A Narrative Review of the Diagnosis and Treatment of Sarcopenia and Malnutrition in Patients with Heart Failure
Abstract
1. Introduction
- HFrEF (heart failure with reduced ejection fraction): Symptomatic HF with LVEF ≤ 40%.
- HFmrEF (heart failure with mildly reduced ejection fraction): Symptomatic HF with LVEF 41–49%.
- HFpEF (heart failure with preserved ejection fraction): Symptomatic HF with LVEF ≥ 50%.
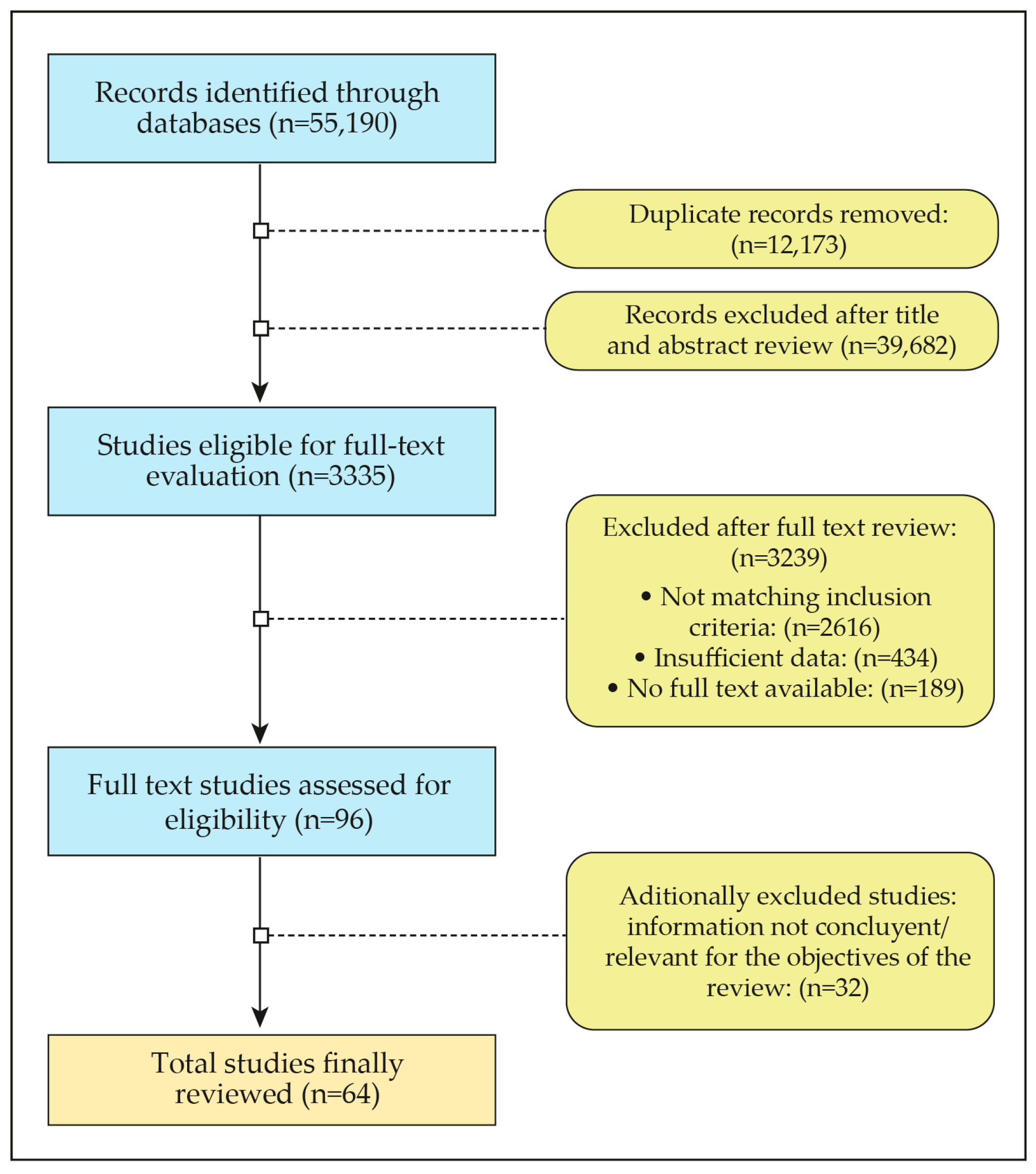
2. Methodology
3. Understanding the Disease (Epidemiology, Pathophysiology and Prognosis)
3.1. Epidemiology
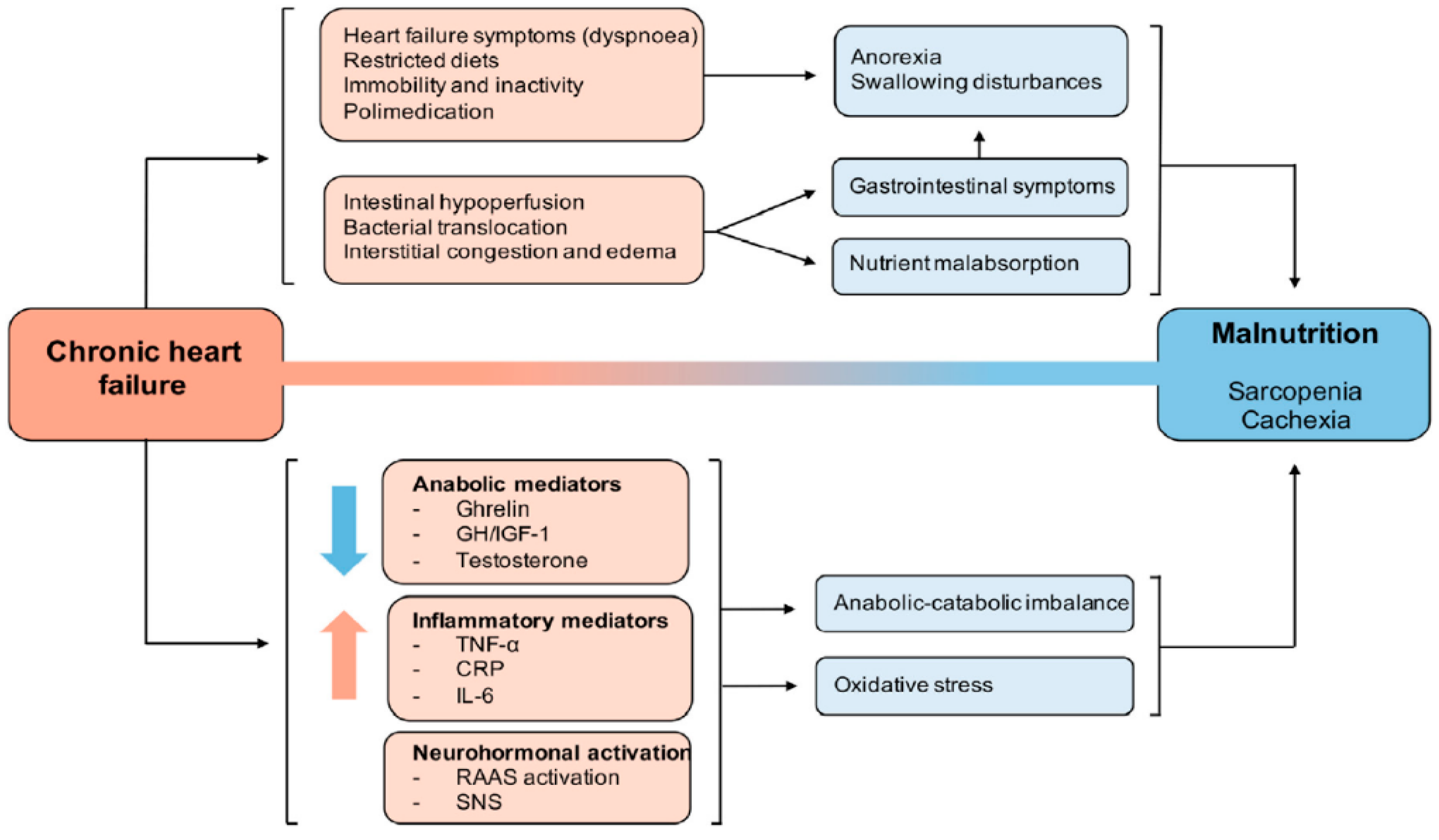
3.2. Pathophysiology: The Cardiac-Skeletal Muscle Binomial
3.3. Prognosis
4. Diagnostic Methods
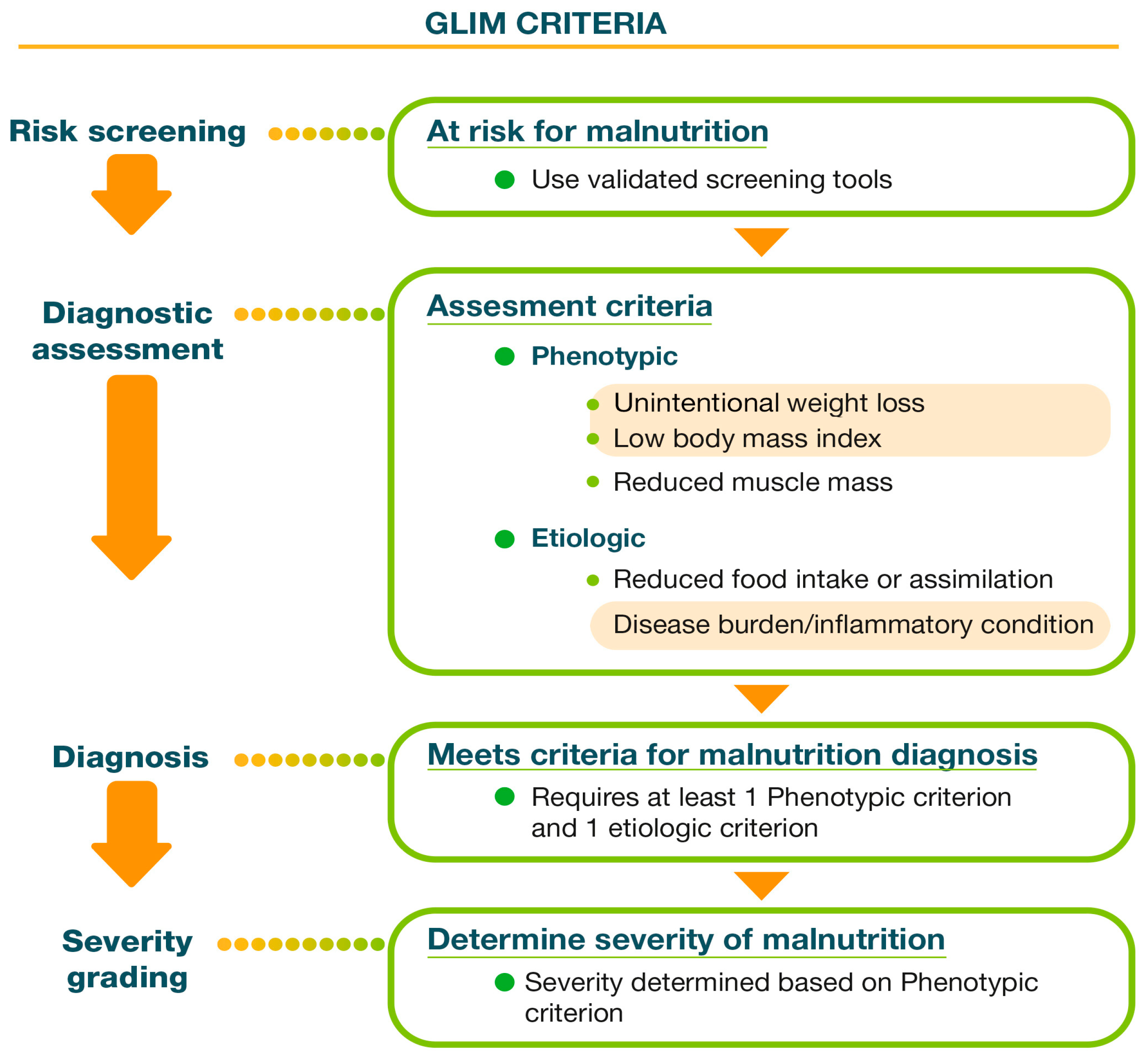
4.1. Nutritional Assessment: Global Leadership Initiative on Malnutrition (GLIM) Criteria
4.1.1. Screening
4.1.2. Diagnosis
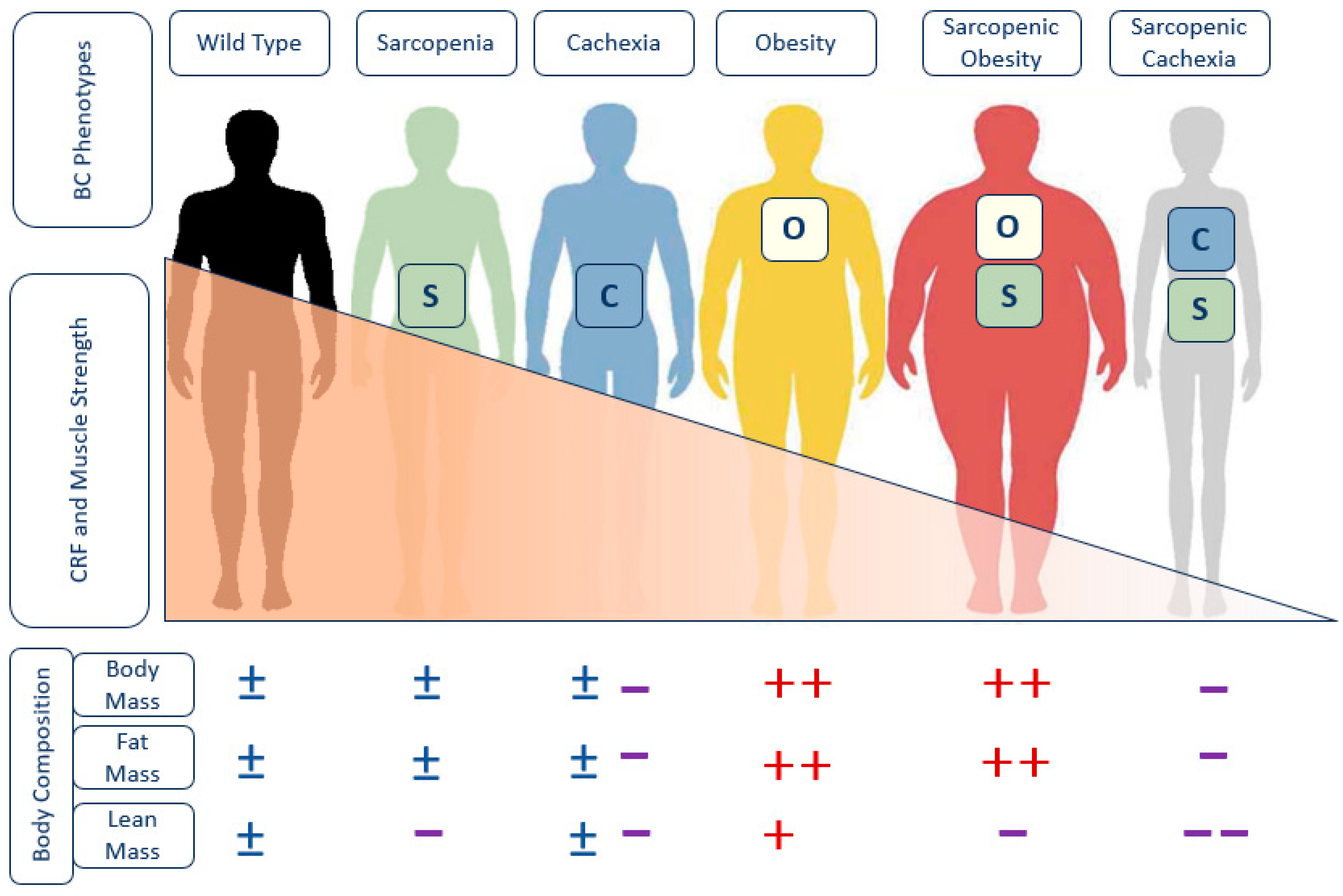
Phenotypic Criteria, a Challenge in Patients with HF
- BIA, a fast, safe, affordable and easy-to-use option, estimates body composition based on mathematical calculations. While there are publications indicating that BIA is safe for patients with cardiac implants [28], some caution is still warranted. BIA measurements before and after cardiac device implantation reveal significant differences in terms of resistance and reactance values, suggesting changes in total body water (TBW), extracellular fluid (ECF), FFM, and fat mass (FM). However, the impact of hydration status changes on these results cannot be ruled out [29]. Manufacturers remain cautious about BIA measurements in patients with implanted cardiac devices due to potential interference