Therapeutic Potential of Various Intermittent Fasting Regimens in Alleviating Type 2 Diabetes Mellitus and Prediabetes: A Narrative Review
Abstract
1. Introduction
2. Effects of IF on Metabolic Complications
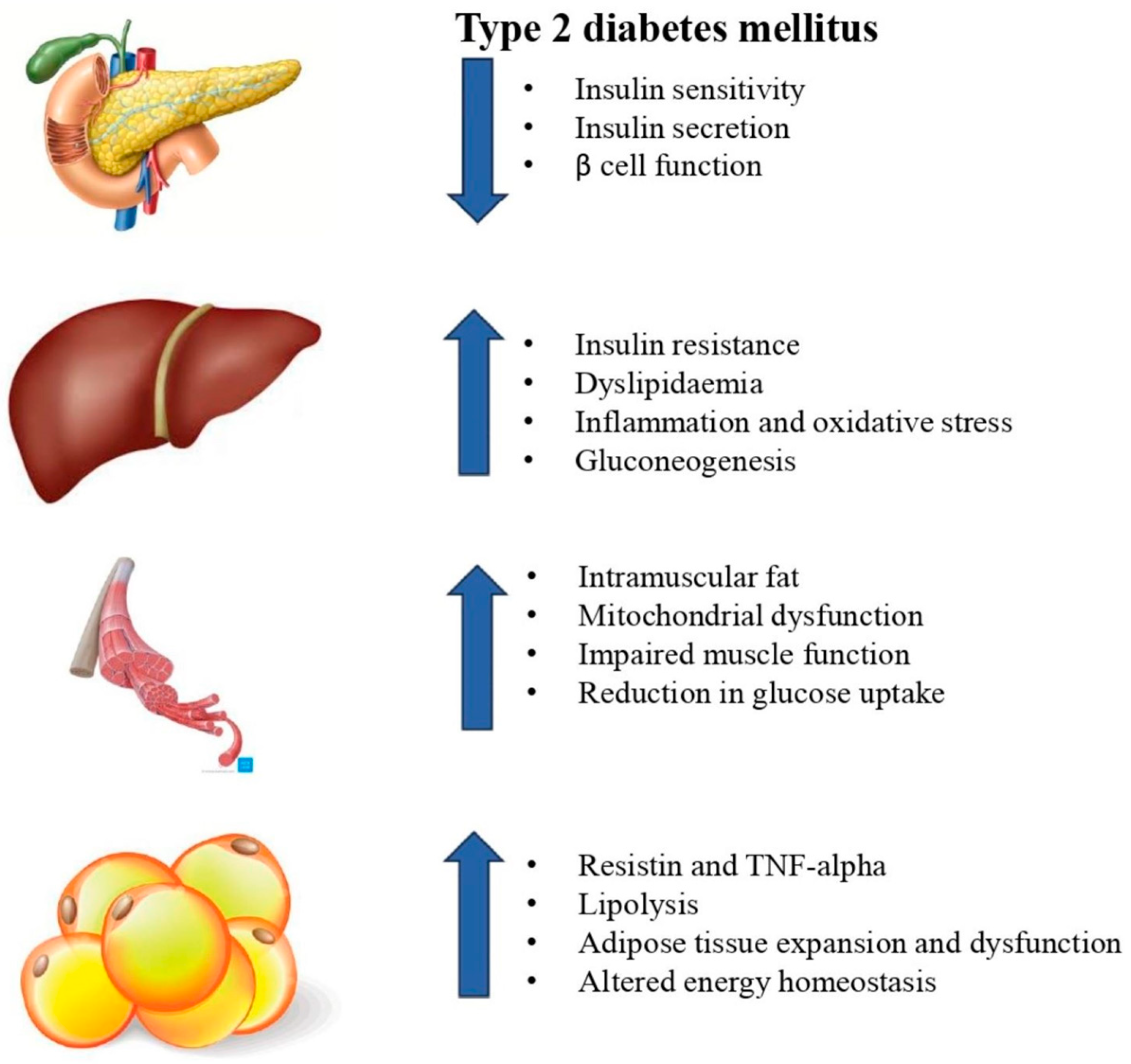
3. Type 2 Diabetes Mellitus
4. Conventional Management of T2DM
4.1. Insulin Therapy
4.2. Glucagon-like Peptide-1 Receptor Agonists
4.3. Dipeptidyl Peptidase-4 Inhibitors
4.4. Sodium-Glucose Co-Transport 2 Inhibitors
4.5. Biguanide (Metformin)
5. Lifestyle Intervention
5.1. Dietary Intervention
5.2. Increased Physical Activity
6. Effect of Intermittent Fasting on T2DM
6.1. Alternate Day Fasting
6.2. The 5:2 Fasting Diet
6.3. Time-Restricted Feeding
7. Prediabetes
HOMA-IR
8. Prediabetes Management
8.1. Biguanides (Metformin)
8.2. Lifestyle Modification
8.3. Intermittent Fasting
8.3.1. Alternate-Day Fasting
8.3.2. The 5:2 Fasting Diet
8.3.3. Time-Restricted Feeding
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Intermittent Fasting | IF |
| Alternate-Day Fasting | ADF |
| Time-Restricted Feeding | TRF |
| Type 2 Diabetes Mellitus | T2DM |
| Body Mass Index | BMI |
| Insulin Resistance | IR |
| Β | Beta |
| American Diabetes Association | ADA |
| World Health Organization | WHO |
| Oral Glucose Tolerance Test | OGTT |
| Glycated Hemoglobin | HbA1c |
| Fasting Glucose | FG |
| Glucagon-Like Peptide 1 Receptor Agonists | GLP-1RA |
| Glucagon-Like Peptide 1 | GLP-1 |
| Dipeptidyl Peptidase-4 Inhibitors | DPP4i |
| Dipeptidyl Peptidase 4 | DPP4 |
| Calorie Restriction | CR |
| Glucose Tolerance | GT |
| Sodium-Glucose Co-Transporter 2 | SGLT 2 |
| Sodium-Glucose Co-Transporter 2 Inhibitor | SGLT-2i |
| Glucose Transporter 4 | GLUT-4 |
| High Fats Ad Libitum | HF-AL |
| Diabetes Prevention Programme | DPP |
| Impaired Glucose Tolerance | IGT |
| Impaired Fasting Glucose | IFG |
| High-Fat Alternate-Day Fasting | HF-ADF |
| Very Low-Calorie Diet | VLCD |
| High-Density Lipoprotein | HDL |
| High-Density Lipoprotein Cholesterol | HDL-C |
| Homeostatic Model Assessment of Insulin Resistance | HOMA-IR |
| Homeostatic Model Assessment of Beta Cell Function | HOMA-β |
| Insulin Receptor Substrate 1 | IRS1 |
| Nonalcoholic Fatty Liver Disease | NAFLD |
| Superoxide Dismutase | SOD |
| Hydrogen Peroxide | H2O2 |
| Superoxide | O2 |
Glossary
References
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.B.; Ramsey, J.J. Honoring Clive McCay and 75 Years of Calorie Restriction Research. J. Nutr. 2010, 140, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Zhang, J.; Xu, J. Intermittent Versus Continuous Energy Restriction for Weight Loss and Metabolic Improvement: A Meta-Analysis and Systematic Review. Obesity 2021, 29, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Cook, F.; Langdon-Daly, J.; Serpell, L. Compliance of participants undergoing a ‘5-2’ intermittent fasting diet and impact on body weight. Clin. Nutr. ESPEN 2022, 52, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Calorie restriction for long-term remission of type 2 diabetes. Clin. Med. 2019, 19, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, A.; Ismail-Beigi, F. Intermittent fasting and ‘metabolic switch’: Effects on metabolic syndrome, prediabetes and type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 1496–1510. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.I.; Direito, M.; Pinto-Ribeiro, F.; Ludovico, P.; Sampaio-Marques, B. Effects of Intermittent Fasting on Regulation of Metabolic Homeostasis: A Systematic Review and Meta-Analysis in Health and Metabolic-Related Disorders. J. Clin. Med. 2023, 12, 3699. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Belinova, L.; Malinska, H.; Oliyarnyk, O.; Trnovska, J.; Skop, V.; Kazdova, L.; Dezortova, M.; Hajek, M.; Tura, A.; et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: A randomised crossover study. Diabetologia 2014, 57, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kimita, W.; Skudder-Hill, L.; Li, X.; Priya, S.; Bharmal, S.H.; Cho, J.; Petrov, M.S. Dietary carbohydrate intake and insulin traits in individuals after acute pancreatitis: Effect modification by intra-pancreatic fat deposition. Pancreatology 2021, 21, 353–362. [Google Scholar] [CrossRef]
