler, N.S.; Wang, G.; McCray, P.B., Jr.; Ritchie, T.C.; Hunninghake, G.W.; Zandi, E.; Engelhardt, J.F. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-α secretion through IKK regulation of NF-κB. J. Biol. Chem. 2001, 276, 30188–30198. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.B.; Park, D.J.; Shah, M.A.; Kim, M.O.; Koh, P.O. Lipopolysaccharide induces neuroglia activation and NF-κB activation in cerebral cortex of adult mice. Lab. Anim. Res. 2019, 35, 19. [Google Scholar] [CrossRef]
- Delhalle, S.; Deregowski, V.; Benoit, V.; Merville, M.P.; Bours, V. NF-kappaB-dependent MnSOD expression protects adenocarcinoma cells from TNF-alpha-induced apoptosis. Oncogene 2002, 21, 3917–3924. [Google Scholar] [CrossRef]
- Pham, C.G.; Bubici, C.; Zazzeroni, F.; Papa, S.; Jones, J.; Alvarez, K.; Jayawardena, S.; De Smaele, E.; Cong, R.; Beaumont, C.; et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 2004, 119, 529–542. [Google Scholar] [CrossRef]
- Schulze-Osthoff, K.; Ferrari, D.; Los, M.; Wesselborg, S.; Peter, M.E. Apoptosis signaling by death receptors. Eur. J. Biochem. 1998, 254, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Bauer, M.K.; Vogt, M.; Wesselborg, S. Oxidative stress and signal transduction. Int. J. Vitam. Nutr. Res. 1997, 67, 336–342. [Google Scholar]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.H.; Chen, X.; Sang, S.; Lee, M.J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: Effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Plummer, S.M.; Holloway, K.A.; Manson, M.M.; Munks, R.J.; Kaptein, A.; Farrow, S.; Howells, L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene 1999, 18, 6013–6020. [Google Scholar] [CrossRef]
- Brouet, I.; Ohshima, H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem. Biophys. Res. Commun. 1995, 206, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Papagiouvanis, G.; Pantelidou, M.; Kourounakis, P.N.; Athanasekou, C.; Rekka, E.A. Design, synthesis and study of nitrogen monoxide donors as potent hypolipidaemic and anti-inflammatory agents. Molecules 2019, 25, 19. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Holvoet, P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh. K. Acad. Geneeskd. Belg. 2008, 70, 193–219. [Google Scholar]
- Siasos, G.; Tsigkou, V.; Kosmopoulos, M.; Theodosiadis, D.; Simantiris, S.; Tagkou, N.M.; Tsimpiktsioglou, A.; Stampouloglou, P.K.; Oikonomou, E.; Mourouzis, K.; et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann. Transl. Med. 2018, 6, 256. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef]
- Zhang, P.N.; Zhou, M.Q.; Guo, J.; Zheng, H.J.; Tang, J.; Zhang, C.; Liu, Y.N.; Liu, W.J.; Wang, Y.X. Mitochondrial Dysfunction and Diabetic Nephropathy: Nontraditional Therapeutic Opportunities. J. Diabetes Res. 2021, 2021, 1010268. [Google Scholar] [CrossRef]
- Chung, S.S.; Ho, E.C.; Lam, K.S.; Chung, S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003, 14, S233–S236. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: Review of the literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Basta, G.; Del Turco, S.; De Caterina, R. Advanced glycation endproducts: Implications for accelerated atherosclerosis in diabetes. Recent. Prog. Med. 2004, 95, 67–80. [Google Scholar]
- Wautier, M.P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Kwon, Y.H. Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice. Nutr. Res. Pract. 2016, 10, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Ghosh, M. Protective role of phytosterol esters in combating oxidative hepatocellular injury in hypercholesterolemic rats. Pak. J. Biol. Sci. 2013, 16, 59–66. [Google Scholar]
- Del Ben, M.; Angelico, F.; Cangemi, R.; Loffredo, L.; Carnevale, R.; Augelletti, T.; Baratta, F.; Polimeni, L.; Pignatelli, P.; Violi, F. Moderate weight loss decreases oxidative stress and increases antioxidant status in patients with metabolic syndrome. ISRN Obes. 2012, 2012, 960427. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Katritsis, D.; Raggi, P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014, 233, 104–112. [Google Scholar] [CrossRef]
- Bradley, R.D.; Fitzpatrick, A.L.; Jacobs, D.R., Jr.; Lee, D.H.; Swords, J.N.; Herrington, D. Associations between γ-glutamyltransferase (GGT) and biomarkers of atherosclerosis: The multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 2014, 233, 387–393. [Google Scholar] [CrossRef]