from metal implants. Therefore, interpreting BIA results in this patient group should be performed carefully, considering the presence of large metal implants that may affect the flow of electrical current [30]. However, it requires validation in the specific population being studied and may be of limited use in patients with hydration disorders [5]. In HF patients, BIA body composition (BC) data provide information about the different body compartments (FFM and FM) and about hydration: TBW, ECW and ICW. No clinically evident edema, identified through BIA as an elevated ratio of ECW to TBW, may serve as an early indicator of preclinical cardiovascular (CV) disease. Additionally, this condition is associated with increased CV mortality. Notably, higher TBW has been observed in patients with atrial fibrillation. Furthermore, assessing the proportions between TBW compartments in CV patients reveals that left ventricle ejection fraction correlates positively with ICW and negatively with ECW in individuals with coronary artery disease [30]. The ratio of ECW to TBW could be obtained from BIA, and an edema index of >0.39 is clinically recognized as the threshold for fluid overload [31].We can also obtain direct muscle data from parameters such as the Appendicular Skeletal Muscle Index (ASMI), Fat-Free Mass Index (FFMI) and Appendicular Lean Mass (ALM). All of these parameters have been of high value because they focus on the distribution of body compartments, which are of great relevance in nutritional assessments in the GLIM criteria. In cases of overhydration, raw impedance parameters, such as resistance (Rz), reactance (Xc) and