- Niki, A.; Baden, M.Y.; Kato, S.; Mitsushio, K.; Horii, T.; Ozawa, H.; Ishibashi, C.; Fujita, S.; Kimura, T.; Fujita, Y.; et al. Consumption of two meals per day is associated with increased intrapancreatic fat deposition in patients with type 2 diabetes: A retrospective study. BMJ Open Diabetes Res. Care 2022, 10, e002926. [Google Scholar] [CrossRef]
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Cienfuegos, S.; McStay, M.; Gabel, K.; Varady, K.A. Time restricted eating for the prevention of type 2 diabetes. J. Physiol. 2022, 600, 1253–1264. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Gilhooly, C.H.; Golden, J.K.; Pittas, A.G.; Fuss, P.J.; Cheatham, R.A.; Tyler, S.; Tsay, M.; McCrory, M.A.; Lichtenstein, A.H.; et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: A 1-y randomized controlled trial. Am. J. Clin. Nutr. 2007, 85, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Hellerstein, M.K. Alternate-day fasting and chronic disease prevention: A review of human and animal trials. Am. J. Clin. Nutr. 2007, 86, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Przulj, D.; Pesola, F.; McRobbie, H.; Peerbux, S.; Phillips-Waller, A.; Bisal, N.; Myers Smith, K. A randomised controlled trial of the 5:2 diet. PLoS ONE 2021, 16, e0258853. [Google Scholar] [CrossRef]
- Aoun, A.; Ghanem, C.; Hamod, N.; Sawaya, S. The Safety and Efficacy of Intermittent Fasting for Weight Loss. Nutr. Today 2020, 55, 270–277. [Google Scholar] [CrossRef]
- Kunduraci, Y.E.; Ozbek, H. Does the Energy Restriction Intermittent Fasting Diet Alleviate Metabolic Syndrome Biomarkers? A Randomized Controlled Trial. Nutrients 2020, 12, 3213. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef]
- Chobot, A.; Górowska-Kowolik, K.; Sokołowska, M.; Jarosz-Chobot, P. Obesity and diabetes—Not only a simple link between two epidemics. Diabetes/Metab. Res. Rev. 2018, 34, e3042. [Google Scholar] [CrossRef] [PubMed]
- Al-Sulaiti, H.; Diboun, I.; Agha, M.V.; Mohamed, F.F.S.; Atkin, S.; Domling, A.S.; Elrayess, M.A.; Mazloum, N.A. Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J. Transl. Med. 2019, 17, 348. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Lu, W.; Zong, X.F.; Ruan, H.Y.; Liu, Y. Obesity and hypertension. Exp. Ther. Med. 2016, 12, 2395–2399. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, F.X. Obesity: Criteria and classification. Proc. Nutr. Soc. 2000, 59, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J. Physiol. Pharmacol. 2018, 69, 663–683. [Google Scholar] [CrossRef]
- Casanova, F.; Gooding, K.M.; Shore, A.C.; Adingupu, D.D.; Mawson, D.; Ball, C.; Anning, C.; Aizawa, K.; Gates, P.E.; Strain, W.D. Weight change and sulfonylurea therapy are related to 3 year change in microvascular function in people with type 2 diabetes. Diabetologia 2020, 63, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, G. Sodium-Glucose Co-Transporter 2 Inhibitors Compared with Sulfonylureas in Patients with Type 2 Diabetes Inadequately Controlled on Metformin: A Meta-Analysis of Randomized Controlled Trials. Clin. Drug Investig. 2019, 39, 521–531. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Loria-Kohen, V.; Zulet, M.A.; Martinez, J.A.; Reglero, G.; Ramirez de Molina, A. Dietary Strategies Implicated in the Prevention and Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 1877. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of Intermittent Fasting Diet on Glucose and Lipid Metabolism and Insulin Resistance in Patients with Impaired Glucose and Lipid Metabolism: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2022, 2022, 6999907. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Wozniak, A.; Taub, P.R. Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives. Nutrients 2021, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cai, T.; Zhou, Z.; Mu, Y.; Lu, Y.; Gao, Z.; Wu, J.; Zhang, Y. Health Effects of Alternate-Day Fasting in Adults: A Systematic Review and Meta-Analysis. Front. Nutr. 2020, 7, 586036. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Kroeger, C.M.; Trepanowski, J.F.; Hoddy, K.K.; Cienfuegos, S.; Kalam, F.; Varady, K.A. Differential Effects of Alternate-Day Fasting versus Daily Calorie Restriction on Insulin Resistance. Obesity 2019, 27, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Poe, M.; Mohr, A.E.; Ives, S.J.; Arciero, A.; Sweazea, K.L.; Gumpricht, E.; Arciero, K.M. Intermittent fasting and protein pacing are superior to caloric restriction for weight and visceral fat loss. Obesity 2023, 31 (Suppl. S1), 139–149. [Google Scholar] [CrossRef] [PubMed]
- Bilibio, B.L.E.; Dos Reis, W.R.; Compagnon, L.; de Batista, D.G.; Sulzbacher, L.M.; Pinheiro, J.F.; Ludwig, M.S.; Frizzo, M.N.; Cruzat, V.; Heck, T.G. Effects of alternate-day fasting and time-restricted feeding in obese middle-aged female rats. Nutrition 2023, 116, 112198. [Google Scholar] [CrossRef]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: A randomised controlled trial. Nutr. Metab. 2021, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Yun, N.; Nah, J.; Lee, M.N.; Wu, D.; Pae, M. Post-Effects of Time-Restricted Feeding against Adipose Tissue Inflammation and Insulin Resistance in Obese Mice. Nutrients 2023, 15, 2617. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Laakso, M.; Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 2014, 10, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, A.; Tohidi, M.; Arshi, B.; Khalili, D.; Azizi, F.; Hadaegh, F. Relationship of hyperinsulinaemia, insulin resistance and beta-cell dysfunction with incident diabetes and pre-diabetes: The Tehran Lipid and Glucose Study. Diabet. Med. 2015, 32, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Collaboration NCDRF. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- van Dieren, S.; Beulens, J.W.; van der Schouw, Y.T.; Grobbee, D.E.; Neal, B. The global burden of diabetes and its complications: An emerging pandemic. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Compensatory hyperinsulinemia and the development of an atherogenic lipoprotein profile: The price paid to maintain glucose homeostasis in insulin-resistant individuals. Endocrinol. Metab. Clin. N. Am. 2005, 34, 49–62. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Farquhar, J.W.; Reaven, G.M. Reappraisal of the role of insulin in hypertriglyceridemia. Am. J. Med. 1974, 57, 551–560. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef]
- Ojo, T.K.; Joshua, O.O.; Ogedegbe, O.J.; Oluwole, O.; Ademidun, A.; Jesuyajolu, D. Role of Intermittent Fasting in the Management of Prediabetes and Type 2 Diabetes Mellitus. Cureus 2022, 14, e28800. [Google Scholar] [CrossRef]
- Phosat, C.; Panprathip, P.; Chumpathat, N.; Prangthip, P.; Chantratita, N.; Soonthornworasiri, N.; Puduang, S.; Kwanbunjan, K. Elevated C-reactive protein, interleukin 6, tumor necrosis factor alpha and glycemic load associated with type 2 diabetes mellitus in rural Thais: A cross-sectional study. BMC Endocr. Disord. 2017, 17, 44. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Shah, R.B.; Patel, M.; Maahs, D.M.; Shah, V.N. Insulin delivery methods: Past, present and future. Int. J. Pharm. Investig. 2016, 6, 1. [Google Scholar] [CrossRef]
- Rys, P.; Wojciechowski, P.; Rogoz-Sitek, A.; Niesyczynski, G.; Lis, J.; Syta, A.; Malecki, M.T. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015, 52, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Clissold, R.; Clissold, S. Insulin glargine in the management of diabetes mellitus: An evidence-based assessment of its clinical efficacy and economic value. Core Evid. 2007, 2, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Hajos, T.R.; Pouwer, F.; de Grooth, R.; Holleman, F.; Twisk, J.W.; Diamant, M.; Snoek, F.J. Initiation of insulin glargine in patients with Type 2 diabetes in suboptimal glycaemic control positively impacts health-related quality of life. A prospective cohort study in primary care. Diabet. Med. 2011, 28, 1096–1102. [Google Scholar] [CrossRef]