- Li, N.; Fu, J.; Koonen, D.P.; Kuivenhoven, J.A.; Snieder, H.; Hofker, M.H. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis 2014, 233, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Lichtenstein, A.H.; Ip, S.; Lau, J.; Balk, E.M. Comparability of methods for LDL subfraction determination: A systematic review. Atherosclerosis 2009, 20, 342–348. [Google Scholar] [CrossRef]
- Mikhailidis, D.P.; Elisaf, M.; Rizzo, M.; Berneis, K.; Griffin, B.; Zambon, A.; Athyros, V.; de Graaf, J.; März, W.; Parhofer, K.G.; et al. “European panel on low density lipoprotein (LDL) subclasses”: A statement on the pathophysiology, atherogenicity and clinical significance of LDL subclasses. Curr. Vasc. Pharmacol. 2011, 9, 533–571. [Google Scholar] [CrossRef]
- Younis, N.; Charlton, M.V.; Sharma, R.; Soran, H.; Durrington, P.N. Glycation of LDL in non-diabetic people: Small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis 2009, 202, 162–168. [Google Scholar] [CrossRef]
- Sutton, G.; Pugh, D.; Dhaun, N. Developments in the role of endothelin-1 in atherosclerosis: A potential therapeutic target? Am. J. Hypertens. 2019, 32, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. A review on vitamin E natural analogues and on the design of synthetic vitamin E derivatives as cytoprotective agents. Mini Rev. Med. Chem. 2021, 21, 10–22. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Glynn, R.J.; Ridker, P.M.; Goldhaber, S.Z.; Zee, R.Y.L.; Buring, J.E. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism. Circulation 2007, 116, 1497–1503. [Google Scholar] [CrossRef]
- Beharka, A.A.; Wu, D.; Serafini, M.; Meydani, S.N. Mechanism of vitamin E inhibition of cyclooxygenase activity in macrophages from old mice: Role of peroxynitrite. Free Radic. Biol. Med. 2002, 32, 503–511. [Google Scholar] [CrossRef]
- Tsiakitzis, K.; Kourounakis, A.P.; Tani, E.; Rekka, E.A.; Kourounakis, P.N. Stress and active oxygen species—Effect of alpha-tocopherol on stress response. Arch. Pharm. 2005, 338, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Azzi, A. Present trends in vitamin E research. Biofactors 2010, 36, 33–42. [Google Scholar] [CrossRef]
- Sarir, H.; Emdadifard, G.; Farhangfar, H.; TaheriChadorneshin, H. Effect of vitamin E succinate on inflammatory cytokines induced by high-intensity interval training. J. Res. Med. Sci. 2015, 20, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Athanasekou, C.; Rekka, E.A. Dual antioxidant structures with potent anti-inflammatory, hypolipidemic and cytoprotective properties. Bioorg. Med. Chem. Lett. 2017, 27, 4800–4804. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Clarendon Press: Oxford, UK, 1999; pp. 208–219. [Google Scholar]
- Rietjens, I.; Boersma, M.; De Haan, L. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2001, 11, 321–333. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E and K interactions—A 50-year-old problem. Nutr. Rev. 2008, 66, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, R.S.; Hollar, D.; Hebert, P.R.; Lamas, G.A.; Hennekens, C.H. Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch. Intern. Med. 2004, 164, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; de Serna, D.G.; Gutierrez, A.; Schade, D.S. Vitamin E in humans: An explanation of clinical trial failure. Endocr. Pract. 2006, 12, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Takemura, Y.; Satoh, M.; Satoh, K.; Hamada, H.; Sekido, Y.; Kubota, S. High dose of ascorbic acid induces cell death in mesothelioma cells. Biochem. Biophys. Res. Commun. 2010, 394, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Farbstein, D.; Kozak-Blickstein, A.; Levy, A.P. Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules 2010, 15, 8098–8110. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, E.; Hasegawa, K.; Irie, J.; Ide, S.; Ushiki, J.; Yamaguchi, K.; Oda, S.; Matsuda, Y. L-ascorbic acid stimulates expression of smooth muscle-specific markers in smooth muscle cells both in vitro and in vivo. J. Cardiovasc. Pharmacol. 2003, 42, 745–751. [Google Scholar] [CrossRef] [PubMed]