the phase angle (PhA), can be obtained using the following:PhA = arc tangent (Xc/R) × 180°/π.PhA is positively associated with tissue reactance (associated with cell mass, integrity, function and composition) and negatively associated with resistance, which depends mainly on the degree of tissue hydration [32]. PhA serves as a valuable prognostic factor. Research has demonstrated a strong correlation between low PhA values and increased CV risk [33]. Among patients with chronic HF, lower PhA levels are evident, particularly in those indicated as having a higher New York Heart Association (NYHA) class. Consequently, PhA can be utilized when assessing the severity of chronic HF. Furthermore, higher PhA values are associated with improved prognosis in chronic HF cases. Notably, during acute HF episodes, PhA values tend to be lower compared to chronic HF, and reduced PhA is linked to higher mortality during acute decompensated HF [30]. A derivative of BIA, bioelectrical impedance vector analysis (BIVA), can be used as an alternative [24]. It seems to provide more accurate values in terms of screening, managing and monitoring HF. BIVA and hydrograph techniques hold promise in the management of heart failure. BIVA aids in identifying both chronic and acute heart failure, while hydrograph facilitates treatment adjustments by monitoring fluid status. The American Heart Association recommends the use of BIVA to optimize fluid therapy and prevent cardiorenal syndrome. Combining BIVA with serum B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) levels enhances treatment guidance and prognosis prediction in heart failure patients [34].