- Wang, Z.; Hedrington, M.S.; Gogitidze Joy, N.; Briscoe, V.J.; Richardson, M.A.; Younk, L.; Nicholson, W.; Tate, D.B.; Davis, S.N. Dose-response effects of insulin glargine in type 2 diabetes. Diabetes Care 2010, 33, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; El-Shamy, K.A. Physiological process of fat loss. Bull. Natl. Res. Cent. 2019, 43, 208. [Google Scholar] [CrossRef]
- Davies, M.; Khunti, K. Insulin management in overweight or obese type 2 diabetes patients: The role of insulin glargine. Diabetes Obes. Metab. 2008, 10, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Knop, F.K.; Vilsboll, T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: Differences and similarities. Eur. J. Intern. Med. 2014, 25, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Ajabnoor, G.M.A.; Hashim, K.T.; Alzahrani, M.M.; Alsuheili, A.Z.; Alharbi, A.F.; Alhozali, A.M.; Enani, S.; Eldakhakhny, B.; Elsamanoudy, A. The Possible Effect of the Long-Term Use of Glucagon-like Peptide-1 Receptor Agonists (GLP-1RA) on Hba1c and Lipid Profile in Type 2 Diabetes Mellitus: A Retrospective Study in KAUH, Jeddah, Saudi Arabia. Diseases 2023, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Tofé, S.; Argüelles, I.; Mena, E.; Serra, G.; Codina, M.; Urgeles, J.R.; García, H.; Pereg, V. Real-world GLP-1 RA therapy in type 2 diabetes: A long-term effectiveness observational study. Endocrinol. Diabetes Metab. 2019, 2, e00051. [Google Scholar] [CrossRef]
- Kaneto, H.; Kimura, T.; Shimoda, M.; Obata, A.; Sanada, J.; Fushimi, Y.; Nakanishi, S.; Mune, T.; Kaku, K. Favorable Effects of GLP-1 Receptor Agonist against Pancreatic β-Cell Glucose Toxicity and the Development of Arteriosclerosis: “The Earlier, the Better” in Therapy with Incretin-Based Medicine. Int. J. Mol. Sci. 2021, 22, 7917. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.Y. Glucagon-like peptide-1 and glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bettge, K.; Kahle, M.; Abd El Aziz, M.S.; Meier, J.J.; Nauck, M.A. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials. Diabetes Obes. Metab. 2017, 19, 336–347. [Google Scholar] [CrossRef]
- Gether, I.M.; Nexoe-Larsen, C.; Knop, F.K. New Avenues in the Regulation of Gallbladder Motility-Implications for the Use of Glucagon-Like Peptide-Derived Drugs. J. Clin. Endocrinol. Metab. 2019, 104, 2463–2472. [Google Scholar] [CrossRef]
- Deacon, C.F. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Garduno-Garcia Jde, J.; Adams, J.; DeFronzo, R.A.; Cersosimo, E. Mechanisms of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin when used alone or with metformin in type 2 diabetes: A double-tracer study. Diabetes Care 2013, 36, 2756–2762. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J.; Feinglos, M.N.; Lunceford, J.K.; Johnson, J.; Williams-Herman, D.E. Effect of Initial Combination Therapy with Sitagliptin, a Dipeptidyl Peptidase-4 Inhibitor, and Metformin on Glycemic Control in Patients with Type 2 Diabetes. Diabetes Care 2007, 30, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Safety of dipeptidyl peptidase-4 inhibitors for treating type 2 diabetes. Expert Opin. Drug Saf. 2015, 14, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.L.; Lima, F.J.C.; Sousa-Rodrigues, C.F.; Barbosa, F.T. Use of SGLT-2 inhibitors in the treatment of type 2 diabetes mellitus. Rev. Assoc. Med. Bras. 2017, 63, 636–641. [Google Scholar] [CrossRef]
- Pinto, L.C.; Rados, D.V.; Remonti, L.R.; Kramer, C.K.; Leitao, C.B.; Gross, J.L. Efficacy of SGLT2 inhibitors in glycemic control, weight loss and blood pressure reduction: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2015, 7, A58. [Google Scholar] [CrossRef]
- Colosimo, S.; Tan, G.D.; Petroni, M.L.; Marchesini, G.; Tomlinson, J.W. Improved glycaemic control in patients with type 2 diabetes has a beneficial impact on NAFLD, independent of change in BMI or glucose lowering agent. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 640–648. [Google Scholar] [CrossRef]