- El-Aal, A.A.; El-Ghffar, E.A.A.; Ghali, A.A.; Zughbur, M.R.; Sirdah, M.M. The effect of vitamin C and/or E supplementations on type 2 diabetic adult males under metformin treatment: A single-blinded randomized controlled clinical trial. Diabetes Metab. Syndr. 2018, 12, 483–489. [Google Scholar] [CrossRef]
- Nosratabadi, S.; Ashtary-Larky, D.; Hosseini, F.; Namkhah, Z.; Mohammadi, S.; Salamat, S.; Nadery, M.; Yarmand, S.; Zamani, M.; Wong, A.; et al. The effects of vitamin C supplementation on glycemic control in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2023, 17, 102824. [Google Scholar] [CrossRef]
- Shimizu, H.; Tsubota, T.; Kanki, K.; Shiota, G. All-trans retinoic acid ameliorates hepatic stellate cell activation via suppression of thioredoxin interacting protein expression. J. Cell. Physiol. 2018, 233, 607–616. [Google Scholar] [CrossRef]
- Urvalek, A.M.; Gudas, L.J. Retinoic acid and histone deacetylases regulate epigenetic changes in embryonic stem cells. J. Biol. Chem. 2014, 289, 19519–19530. [Google Scholar] [CrossRef]
- Gad, A.; Abu Hamed, S.; Khalifa, M.; Amin, A.; El-Sayed, A.; Swiefy, S.A.; El-Assal, S. Retinoic acid improves maturation rate and upregulates the expression of antioxidant-related genes in in vitro matured buffalo (Bubalus bubalis) oocytes. Int. J. Vet. Sci. Med. 2018, 6, 279–285. [Google Scholar] [CrossRef]
- de Oliveira, M.R. Vitamin A and retinoids as mitochondrial toxicants. Oxidative Med. Cell. Longev. 2015, 2015, 140267. [Google Scholar] [CrossRef]
- Malivindi, R.; Rago, V.; De Rose, D.; Gervasi, M.C.; Cione, E.; Russo, G.; Santoro, M.; Aquila, S. Influence of all-trans retinoic acid on sperm metabolism and oxidative stress: Its involvement in the physiopathology of varicocele-associated male infertility. J. Cell. Physiol. 2018, 233, 9526–9537. [Google Scholar] [CrossRef]
- Trasino, S.E.; Benoit, Y.D.; Gudas, L.J. Vitamin A deficiency causes hyperglycemia and loss of pancreatic β-cell mass. J. Biol. Chem. 2015, 290, 1456–1473. [Google Scholar] [CrossRef]
- Trasino, S.E.; Gudas, L.J. Vitamin A: A missing link in diabetes? Diabetes Manag. 2015, 5, 359–367. [Google Scholar] [CrossRef]
- Amisten, S.; Mohammad Al-Amily, I.; Soni, A.; Hawkes, R.; Atanes, P.; Persaud, S.J.; Rorsman, P.; Salehi, A. Anti-diabetic action of all-trans retinoic acid and the orphan G protein coupled receptor GPRC5C in pancreatic β-cells. Endocr. J. 2017, 64, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Shroff, M.R.; Chen, X.; Beydoun, H.A.; Wang, Y.; Zonderman, A.B. Serum antioxidant status is associated with metabolic syndrome among U.S. Adults in recent national surveys. J. Nutr. 2011, 141, 903–913. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Quang, D.D. Unraveling the antioxidant potential of thiamine: Thermochemical and kinetics studies in aqueous phase using DFT. Vietnam. J. Chem. 2019, 57, 485–490. [Google Scholar] [CrossRef]
- Bâ, A. Metabolic and structural role of thiamine in nervous tissues. Cell. Mol. Neurobiol. 2008, 28, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, E.; Mazzeo, A.; Porta, M. Thiamine and diabetes: Back to the future? Acta Diabetol. 2021, 58, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Stirban, A.; Negrean, M.; Stratmann, B.; Gawlowski, T.; Horstmann, T.; Götting, C.; Kleesiek, K.; Mueller-Roesel, M.; Koschinsky, T.; Uribarri, J.; et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care 2006, 29, 2064–2071. [Google Scholar] [CrossRef]
- Lukienko, P.I.; Mel’nichenko, N.G.; Zverinskii, I.V.; Zabrodskaya, S.V. Antioxidant properties of thiamine. Bull. Exp. Biol. Med. 2000, 130, 874–876. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Liu, J.; O’Keefe, J.H. Thiamine and Cardiovascular Disease: A Literature Review. Prog. Cardiovasc. Dis. 2018, 61, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Ghanta, S.N.; Saluja, P.; Chidambaram, V.; Mehta, J.L. The Interplay of Thiamine and Cardiovascular Diseases. In Hydrophilic Vitamins in Health and Disease; Shah, A.K., Tappia, P.S., Dhalla, N.S., Eds.; Advances in Biochemistry in Health and Disease; Springer: Cham, Switzerland, 2024; Volume 29. [Google Scholar]