- DXA, recently defined as the gold standard, is an easy-to-use method with minimal radiation exposure and a lower cost than CT or MRI. However, fluctuations in body water make this measurement not sufficiently reliable for patients suffering from HF.
- Muscle ultrasound has emerged as a promising technique for measuring muscle mass due to its low cost, availability, simplicity and correlation with MRI findings. It allows for the differentiation between edema in subcutaneous tissue and lean tissue. Standardization and cutoff values have recently been published by a DRECO study relating to a Spanish population [35]. However, the validated cutoff points are not yet well defined in the population with HF.
Etiologic Criteria
4.1.3. Other Nutritional Indirect Diagnostic Methods
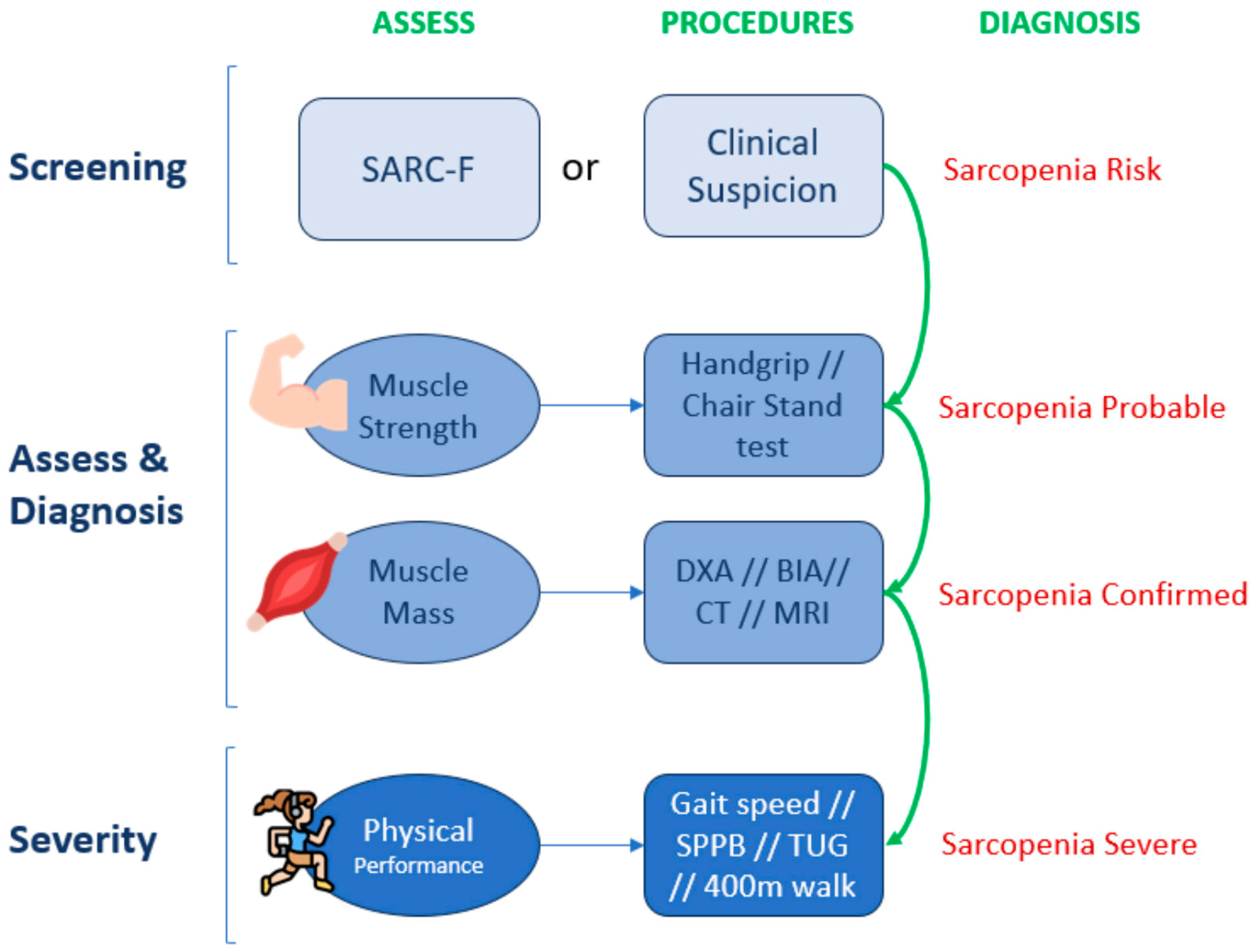
4.2. Sarcopenia Assessment: European Working Group of Sarcopenia in Older People Algorithm (EWGSOP2)
4.2.1. Screening
4.2.2. Diagnosis
5. Therapeutic Approach
5.1. Physical Exercise
Types of Exercise
5.2. Nutrition
5.2.1. Diet
- Most societies support the adoption of a Mediterranean diet (MedDiet) or dietary approaches such as the DASH diet by people with or at risk of developing HF.