- Shigiyama, F.; Kumashiro, N.; Miyagi, M.; Ikehara, K.; Kanda, E.; Uchino, H.; Hirose, T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 2017, 16, 84. [Google Scholar] [CrossRef]
- Satoh, H. Pleiotropic effects of SGLT2 inhibitors beyond the effect on glycemic control. Diabetol. Int. 2018, 9, 212–214. [Google Scholar] [CrossRef]
- Gill, H.K.; Kaur, P.; Mahendru, S.; Mithal, A. Adverse Effect Profile and Effectiveness of Sodium Glucose Co-transporter 2 Inhibitors (SGLT2i)—A Prospective Real-world Setting Study. Indian J. Endocrinol. Metab. 2019, 23, 50–55. [Google Scholar] [CrossRef]
- Wang, G.S.; Hoyte, C. Review of Biguanide (Metformin) Toxicity. J. Intensive Care Med. 2019, 34, 863–876. [Google Scholar] [CrossRef] [PubMed]
- He, L. Metformin and Systemic Metabolism. Trends Pharmacol. Sci. 2020, 41, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Horakova, O.; Kroupova, P.; Bardova, K.; Buresova, J.; Janovska, P.; Kopecky, J.; Rossmeisl, M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019, 9, 6156. [Google Scholar] [CrossRef] [PubMed]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Wakeman, M.; Archer, D.T. Metformin and Micronutrient Status in Type 2 Diabetes: Does Polypharmacy Involving Acid-Suppressing Medications Affect Vitamin B12 Levels? Diabetes Metab. Syndr. Obes. 2020, 13, 2093–2108. [Google Scholar] [CrossRef] [PubMed]
- Kozyraki, R.; Cases, O. Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Zhang, P.; Wang, J.; Ma, J.; An, Y.; Chen, Y.; Zhang, B.; Feng, X.; Li, H.; Chen, X.; et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019, 7, 452–461. [Google Scholar] [CrossRef] [PubMed]
- The 10-Year Cost-Effectiveness of Lifestyle Intervention or Metformin for Diabetes Prevention. Diabetes Care 2012, 35, 723–730. [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group; Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef]
- Cardona-Morrell, M.; Rychetnik, L.; Morrell, S.L.; Espinel, P.T.; Bauman, A. Reduction of diabetes risk in routine clinical practice: Are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health 2010, 10, 653. [Google Scholar] [CrossRef]
- Lean, M.E.J. Banting Memorial Lecture 2021—Banting, banting, banter and bravado: Convictions meet evidence in the scientific process. Diabet. Med. 2021, 38, e14643. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Vining, E.P.G. Clinical Aspects of the Ketogenic Diet. Epilepsia 2007, 48, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R. Importance of functional foods in the Mediterranean diet. Public Health Nutr. 2006, 9, 1136–1140. [Google Scholar] [CrossRef]
- Kossoff, E.H.; McGrogan, J.R. Worldwide Use of the Ketogenic Diet. Epilepsia 2005, 46, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O.; Wainstein, J.; Boaz, M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids 2012, 77, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Banting Memorial Lecture 2012 Reversing the twin cycles of Type 2 diabetes. Diabet. Med. 2013, 30, 267–275. [Google Scholar] [CrossRef]
- Yancy, W.S.; Foy, M.; Chalecki, A.M.; Vernon, M.C.; Westman, E.C. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2005, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: A meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014, 63, 903–911. [Google Scholar] [CrossRef]
- Bao, W.; Tobias, D.K.; Bowers, K.; Chavarro, J.; Vaag, A.; Grunnet, L.G.; Strøm, M.; Mills, J.; Liu, A.; Kiely, M.; et al. Physical Activity and Sedentary Behaviors Associated with Risk of Progression from Gestational Diabetes Mellitus to Type 2 Diabetes Mellitus. JAMA Intern. Med. 2014, 174, 1047. [Google Scholar] [CrossRef]
- Hrubeniuk, T.J.; Bouchard, D.R.; Goulet, E.D.B.; Gurd, B.; Sénéchal, M. The ability of exercise to meaningfully improve glucose tolerance in people living with prediabetes: A meta-analysis. Scand. J. Med. Sci. Sports 2020, 30, 209–216. [Google Scholar] [CrossRef]