- Jain, A.; Mehta, R.; Al-Ani, M.; Hill, J.A.; Winchester, D.E. Determining the role of thiamine deficiency in systolic heart failure: A meta-analysis and systematic review. J. Card. Fail. 2015, 21, 1000–1007. [Google Scholar] [CrossRef]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Lu, X.; Zhao, X. Riboflavin alleviates cardiac failure in Type I diabetic cardiomyopathy. Heart Int. 2011, 6, e21. [Google Scholar] [CrossRef]
- Toyosaki, T. Antioxidant effect of riboflavin in enzymic lipid peroxidation. J. Agric. Food Chem. 1992, 40, 1727–1730. [Google Scholar] [CrossRef]
- Hultquist, D.E.; Xu, F.; Quandt, K.S.; Shlafer, M.; Mack, C.P.; Till, G.O.; Seekamp, A.; Betz, A.L.; Ennis, S.R. Evidence that NADPH-dependent methemoglobin reductase and administered riboflavin protect tissues from oxidative injury. Am. J. Hematol. 1993, 42, 13–18. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Verdrengh, M.; Tarkowski, A. Riboflavin in innate and acquired immune responses. Inflamm. Res. 2005, 9, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Leskova, E.J.; Kubikova, E.; Kovacikova, K. Vitamin losses: Retention during heat treatment and continual changes expressed by mathematical models. J. Food Comp. Anal. 2006, 19, 252–276. [Google Scholar] [CrossRef]
- Tupe, R.S.; Tupe, S.G.; Agte, V.V. Dietary nicotinic acid supplementation improves hepatic zinc uptake and offers hepatoprotection against oxidative damage. Br. J. Nutr. 2011, 105, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kim, H.J.; Rodriguez-Iturbe, B.; Vaziri, N.D. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am. J. Physiol. Ren. Physiol. 2009, 297, F106–F113. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.H.; Kashyap, M.L.; Kamanna, V.S. Niacin inhibits fat accumulation, oxidative stress, and inflammatory cytokine IL-8 in cultured hepatocytes: Impact on non-alcoholic fatty liver disease. Metabolism 2015, 64, 982–990. [Google Scholar] [CrossRef]
- Taylor, J.K.; Plaisance, E.P.; Mahurin, A.J.; Mestek, M.L.; Moncada-Jimenez, J.; Grandjean, P.W. Paraoxonase responses to exercise and niacin therapy in men with metabolic syndrome. Redox Rep. 2015, 20, 42–48. [Google Scholar] [CrossRef]
- Trueblood, N.A.; Ramasamy, R.; Wang, L.F.; Schaefer, S. Niacin protects the isolated heart from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H764–H771. [Google Scholar] [CrossRef]
- Yan, X.; Wang, S. The efficacy of niacin supplementation in type 2 diabetes patients: Study protocol of a randomized controlled trial. Medicine 2021, 100, e22272. [Google Scholar] [CrossRef]
- Abdullah, K.M.; Alam, M.M.; Iqbal, Z.; Naseem, I. Therapeutic effect of vitamin B3 on hyperglycemia, oxidative stress and DNA damage in alloxan induced diabetic rat model. Biomed. Pharmacother. 2018, 105, 1223–1231. [Google Scholar] [CrossRef]
- Harris, E. High Niacin Levels May Raise Cardiovascular Disease Risk. JAMA 2024, 331, 1001. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, L.; Slyshenkov, V.S. Protection by pantothenic acid against apoptosis and cell damage by oxygen free radicals—The role of glutathione. Biofactors 2003, 17, 61–73. [Google Scholar] [CrossRef]
- Demirci, B.; Demir, O.; Dost, T.; Birincioglu, M. Protective effect of vitamin B5 (dexpanthenol) on cardiovascular damage induced by streptozocin in rats. Bratisl. Lek. Listy 2014, 115, 190–196. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.K.; Choi, B.Y. The long-term relationship between dietary pantothenic acid (vitamin B5) intake and C-reactive protein concentration in adults aged 40 years and older. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Araki, A.; Yoshimura, Y.; Sakurai, T.; Umegaki, H.; Kamada, C.; Iimuro, S.; Ohashi, Y.; Ito, H. Japanese Elderly Diabetes Intervention Trial Research Group. Low intakes of carotene, vitamin B2, pantothenate and calcium predict cognitive decline among elderly patients with diabetes mellitus: The Japanese Elderly Diabetes Intervention Trial. Geriatr. Gerontol. Int. 2017, 17, 1168–1175. [Google Scholar] [CrossRef]
- Evans, M.; Rumberger, J.A.; Azumano, I.; Napolitano, J.J.; Citrolo, D.; Kamiya, T. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: A triple-blinded placebo and diet-controlled investigation. Vasc. Health Risk Manag. 2014, 10, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Weng, H.; Fan, F.; Zhang, N.; Liu, Z.; Chen, P.; Jia, J.; Zheng, B.; Yi, T.; Li, Y.; et al. Association between plasma vitamin B5 and coronary heart disease: Results from a case-control study. Front. Cardiovasc. Med. 2022, 9, 906232. [Google Scholar] [CrossRef] [PubMed]