- A Mediterranean diet has been shown to protect against the development of cardiovascular diseases despite the fact that there are currently conflicting results regarding its ability to reduce the risk of HF. Recent studies indicate that greater compliance with this diet can lead to a decrease in hospital readmissions due to this cause.
- On the other hand, regarding the DASH diet, small clinical trials focusing on patients with hypertensive HF with a preserved ejection fraction have shown a reduction in blood pressure, arterial stiffness and oxidative stress and improved left ventricular diastolic function. The DASH diet has also been associated with fewer readmissions after hospitalization due to HF and lower mortality.
5.2.2. Nutritional Supplements
- Hypercaloric supplements (1.52 kcal/mL) and hyperproteic supplements are generally advised, potentially improving inflammation status, quality of life and survival in HF patients. A high protein goal of 1.1 g/kg or more could be necessary, especially when considering the results of a study focusing on the nitrogen balance of 57 non-obese patients with HF versus 49 controls [5]. Although there is no scientific literature to support screening for and treatment of cardiac cachexia, the strong association between cachexia and mortality suggests that it is reasonable to try to adapt the inadequate intake of proteins and calories in selected patients and that functional status may be improved when supplying a protein intake that exceeds the 0.8 g/kg/day value recommended for the general population [3,24,37]. A protein intake goal of 1.1 g/kg daily is reasonable (as per PROT-AGE and Academy of Nutrition and Dietetics recommendations) [4].
- Hypercaloric and hyperproteic oral nutritional supplementation (ONS) enriched with β-Hydroxy-β-MethylButyrate (HMB) demonstrated significant improvement in terms of quality of life, handgrip strength and nutritional status and also reduced mortality risk in patients hospitalized for cardiovascular and pulmonary events, such as congestive HF [50,52,53].
- ESC guidelines propose omega-3 polyunsaturated fatty acid (PUFA) supplementation, considering its potential benefits in reducing hospitalization due to cardiovascular causes and death in HF patients, with level-B evidence in terms of recommendation: class IIb [1].
- Branched-chain amino acid (BCAA) preparations, which are essential for skeletal muscle formation, may be beneficial in HF, promoting postoperative wound healing and recovery from muscle fatigue after exercise, as well as improving muscle strength. The pros and cons of BCAA supplementation may vary depending on the patient and their specific conditions. BCAA supplementation for patients with cardiac dysfunction, in whom metabolic dysfunction could easily be presumed, should be carefully considered [4].
5.2.3. Micronutrients
5.3. Drugs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.; Ebner, N.; Anker, M.S. Cardiac cachexia. Eur. Heart J. Suppl. 2019, 21, L24–L27. [Google Scholar] [CrossRef] [PubMed]
- Vest, A.R.; Chan, M.; Deswal, A.; Givertz, M.M.; Lekavich, C.; Lennie, T.; Litwin, S.E.; Parsly, L.; Rodgers, J.E.; Rich, M.W.; et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J. Card. Fail. 2019, 25, 380–400. [Google Scholar] [CrossRef]
- Springer, J.; Springer, J.-I.; Anker, S.D. Muscle wasting and sarcopenia in heart failure and beyond: Update 2017. ESC Heart Fail. 2017, 4, 492–498. [Google Scholar] [CrossRef]
- Carbone, S.; Billingsley, H.E.; Rodriguez-Miguelez, P.; Kirkman, D.L.; Garten, R.; Franco, R.L.; Lee, D.C.; Lavie, C.J. Lean Mass Abnormalities in Heart Failure: The Role of Sarcopenia, Sarcopenic Obesity, and Cachexia. Curr. Probl. Cardiol. 2020, 45, 100417. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 10, 207–217. [Google Scholar]
- Fernández-Pombo, A.; Rodríguez-Carnero, G.; Castro, A.I.; Cantón-Blanco, A.; Seoane, L.M.; Casanueva, F.F.; Crujeiras, A.B.; Martínez-Olmos, M.A. Relevance of nutritional assessment and treatment to counteract cardiac cachexia and sarcopenia in chronic heart failure. Clin. Nutr. 2021, 40, 5141–5155. [Google Scholar] [CrossRef] [PubMed]
- Curcio, F.; Testa, G.; Liguori, I.; Papillo, M.; Flocco, V.; Panicara, V.; Galizia, G.; Della-Morte, D.; Gargiulo, G.; Cacciatore, F.; et al. Sarcopenia and Heart Failure. Nutrients 2020, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Salom Vendrell, C.; García Tercero, E.; Moro Hernández, J.B.; Cedeno-Veloz, B.A. Sarcopenia as a Little-Recognized Comorbidity of Type II Diabetes Mellitus: A Review of the Diagnosis and Treatment. Nutrients 2023, 15, 4149. [Google Scholar] [CrossRef]
- Sasaki, K.I.; Fukumoto, Y. Sarcopenia as a comorbidity of cardiovascular disease. J. Cardiol. 2022, 79, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.D.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.R.; Mounier, R.; Plomgaard, P.; Mortensen, O.H.; Penkowa, M.; Speerschneider, T.; Pilegaard, H.; Pedersen, B.K. Expression of interleukin-15 in human skeletal muscle—Effect of exercise and muscle fibre type composition. J. Physiol. 2007, 584, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Ishida, J.; Doehner, W.; Von Haehling, S.; Anker, M.S.; Coats, A.J.S.; Anker, S.D.; Springer, J. Sarcopenia, cachexia, and muscle performance in heart failure: Review update 2016. Int. J. Cardiol. 2017, 238, 5–11. [Google Scholar] [CrossRef]
- Konishi, M.; Kagiyama, N.; Kamiya, K.; Saito, H.; Saito, K.; Ogasahara, Y.; Maekawa, E.; Misumi, T.; Kitai, T.; Iwata, K.; et al. Impact of sarcopenia on prognosis in patients with heart failure with reduced and preserved ejection fraction. Eur. J. Prev. Cardiol. 2021, 28, 1022–1029. [Google Scholar] [CrossRef]