- Agboola, S.; Jethwani, K.; Lopez, L.; Searl, M.; O’Keefe, S.; Kvedar, J. Text to Move: A Randomized Controlled Trial of a Text-Messaging Program to Improve Physical Activity Behaviors in Patients with Type 2 Diabetes Mellitus. J. Med. Internet Res. 2016, 18, e307. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Dendale, P.; Jonkers, R.A.M.; Beelen, M.; Manders, R.J.F.; Corluy, L.; Mullens, A.; Berger, J.; Meeusen, R.; Van Loon, L.J.C. Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA1c in obese type 2 diabetes patients. Diabetologia 2009, 52, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Medagama, A.; Galgomuwa, M. Lack of infrastructure, social and cultural factors limit physical activity among patients with type 2 diabetes in rural Sri Lanka, a qualitative study. PLoS ONE 2018, 13, e0192679. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults. JAMA Intern. Med. 2017, 177, 930. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl. Res. 2014, 164, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, Y.; Zhang, Y.; Xu, W.; Zhang, H.; Yan, Q.; Gao, L.; Shang, L. Dietary recommendations for fasting days in an alternate-day intermittent fasting pattern: A randomized controlled trial. Nutrition 2022, 102, 111735. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Qin, Y.-L.; Shi, Z.-Y.; Chen, J.-H.; Zeng, M.-J.; Zhou, W.; Chen, R.-Q.; Chen, Z.-Y. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Civitarese, A.E.; Bogacka, I.; Smith, S.R.; Hulver, M.; Ravussin, E. Glucose Tolerance and Skeletal Muscle Gene Expression in Response to Alternate Day Fasting. Obes. Res. 2005, 13, 574–581. [Google Scholar] [CrossRef]
- Higashida, K.; Fujimoto, E.; Higuchi, M.; Terada, S. Effects of alternate-day fasting on high-fat diet-induced insulin resistance in rat skeletal muscle. Life Sci. 2013, 93, 208–213. [Google Scholar] [CrossRef]
- Swoap, S.J.; Bingaman, M.J.; Hult, E.M.; Sandstrom, N.J. Alternate-day feeding leads to improved glucose regulation on fasting days without significant weight loss in genetically obese mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2019, 317, R461–R469. [Google Scholar] [CrossRef] [PubMed]
- Beigy, M.; Vakili, S.; Berijani, S.; Aminizade, M.; Ahmadi-Dastgerdi, M.; Meshkani, R. Alternate-day fasting diet improves fructose-induced insulin resistance in mice. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Ingersen, A.; Helset, H.R.; Calov, M.; Chabanova, E.; Harreskov, E.G.; Jensen, C.; Hansen, C.N.; Prats, C.; Helge, J.W.; Larsen, S.; et al. Metabolic effects of alternate-day fasting in males with obesity with or without type 2 diabetes. Front. Physiol. 2022, 13, 1061063. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, C.M.; Trepanowski, J.F.; Klempel, M.C.; Barnosky, A.; Bhutani, S.; Gabel, K.; Varady, K.A. Eating behavior traits of successful weight losers during 12 months of alternate-day fasting: An exploratory analysis of a randomized controlled trial. Nutr. Health 2018, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kalam, F.; Gabel, K.; Cienfuegos, S.; Wiseman, E.; Ezpeleta, M.; Pavlou, V.; Varady, K.A. Changes in subjective measures of appetite during 6 months of alternate day fasting with a low carbohydrate diet. Clin. Nutr. ESPEN 2021, 41, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent fasting in Type 2 diabetes mellitus and the risk of hypoglycaemia: A randomized controlled trial. Diabet. Med. 2018, 35, 588–594. [Google Scholar] [CrossRef]
- Carter, S.; Clifton, P.M.; Keogh, J.B. Intermittent energy restriction in type 2 diabetes: A short discussion of medication management. World J. Diabetes 2016, 7, 627–630. [Google Scholar] [CrossRef]