- Mosharov, E.; Cranford, M.R.; Banerjee, R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 2000, 39, 13005–13011. [Google Scholar] [CrossRef]
- Dalto, D.B.; Matte, J.J. Pyridoxine (vitamin B6) and the glutathione peroxidase system; a link between one-carbon metabolism and antioxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2O2-treated U937 monocytes. Free Radic. Biol. Med. 2004, 36, 423–428. [Google Scholar] [CrossRef]
- Matxain, J.M.; Ristilä, M.; Strid, A.; Eriksson, L.A. Theoretical study of the antioxidant properties of pyridoxine. J. Phys. Chem. A 2006, 110, 13068–13072. [Google Scholar] [CrossRef]
- Ohta, B.K.; Foote, C.S. Characterisation of endoperoxide and hydroperoxide intermediates in the reaction of pyridoxine with singlet oxygen. J. Am. Chem. Soc. 2002, 124, 12064–12065. [Google Scholar] [CrossRef]
- Hakola, L.; Mramba, L.K.; Uusitalo, U.; Andrén Aronsson, C.; Hummel, S.; Niinistö, S.; Erlund, I.; Yang, J.; Rewers, M.J.; Akolkar, B.; et al. TEDDY Study Group. Intake of B vitamins and the risk of developing islet autoimmunity and type 1 diabetes in the TEDDY study. Eur. J. Nutr. 2024, 63, 1329–1338. [Google Scholar] [CrossRef]
- Khobrani, M.; Kandasamy, G.; Vasudevan, R.; Alhossan, A.; Puvvada, R.C.; Devanandan, P.; Dhurke, R.; Naredla, M. Impact of vitamin B6 deficiency on the severity of diabetic peripheral neuropathy—A cross sectional study. Saudi Pharm. J. 2023, 31, 655–658. [Google Scholar] [CrossRef]
- Friso, S.; Lotto, V.; Corrocher, R.; Choi, S.W. Vitamin B6 and cardiovascular disease. Subcell. Biochem. 2012, 56, 265–290. [Google Scholar]
- Penberthy, W.T.; Sadri, M.; Zempleni, J. Biotin. In Present Knowledge in Nutrition, 11th ed.; Marriott, B.P., Birt, D.F., Stallings, V.A., Yates, A.A., Eds.; Academic Press (Elsevier): London, UK, 2020; pp. 289–304. [Google Scholar]
- Feng, L.; Zhao, S.; Chen, G.; Jiang, W.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.; Zhou, X. Antioxidant status of serum, muscle, intestine and hepatopancreas for fish fed graded levels of biotin. Fish. Physiol. Biochem. 2014, 40, 499–510. [Google Scholar] [CrossRef]
- Sghaier, R.; Zarrouk, A.; Nury, T.; Badreddine, I.; O’Brien, N.; Mackrill, J.J.; Vejux, A.; Samadi, M.; Nasser, B.; Caccia, C.; et al. Biotin attenuation of oxidative stress, mitochondrial dysfunction, lipid metabolism alteration and 7β-hydroxycholesterol-induced cell death in 158N murine oligodendrocytes. Free Radic. Res. 2019, 53, 535–561. [Google Scholar] [CrossRef]
- Riverón-Negrete, L.; Sicilia-Argumedo, G.; Álvarez-Delgado, C.; Coballase-Urrutia, E.; Alcántar-Fernández, J.; Fernandez-Mejia, C. Dietary biotin supplementation modifies hepatic morphology without changes in liver toxicity markers. BioMed Res. Int. 2016, 2016, 7276463. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Fan, Y.; Xu, Y.; Lu, Y.; Zhai, L.; Wang, L. Influence of biotin intervention on glycemic control and lipid profile in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 1046800. [Google Scholar] [CrossRef]
- McCarty, M.F. In type 1 diabetics, high-dose biotin may compensate for low hepatic insulin exposure, promoting a more normal expression of glycolytic and gluconeogenic enyzymes and thereby aiding glycemic control. Med. Hypotheses 2016, 95, 45–48. [Google Scholar] [CrossRef]
- Rodriguez-Melendez, R.; Zempleni, J. Nitric oxide signaling depends on biotin in Jurkat human lymphoma cells. J. Nutr. 2009, 139, 429–433. [Google Scholar] [CrossRef]
- Ho, R.C.; Cordain, L. The potential role of biotin insufficiency on essential fatty acid metabolism and cardiovascular disease risk. Nutr. Res. 2000, 20, 1201–1212. [Google Scholar] [CrossRef]
- Levy, E.J.; Anderson, M.E.; Meister, A. Transport of glutathione diethyl ester into human cells. Proc. Natl. Acad. Sci. USA 1993, 90, 9171–9175. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Waly, M.I.; Taranikanti, V.; Guizani, N.; Ali, A.; Rahman, M.S.; Al-Attabi, Z.; Al-Malky, R.N.; Al-Maskari, S.N.M.; Al-Ruqaishi, B.R.S.; et al. Folate/vitamin B12 supplementation combats oxidative stress-associated carcinogenesis in a rat model of colon cancer. Nutr. Cancer 2019, 71, 100–110. [Google Scholar] [CrossRef]