- Emami, A.; Saitoh, M.; Valentova, M.; Sandek, A.; Evertz, R.; Ebner, N.; Loncar, G.; Springer, J.; Doehner, W.; Lainscak, M.; et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: Results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur. J. Heart Fail. 2018, 20, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández, A.; Villar-Taibo, R.; Alejo, M.; Arroyo, D.; Bonilla Palomas, J.L.; Cachero, M.; Joaquin, C.; Méndez Bailón, M.; Pérez-Rivera, J.Á.; Romero-Vigara, J.C.; et al. Diagnosis and Management of Malnutrition in Patients with Heart Failure. J. Clin. Med. 2023, 12, 3320. [Google Scholar] [CrossRef] [PubMed]
- Horwich, T.B.; Fonarow, G.C.; Hamilton, M.A.; MacLellan, W.R.; Woo, M.A.; Tillisch, J.H. The relationship between obesity and mortality in patients with heart failure. J. Am. Coll. Cardiol. 2001, 38, 789–795. [Google Scholar] [CrossRef]
- Fonseca, G.W.P.D.; Dos Santos, M.R.; De Souza, F.R.; Takayama, L.; Rodrigues Pereira, R.M.; Negrão, C.E.; Alves, M.N.N. Discriminating sarcopenia in overweight/obese male patients with heart failure: The influence of body mass index. ESC Heart Fail. 2020, 7, 85–92. [Google Scholar] [CrossRef]
- Abe, T.; Thiebaud, R.S.; Loenneke, J.P.; Bemben, M.G.; Loftin, M.; Fukunaga, T. Influence of Severe Sarcopenia on Cardiovascular Risk Factors in Nonobese Men. Metab. Syndr. Relat. Disord. 2012, 10, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.; Anker, M.S.; Springer, J. Muscle Wasting and Sarcopenia in Heart Failure—The Current State of Science. Int. J. Mol. Sci. 2020, 21, 6549. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Maeda, K.; Nonogaki, T.; Shimizu, A.; Yamanaka, Y.; Matsuyama, R.; Kato, R.; Mori, N. Impact of edema on length of calf circumference in older adults. Geriatr. Gerontol. Int. 2019, 19, 993–998. [Google Scholar] [CrossRef]
- Prado, C.M.; Landi, F.; Chew, S.T.H.; Atherton, P.J.; Molinger, J.; Ruck, T.; Gonzalez, M.C. Advances in muscle health and nutrition: A toolkit for healthcare professionals. Clin. Nutr. 2022, 41, 2244–2263. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef]
- Meyer, P.; Makhlouf, A.; Mondouagne Engkolo, L.P.; Trentaz, F.; Thibault, R.; Pichard, C.; Burri, H. Safety of Bioelectrical Impedance Analysis in Patients Equipped With Implantable Cardioverter Defibrillators. J. Parenter. Enter. Nutr. 2017, 41, 981–985. [Google Scholar] [CrossRef]
- Garlini, L.M.; Alves, F.D.; Kochi, A.; Zuchinali, P.; Zimerman, L.; Pimentel, M.; Perry, I.S.; Souza, G.C.; Clausell, N. Safety and Results of Bioelectrical Impedance Analysis in Patients with Cardiac Implantable Electronic Devices. Braz. J. Cardiovasc. Surg. 2020, 35, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Szczygiel, K. Bioelectrical Impedance Analysis and Body Composition in Cardiovascular Diseases. Curr. Probl. Cardiol. 2023, 48, 101911. [Google Scholar] [CrossRef]
- Lyons, K.J.; Bischoff, M.K.; Fonarow, G.C.; Horwich, T.B. Noninvasive Bioelectrical Impedance for Predicting Clinical Outcomes in Outpatients With Heart Failure. Crit. Pathw. Cardiol. 2017, 16, 32–36. [Google Scholar] [CrossRef]
- Kyle, U. Bioelectrical impedance analysis? part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.A.; Jorge, A.J.; De Andrade Martins, W.; Cardoso, G.P.; Dos Santos, M.M.; Rosa, M.L.; Lima, G.A.; de Moraes, R.Q.; da Cruz Filho, R.A. Phase angle measured by electrical bioimpedance and global cardiovascular risk in older adults. Geriatr. Gerontol. Int. 2018, 18, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Thanapholsart, J.; Khan, E.; Lee, G.A. A Current Review of the Uses of Bioelectrical Impedance Analysis and Bioelectrical Impedance Vector Analysis in Acute and Chronic Heart Failure Patients: An Under-valued Resource? Biol. Res. Nurs. 2023, 25, 240–249. [Google Scholar] [CrossRef]
- de Luis Roman, D.; García Almeida, J.M.; Bellido Guerrero, D.; Guzmán Rolo, G.; Martín, A.; Primo Martín, D.; García-Delgado, Y.; Guirado-Peláez, P.; Palmas, F.; Tejera Pérez, C.; et al. Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk. Nutrients 2024, 16, 1552. [Google Scholar] [CrossRef]
- Aminuddin, A.; Noor Hashim, M.F.; Mohd Zaberi, N.A.S.; Zheng Wei, L.; Ching Chu, B.; Jamaludin, N.A.; Salamt, N.; Che Roos, N.A.; Ugusman, A. The Association Between Arterial Stiffness and Muscle Indices Among Healthy Subjects and Subjects With Cardiovascular Risk Factors: An Evidence-Based Review. Front. Physiol. 2021, 12, 742338. [Google Scholar] [CrossRef]
- Driggin, E.; Cohen, L.P.; Gallagher, D.; Karmally, W.; Maddox, T.; Hummel, S.L.; Carbone, S.; Maurer, M.S. Nutrition Assessment and Dietary Interventions in Heart Failure. J. Am. Coll. Cardiol. 2022, 79, 1623–1635. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; Micciolo, R.; Rubele, S.; Fantin, F.; Caliari, C.; Zoico, E.; Mazzali, G.; Ferrari, E.; Volpato, S.; Zamboni, M. Assessing the risk of sarcopenia in the elderly: The Mini Sarcopenia Risk Assessment (MSRA) questionnaire. J. Nutr. Health Aging. 2017, 21, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes: SARC-F. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Misra, A.; Vaish, A.; Ursino, N.; D’Ambrosi, R. Hand grip strength as a proposed new vital sign of health: A narrative review of evidences. J. Health Popul. Nutr. 2024, 43, 7. [Google Scholar] [CrossRef]
- Yoshiko, A.; Ogawa, M.; Shimizu, K.; Radaelli, R.; Neske, R.; Maeda, H.; Maeda, K.; Teodoro, J.; Tanaka, N.; Pinto, R.S.; et al. Chair sit-to-stand performance is associated with diagnostic features of sarcopenia in older men and women. Arch. Gerontol. Geriatr. 2021, 96, 104463. [Google Scholar] [CrossRef] [PubMed]
- Izawa, K.P.; Watanabe, S.; Oka, K.; Kasahara, Y.; Morio, Y.; Hiraki, K.; Hirano, Y.; Omori, Y.; Suzuki, N.; Kida, K.; et al. Sarcopenia and physical activity in older male cardiac patients. Int. J. Cardiol. 2016, 222, 457–461. [Google Scholar] [CrossRef]
- Collamati, A.; Marzetti, E.; Calvani, R.; Tosato, M.; D’Angelo, E.; Sisto, A.N.; Landi, F. Sarcopenia in heart failure: Mechanisms and therapeutic strategies. J. Geriatr. Cardiol. 2016, 13, 615–624. [Google Scholar]
- Guía ESC 2020 Sobre Cardiología Del Deporte Y El Ejercicio En Pacientes Con Enfermedad Cardiovascular. Available online: https://www.revespcardiol.org/es-pdf-S0300893221000750 (accessed on 31 May 2024).