- Baker, S.; Jerums, G.; Proietto, J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res. Clin. Pract. 2009, 85, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; White, K.; Maw, M.T.T.; Charleston, J.; Zhao, D.; Guallar, E.; Appel, L.J.; Clark, J.M.; Maruthur, N.M.; Pilla, S.J. Adherence to Diet and Meal Timing in a Randomized Controlled Feeding Study of Time-Restricted Feeding. Nutrients 2022, 14, 2283. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- de Goede, P.; Foppen, E.; Ritsema, W.; Korpel, N.L.; Yi, C.X.; Kalsbeek, A. Time-Restricted Feeding Improves Glucose Tolerance in Rats, but Only When in Line with the Circadian Timing System. Front. Endocrinol. 2019, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Johnston, J.D.; Robertson, M.D. Early versus late time-restricted feeding in adults at increased risk of developing type 2 diabetes: Is there an optimal time to eat for metabolic health? Nutr. Bull. 2021, 46, 69–76. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men with Overweight and Obesity. JAMA Intern. Med. 2020, 180, 1491. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Devlin, B.L.; Radford, B.E.; Hawley, J.A. A Delayed Morning and Earlier Evening Time-Restricted Feeding Protocol for Improving Glycemic Control and Dietary Adherence in Men with Overweight/Obesity: A Randomized Controlled Trial. Nutrients 2020, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Tsameret, S.; Chapnik, N.; Froy, O. Effect of early vs. late time-restricted high-fat feeding on circadian metabolism and weight loss in obese mice. Cell. Mol. Life Sci. 2023, 80, 180. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Zhao, L.; Zhou, Y. Effect of 5:2 Fasting Diet on Liver Fat Content in Patients with Type 2 Diabetic with Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat. Disord. 2022, 20, 459–465. [Google Scholar] [CrossRef]
- Parvaresh, A.; Razavi, R.; Abbasi, B.; Yaghoobloo, K.; Hassanzadeh, A.; Mohammadifard, N.; Safavi, S.M.; Hadi, A.; Clark, C.C.T. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: A randomized clinical trial. Complement. Ther. Med. 2019, 47, 102187. [Google Scholar] [CrossRef]
- Sukkriang, N.; Buranapin, S. Effect of intermittent fasting 16:8 and 14:10 compared with control-group on weight reduction and metabolic outcomes in obesity with type 2 diabetes patients: A randomized controlled trial. J. Diabetes Investig. 2024, 31, 92–104.e5. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Robertson, T.M.; Robertson, M.D.; Johnston, J.D. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J. Nutr. Sci. 2018, 7, e22. [Google Scholar] [CrossRef]
- Perreault, L.; Pan, Q.; Mather, K.J.; Watson, K.E.; Hamman, R.F.; Kahn, S.E.; Diabetes Prevention Program Research Group. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: Results from the Diabetes Prevention Program Outcomes Study. Lancet 2012, 379, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.M.; Fox, C.S. Implications of rising prediabetes prevalence. Diabetes Care 2013, 36, 2139–2141. [Google Scholar] [CrossRef]
- Gong, R.; Liu, Y.; Luo, G.; Liu, W.; Jin, Z.; Xu, Z.; Li, Z.; Yang, L.; Wei, X. Associations of TG/HDL Ratio with the Risk of Prediabetes and Diabetes in Chinese Adults: A Chinese Population Cohort Study Based on Open Data. Int. J. Endocrinol. 2021, 2021, 9949579. [Google Scholar] [CrossRef] [PubMed]
- Brannick, B.; Wynn, A.; Dagogo-Jack, S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp. Biol. Med. 2016, 241, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36, S67–S74. [CrossRef]
- Rooney, M.R.; Fang, M.; Ogurtsova, K.; Ozkan, B.; Echouffo-Tcheugui, J.B.; Boyko, E.J.; Magliano, D.J.; Selvin, E. Global Prevalence of Prediabetes. Diabetes Care 2023, 46, 1388–1394. [Google Scholar] [CrossRef]
- Lomonaco, R.; Ortiz-Lopez, C.; Orsak, B.; Webb, A.; Hardies, J.; Darland, C.; Finch, J.; Gastaldelli, A.; Harrison, S.; Tio, F.; et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 1389–1397. [Google Scholar] [CrossRef]