- Hajrezaie, M.; Shams, K.; Moghadamtousi, S.Z.; Karimian, H.; Hassandarvish, P.; Emtyazjoo, M.; Zahedifard, M.; Majid, N.A.; Mohd Ali, H.; Abdulla, M.A. Chemoprevention of colonic aberrant crypt foci by novel Schiff based dichlorido(4-methoxy-2-{[2-(piperazin-4-ium-1-yl)ethyl]iminomethyl}phenolate)Cd complex in azoxymethane-induced colorectal cancer in rats. Sci. Rep. 2015, 5, 12379. [Google Scholar] [CrossRef]
- van de Lagemaat, E.E.; de Groot, L.C.P.G.M.; van den Heuvel, E.G.H.M. Vitamin B12 in relation to oxidative stress: A systematic review. Nutrients 2019, 11, 482. [Google Scholar] [CrossRef]
- Politis, A.; Olgiati, P.; Malitas, P.; Albani, D.; Signorini, A.; Polito, L.; De Mauro, S.; Zisaki, A.; Piperi, C.; Stamouli, E.; et al. Vitamin B12 levels in Alzheimer’s disease: Association with clinical features and cytokine production. J. Alzheimer’s Dis. 2010, 19, 481–488. [Google Scholar] [CrossRef]
- Obeid, R.; Shannan, B.; Herrmann, W. Advanced glycation end products overload might explain intracellular cobalamin deficiency in renal dysfunction, diabetes and aging. Med. Hypotheses 2011, 77, 884–888. [Google Scholar] [CrossRef]
- Nakano, E.; Higgins, J.A.; Powers, H.J. Folate protects against oxidative modification of human LDL. Br. J. Nutr. 2001, 86, 637–639. [Google Scholar] [CrossRef]
- Joshi, R.; Adhikari, S.; Patro, B.S.; Chattopadhyay, S.; Mukherjee, T. Free radical scavenging behavior of folic acid: Evidence for possible antioxidant activity. Free Radic. Biol. Med. 2001, 30, 1390–1399. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Zheng, Y.; Muka, T.; Troup, J.; Hu, F.B. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e003768. [Google Scholar] [CrossRef]
- Satapathy, S.; Bandyopadhyay, D.; Patro, B.K.; Khan, S.; Naik, S. Folic acid and vitamin B12 supplementation in subjects with type 2 diabetes mellitus: A multi-arm randomized controlled clinical trial. Complement. Ther. Med. 2020, 53, 102526. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Kovatcheva, M.; Casas, R.; Toledo, E.; Fitó, M.; Ros, E.; Estruch, R.; Serrano, M.; Lamuela-Raventós, R.M. Higher circulating vitamin B12 is associated with lower levels of inflammatory markers in individuals at high cardiovascular risk and in naturally aged mice. J. Sci. Food Agric. 2024, 104, 875–882. [Google Scholar] [CrossRef]
- Liu, K.; Yang, Z.; Lu, X.; Zheng, B.; Wu, S.; Kang, J.; Sun, S.; Zhao, J. The origin of vitamin B12 levels and risk of all-cause, cardiovascular and cancer specific mortality: A systematic review and dose-response meta-analysis. Arch. Gerontol. Geriatr. 2024, 117, 105230. [Google Scholar] [CrossRef]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef]
- Lehmann, B.; Meurer, M. Vitamin D metabolism. Dermatol. Ther. 2010, 23, 2–12. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on oxidative stress, epigenetics, gene regulation, and aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Watanabe, R.; Inoue, D. Current topics on vitamin D. Anti-cancer effects of vitamin D. Clin. Calcium 2015, 25, 373–380. [Google Scholar] [PubMed]
- Codoner-Franch, P.; Tavarez-Alonso, S.; Simo-Jorda, R.; Laporta-Martin, P.; Carratala-Calvo, A.; Alonso-Iglesias, E. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J. Pediatr. 2012, 161, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, S. Metabolic changes in cancer: Beyond the Warburg effect. Acta Biochim. Biophys. Sin. 2013, 45, 18–26. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D receptor is necessary for mitochondrial function and cell health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am. J. Hypertens. 2014, 27, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D: A custodian of cell signalling stability in health and disease. Biochem. Soc. Trans. 2015, 43, 349–358. [Google Scholar] [CrossRef]
- Tseng, A.H.; Shieh, S.S.; Wang, D.L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef]
- Song, C.; Fu, B.; Zhang, J.; Zhao, J.; Yuan, M.; Peng, W.; Zhang, Y.; Wu, H. Sodium fluoride induces nephrotoxicity via oxidative stress-regulated mitochondrial SIRT3 signaling pathway. Sci. Rep. 2017, 7, 672. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D cell signalling in health and disease. Biochem. Biophys. Res. Commun. 2015, 460, 53–71. [Google Scholar] [CrossRef]