- Suzuki, T.; Palus, S.; Springer, J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018, 5, 1099–1107. [Google Scholar] [CrossRef]
- Bekfani, T.; Pellicori, P.; Morris, D.A.; Ebner, N.; Valentova, M.; Steinbeck, L.; Wachter, R.; Elsner, S.; Sliziuk, V.; Schefold, J.C.; et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int. J. Cardiol. 2016, 222, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Hersberger, L.; Dietz, A.; Bürgler, H.; Bargetzi, A.; Bargetzi, L.; Kägi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; Pavlicek, V.; et al. Individualized Nutritional Support for Hospitalized Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Palomas, J.L.; Gámez-López, A.L.; Castillo-Domínguez, J.C.; Moreno-Conde, M.; López Ibáñez, M.C.; Alhambra Expósito, R.; Ramiro Ortega, E.; Anguita-Sánchez, M.P.; Villar-Ráez, A. Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure. Arch. Med. Res. 2016, 47, 535–540. [Google Scholar] [CrossRef]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R.; NOURISH Study Group. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef]
- Streng, K.W.; Hillege, H.L.; ter Maaten, J.M.; van Veldhuisen, D.J.; Dickstein, K.; Ng, L.L.; Samani, N.J.; Metra, M.; Ponikowski, P.; Cleland, J.G.; et al. Clinical implications of low estimated protein intake in patients with heart failure. J. Cachexia Sarcopenia Muscle 2022, 13, 1762–1770. [Google Scholar] [CrossRef]
- Baggs, G.E.; Middleton, C.; Nelson, J.L.; Pereira, S.L.; Hegazi, R.M.; Matarese, L.; Matheson, E.; Ziegler, T.R.; Tappenden, K.A.; Deutz, N. Impact of a specialized oral nutritional supplement on quality of life in older adults following hospitalization: Post-hoc analysis of the NOURISH trial. Clin. Nutr. 2023, 42, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.R.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am. J Clin Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Yera, H.O.; Khan, A.; Akinlade, O.M.; Champsi, A.; Glouzon, V.N.J.; Spencer, C. Improving the Outcome of Patients With Heart Failure: Assessment of Iron Deficiency and Intravenous Iron Replacement. Cureus 2023, 15, e47027. Available online: https://www.cureus.com/articles/179740-improving-the-outcome-of-patients-with-heart-failure-assessment-of-iron-deficiency-and-intravenous-iron-replacement (accessed on 31 May 2024). [CrossRef] [PubMed]
- Billingsley, H.E.; Hummel, S.L.; Carbone, S. The role of diet and nutrition in heart failure: A state-of-the-art narrative review. Prog. Cardiovasc. Dis. 2020, 63, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Cvetinovic, N.; Loncar, G.; Isakovic, A.M.; von Haehling, S.; Doehner, W.; Lainscak, M.; Farkas, J. Micronutrient Depletion in Heart Failure: Common, Clinically Relevant and Treatable. Int. J. Mol. Sci. 2019, 20, 5627. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, Dietary, and Supplemental Vitamin C Intake and Risk of Incident Kidney Stones. Am. J. Kidney Dis. 2016, 67, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.R. Vitamin D toxicity related to its physiological and unphysiological supply. Trends Endocrinol. Metab. 2021, 32, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the Risk of Prostate Cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute Selenium Toxicity Associated With a Dietary Supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef]
- Rae, W.; Kitley, J.; Pinto, A. Selenium Toxicity Associated With Reversible Leukoencephalopathy and Cortical Blindness. JAMA Neurol. 2018, 75, 1282. [Google Scholar] [CrossRef]
- Cicoira, M.; Zanolla, L.; Franceschini, L.; Rossi, A.; Golia, G.; Zeni, P.; Caruso, B.; Zardini, P. Relation of aldosterone “escape” despite angiotensin-converting enzyme inhibitor administration to impaired exercise capacity in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2002, 89, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.A.; McMurdo, M.E.T.; Struthers, A.D. Mineralocorticoid antagonism: A novel way to treat sarcopenia and physical impairment in older people? Mineralocorticoid antagonism. Clin. Endocrinol. 2011, 75, 725–729. [Google Scholar] [CrossRef] [PubMed]
- De Luis Román, D.; Garrachón Vallo, F.; Carretero Gómez, J.; López Gómez, J.J.; Tarazona Santabalbina, F.J.; Guzmán Rolo, G.; García Almeida, J.M.; Sanz Paris, A. Decreased muscle mass in type-2 diabetes. A hidden comorbidity to consider. Nutr. Hosp. 2023, 40, 59–66. Available online: https://www.nutricionhospitalaria.org/articles/04468/show (accessed on 31 May 2024). [CrossRef] [PubMed]
| Name of Myokine | Origin of Myokines | HF-Related Actions |
|---|---|---|
| Decorin | Skeletal Muscles | Downregulated in HF |
| Fibroblasts | ↓ Cardiac hypertrophy | |
| Vascular endothelial cells | ↑ Cardiac fibrosis | |
| Cardiac myocytes | ||
| Smooth muscle cells | ||
| Irisin | Skeletal Muscles | Downregulated in HF |
| Myocardium | ↓ Tolerance for physical activity | |