- Pilz, S.; Marz, W.; Wellnitz, B.; Seelhorst, U.; Fahrleitner-Pammer, A.; Dimai, H.P.; Boehm, B.O.; Dobnig, H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J. Clin. Endocrinol. Metab. 2008, 93, 3927–3935. [Google Scholar] [CrossRef]
- Liu, Y.; Hyde, A.S.; Simpson, M.A.; Barycki, J.J. Emerging regulatory paradigms in glutathione metabolism. Adv. Cancer Res. 2014, 122, 69–101. [Google Scholar] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Tetich, M.; Kutner, A.; Leskiewicz, M.; Budziszewska, B.; Lasoń, W. Neuroprotective effects of (24R)-1,24-dihydroxycholecalciferol in human neuroblastoma SH-SY5Y cell line. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Cojic, M.; Kocic, R.; Klisic, A.; Kocic, G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients with Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front. Endocrinol. 2021, 12, 610893. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2017, 102, 3097–3110. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Rosenberg, P.A. Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes. J. Neurosci. Res. 2009, 87, 1997–2005. [Google Scholar] [CrossRef]
- Ivanova, D.; Zhelev, Z.; Getsov, P.; Nikolova, B.; Aoki, I.; Higashi, T.; Bakalova, R. Vitamin K: Redox-modulation, prevention of mitochondrial dysfunction and anticancer effect. Redox Biol. 2018, 16, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Gant, T.W.; Rao, D.N.; Mason, R.P.; Cohen, G.M. Redox cycling and sulfhydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem. Biol. Interact. 1988, 65, 157–173. [Google Scholar] [CrossRef]
- Chen, G.; Wang, F.; Trachootham, D.; Huang, P. Preferential killing of cancer cells with mitochondrial dysfunction by natural compounds. Mitochondrion 2010, 10, 614–625. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, Y.; Wu, X.; Lu, C.; Zhao, H.; Chen, K.; Zhao, A.; Li, X.; Xu, J. GAS6/Axl is associated with AMPK activation and attenuates H2O2-induced oxidative stress. Apoptosis 2023, 28, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef]
- Taylor, N.L.; Heazlewood, J.L.; Day, D.A.; Millar, A.H. Lipoic acid-dependent oxidative catabolism of alpha-keto acids in mitochondria provides evidence for branched-chain amino acid catabolism in Arabidopsis. Plant Physiol. 2004, 134, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Booker, S.J. Unraveling the pathway of lipoic acid biosynthesis. Chem. Biol. 2004, 11, 10–12. [Google Scholar] [CrossRef][Green Version]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Rekka, E.A. Lipoic acid. Kinetics and pluripotent biological properties and derivatives. Mol. Biol. Rep. 2021, 48, 6539–6550. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.B.; Negrato, C.A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014, 6, 80. [Google Scholar] [CrossRef]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Saljooghi, A.S.; Fatemi, S.J. Cadmium transport in blood serum. Toxicol. Ind. Health 2010, 26, 195–201. [Google Scholar] [CrossRef]
- Morris, T.T.; Keir, J.L.; Boshart, S.J.; Lobanov, V.P.; Ruhland, A.M.; Bahl, N.; Gailer, J. Mobilization of Cd from human serum albumin by small molecular weight thiols. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 958, 16–21. [Google Scholar] [CrossRef]
- Chang, H.; Xu, A.; Chen, Z.; Zhang, Y.; Tian, F.; Li, T. Long-term effects of a combination of D-penicillamine and zinc salts in the treatment of Wilson’s disease in children. Exp. Ther. Med. 2013, 5, 1129–1132. [Google Scholar] [CrossRef]
- Storkey, C.; Davies, M.J.; Pattison, D.I. Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach. Free Radic. Biol. Med. 2014, 73, 60–66. [Google Scholar] [CrossRef]
- Trujillo, M.; Ferrer-Sueta, G.; Thomson, L.; Flohé, L.; Radi, R. Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite. Subcell. Biochem. 2007, 44, 83–113. [Google Scholar]
- Masuda, T.; Inai, M.; Miura, Y.; Masuda, A.; Yamauchi, S.J. Effect of polyphenols on oxymyoglobin oxidation: Prooxidant activity of polyphenols in vitro and inhibition by amino acids. Agric. Food. Chem. 2013, 61, 1097–1104. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Teplova, V.V.; Beloborodova, N.V. The role of thiol antioxidants in restoring mitochondrial functions, modified by microbial metabolites. Biofizika 2012, 57, 820–826. [Google Scholar] [CrossRef]
- Wadhwa, S.; Mumper, R.J. D-penicillamine and other low molecular weight thiols: Review of anticancer effects and related mechanisms. Cancer Lett. 2013, 337, 8–21. [Google Scholar] [CrossRef]
- Bhatia, M. Hydrogen sulfide as a vasodilator. Life 2005, 57, 603–606. [Google Scholar] [CrossRef]
- Sekiguchi, F.; Miyamoto, Y.; Kanaoka, D.; Ide, H.; Yoshida, S.; Ohkubo, T.; Kawabata, A. Endogenous and exogenous hydrogen sulfide facilitates T-type calcium channel currents in Cav3.2-expressing HEK293 cells. Biochem. Biophys. Res. Commun. 2014, 445, 225–229. [Google Scholar] [CrossRef]
- Wallace, J.L. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol. Sci. 2007, 28, 501–505. [Google Scholar] [CrossRef]
- Pekala, J.; Patkowska-Sokoła, B.; Bodkowski, R.; Jamroz, D.; Nowakowski, P.; Lochyński, S.; Librowski, T. L-carnitine--metabolic functions and meaning in humans life. Curr. Drug Metab. 2011, 12, 667–678. [Google Scholar] [CrossRef]
- Terruzzi, I.; Montesano, A.; Senesi, P.; Villa, I.; Ferraretto, A.; Bottani, M.; Vacante, F.; Spinello, A.; Bolamperti, S.; Luzi, L.; et al. L-carnitine reduces oxidative stress and promotes cells differentiation and bone matrix proteins expression in human osteoblast-like cells. BioMed Res. Int. 2019, 2019, 5678548. [Google Scholar] [CrossRef]
- Lee, B.J.; Lin, J.S.; Lin, Y.C.; Lin, P.T. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: A randomized, placebo-controlled trial. Nutr. J. 2014, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Wang, Q.Y.; Luan, H.Y.; Kang, Z.C.; Wang, C.B. Effects of L-carnitine against oxidative stress in human hepatocytes: Involvement of peroxisome proliferator-activated receptor alpha. J. Biomed. Sci. 2012, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Mousavi, S.M.; Asgharzadeh, A.; Yazdani, N. Coenzyme Q10 in the treatment of heart failure: A systematic review of systematic reviews. Indian Heart J. 2018, 70 (Suppl. 1), S111–S117. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, T.; Huang, P.; Cui, S.; Gao, C.; Lin, Y.; Fu, R.; Shen, J.; He, Y.; Tan, Y.; et al. Clinical correlates of decreased plasma coenzyme Q10 levels in patients with multiple system atrophy. Parkinsonism Relat. Disord. 2018, 57, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, J.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032, Erratum in Circulation 2022, 146, e185. [Google Scholar] [CrossRef] [PubMed]
- Schniertshauer, D.; Müller, S.; Mayr, T.; Sonntag, T.; Gebhard, D.; Bergemann, J. Accelerated Regeneration of ATP Level after Irradiation in Human Skin Fibroblasts by Coenzyme Q10. Photochem. Photobiol. 2016, 92, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Kędziora-Kornatowska, K.; Czuczejko, J.; Motyl, J.; Szewczyk-Golec, K.; Kozakiewicz, M.; Pawluk, H.; Kędziora, J.; Błaszczak, R.; Banach, M.; Rysz, J. Effects of coenzyme Q10 supplementation on activities of selected antioxidative enzymes and lipid peroxidation in hypertensive patients treated with indapamide. A pilot study. Arch. Med. Sci. 2010, 6, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R. Safety and efficacy of coenzyme Q10 supplementation in early chronic Peyronie’s disease: A double-blind, placebo-controlled randomized study. Int. J. Impot. Res. 2010, 22, 298–309. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodosis-Nobelos, P.; Rekka, E.A. The Antioxidant Potential of Vitamins and Their Implication in Metabolic Abnormalities. Nutrients 2024, 16, 2740. https://doi.org/10.3390/nu16162740
Theodosis-Nobelos P, Rekka EA. The Antioxidant Potential of Vitamins and Their Implication in Metabolic Abnormalities. Nutrients. 2024; 16(16):2740. https://doi.org/10.3390/nu16162740
Chicago/Turabian StyleTheodosis-Nobelos, Panagiotis, and Eleni A. Rekka. 2024. "The Antioxidant Potential of Vitamins and Their Implication in Metabolic Abnormalities" Nutrients 16, no. 16: 2740. https://doi.org/10.3390/nu16162740
APA StyleTheodosis-Nobelos, P., & Rekka, E. A. (2024). The Antioxidant Potential of Vitamins and Their Implication in Metabolic Abnormalities. Nutrients, 16(16), 2740. https://doi.org/10.3390/nu16162740