| ↑ Skeletal muscle hypotrophy | ||
| Myonectin | Skeletal Muscles | Downregulated in HF |
| Adipose tissue | ↑ Skeletal muscle hypotrophy | |
| Brain Derived Neurotropic Factor (BDNF) | Skeletal Muscles | Downregulated in HF |
| Cardiac myocytes | ↑ Tolerance for physical activity | |
| smooth muscle cells | ||
| Endothelial cells | ||
| Astrocytes | ||
| Growth Differential Factor-11 (GDF-11) | Skeletal Muscles | Downregulated in HF |
| Neural stem cells | ↓ Physical endurance | |
| Cardiac myocytes | ↑ Muscle weakness | |
| ↑ Skeletal muscle hypotrophy | ||
| Myostatin | Skeletal Muscles | Upregulated in HF |
| Cardiac myocytes | ↑ Skeletal muscle hypotrophy | |
| ↓ Tolerance for physical activity | ||
| ↑ Insulin resistance | ||
| ↑ Autophagy | ||
| ↑ Muscle weakness | ||
| Osteonectin | Skeletal Muscles | Upregulated in HF |
| Adipose tissue | Predictor of poor HF outcomes | |
| Cardiac myocytes | ↑ Cardiac contractility | |
| Bones mucosa | ↑ Cardiac reparation at early stage | |
| Vasculature | ↓ Vascular integrity at late stage | |
| Kidney and liver | ↓ Cardiac myocyte survival |
| Name of Myokine | Origin of Myokines | HF-Related Actions |
|---|---|---|
| IL-8 | Mononuclear phagocytes | Upregulated in HF |
| Adipocytes | ↓ Skeletal muscle energy metabolism | |
| Epithelial cells | ↑ Cardiac fibrosis | |
| Endothelial cells | ||
| Mesenchymal Cells | ||
| FGF-21 | Cardiac myocytes | Downregulated in HF |
| Pancreas | ↑ Skeletal muscle mass | |
| Adipose tissue | ↑ Tolerance for physical activity | |
| Liver | ↓ Insulin Resistance | |
| Brain | ||
| Kidney | ||
| IL-15 | Cardiac myocytes | Downregulated in HF |
| Mononuclear phagocytes | ↑ Tolerance for physical activity | |
| ↑ Skeletal muscle mass | ||
| ↓ White adipose tissue | ||
| ↓ Apoptosis of cardiac myocytes and myoblasts |
| Micronutrient | Benefits | Potentially Dangerous Side Effects (in General Adult Population) |
|---|---|---|
| Vitamin D | Improves muscle strength in elderly individuals with serum 25(OH)D concentrations < 30 ng/mL compared to those exceeding 30 ng/mL | Doses > 250 µg/d may produce signs of hypercalcemia, hypercalciuria and soft-tissue calcification. |
| Vitamin E | Possible improvement in markers of oxidative stress. | High doses of supplementation increase the risk of developing HF in patients after heart stroke. Increase the risk of prostate cancer. Rarely elevate creatine levels in urine. Nausea, diarrhea, intestinal cramps, fatigue, weakness, headache, blurred vision, rash, and gonadal dysfunction. |
| Vitamin B1 (thiamine) | Possible improvement in ejection fraction. | Not described |
| Vitamin B12 | Improves respiratory muscle strength. Consider supplementation for patients on diuretics and restrictive diets. | Not described |
| Vitamin C | Improves contractility and endothelial function. | Very large doses (g quantity) may produce oxalate kidney stones in males. |
| Coenzyme Q10 | Reduction in adverse cardiovascular events (mortality, hospitalizations, dyspnea, fatigue, arrhythmias, acute lung edema, nocturia, etc.). Improvement in NYHA functional class, improvement in the 6 min walk test, increase in exercise time. | Not described |
| Iron | Intravenous ferric carboxymaltose improves functional class, exercise capacity, and quality of life (These results are not observed when oral iron is given). | Ingestions ≥ 60 mg/kg of elemental iron are associated with serious toxicity and death. |
| Selenium | Scarce evidence. Seems to reduce serum insulin levels and insulin resistance (measured using the HOMA homeostasis model assessment of insulin resistance), as well as to improve the lipid profile. | Megadoses (>400 µg/d) could produce peripheral neuropathy, hair loss, nail changes, emesis, nausea, diarrhea, mental status changes, and visual loss. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jorge-Huerta, L.; Marco-Alacid, C.; Grande, C.; Velardo Andrés, C. A Narrative Review of the Diagnosis and Treatment of Sarcopenia and Malnutrition in Patients with Heart Failure. Nutrients 2024, 16, 2717. https://doi.org/10.3390/nu16162717
de Jorge-Huerta L, Marco-Alacid C, Grande C, Velardo Andrés C. A Narrative Review of the Diagnosis and Treatment of Sarcopenia and Malnutrition in Patients with Heart Failure. Nutrients. 2024; 16(16):2717. https://doi.org/10.3390/nu16162717
Chicago/Turabian Stylede Jorge-Huerta, Lucía, Cristian Marco-Alacid, Cristina Grande, and Christian Velardo Andrés. 2024. "A Narrative Review of the Diagnosis and Treatment of Sarcopenia and Malnutrition in Patients with Heart Failure" Nutrients 16, no. 16: 2717. https://doi.org/10.3390/nu16162717
APA Stylede Jorge-Huerta, L., Marco-Alacid, C., Grande, C., & Velardo Andrés, C. (2024). A Narrative Review of the Diagnosis and Treatment of Sarcopenia and Malnutrition in Patients with Heart Failure. Nutrients, 16(16), 2717. https://doi.org/10.3390/nu16162717