- Rattarasarn, C. Dysregulated lipid storage and its relationship with insulin resistance and cardiovascular risk factors in non-obese Asian patients with type 2 diabetes. Adipocyte 2018, 7, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, D.; Tamura, Y.; Takeno, K.; Kaga, H.; Someya, Y.; Kakehi, S.; Funayama, T.; Furukawa, Y.; Suzuki, R.; Kadowaki, S.; et al. Clinical Features of Nonobese, Apparently Healthy, Japanese Men with Reduced Adipose Tissue Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2019, 104, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Bacha, F.; Tfayli, H.; Michaliszyn, S.F.; Yousuf, S.; Arslanian, S. Adipose Tissue Insulin Resistance in Youth on the Spectrum from Normal Weight to Obese and from Normal Glucose Tolerance to Impaired Glucose Tolerance to Type 2 Diabetes. Diabetes Care 2019, 42, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.R.; Carbajal, H.A.; Espeche, W.G.; Aizpurúa, M.; Leiva Sisnieguez, C.E.; Leiva Sisnieguez, B.C.; Stavile, R.N.; March, C.E.; Reaven, G.M. Insulin resistance: The linchpin between prediabetes and cardiovascular disease. Diabetes Vasc. Dis. Res. 2016, 13, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Guemes, M.; Rahman, S.A.; Hussain, K. What is a normal blood glucose? Arch. Dis. Child. 2016, 101, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Santaguida, P.; Raina, P.; Morrison, K.M.; Balion, C.; Hunt, D.; Yazdi, H.; Booker, L. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: A systematic overview and meta-analysis of prospective studies. Diabetes Res. Clin. Pract. 2007, 78, 305–312. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Perreault, L.; Ji, L.; Dagogo-Jack, S. Diagnosis and Management of Prediabetes: A Review. JAMA 2023, 329, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Saito, T. Lifestyle Modification and Prevention of Type 2 Diabetes in Overweight Japanese with Impaired Fasting Glucose Levels. Arch. Intern. Med. 2011, 171, 1352. [Google Scholar] [CrossRef]
- Reasner, C.; Olansky, L.; Seck, T.L.; Williams-Herman, D.E.; Chen, M.; Terranella, L.; Johnson-Levonas, A.O.; Kaufman, K.D.; Goldstein, B.J. The effect of initial therapy with the fixed-dose combination of sitagliptin and metformin compared with metformin monotherapy in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2011, 13, 644–652. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Qu, Y.; Lin, M.; Dong, F.; Li, Y.; Cao, L.; Lin, S. Efficacy and safety of combination therapy with vildagliptin and metformin vs. metformin monotherapy for Type 2 Diabetes Mellitus therapy: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2802–2817. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Boule, N.G. Does metformin therapy influence the effects of intensive lifestyle intervention? Exploring the interaction between first line therapies in the Look AHEAD trial. Metabolism 2019, 94, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.S.; Li, T.Y.; Manson, J.E.; Hu, F.B. Adiposity Compared with Physical Inactivity and Risk of Type 2 Diabetes in Women. Diabetes Care 2007, 30, 53–58. [Google Scholar] [CrossRef]
- Hamasaki, H. Daily physical activity and type 2 diabetes: A review. World J. Diabetes 2016, 7, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O. Exercise-induced increase in muscle insulin sensitivity. J. Appl. Physiol. 2005, 99, 338–343. [Google Scholar] [CrossRef]
- Chen, A.K.; Roberts, C.K.; Barnard, R.J. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism 2006, 55, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Durstine, J.L. Effect of aerobic exercise on high-density lipoprotein cholesterol: A meta-analysis. Clin. J. Sport. Med. 2008, 18, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Tanaka, S.; Saito, K.; Shu, M.; Sone, Y.; Onitake, F.; Suzuki, E.; Shimano, H.; Yamamoto, S.; Kondo, K.; et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: A meta-analysis. Arch. Intern. Med. 2007, 167, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.M.; Connelly, D.M. The effects of exercise interventions on physical function tests and glycemic control in adults with type 2 diabetes: A systematic review. J. Bodyw. Mov. Ther. 2021, 28, 283–293. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Wei, J.; Chen, J.; Wei, X.; Xiang, X.; Cheng, Q.; Xu, J.; Xu, S.; Chen, G.; Liu, C. Long-term remission of type 2 diabetes after very-low-calorie restriction and related predictors. Front. Endocrinol. 2022, 13, 968239. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, L.M.; Napolitano, M.A.; Pogach, L.M.; Quigley, K.S.; Shantz, K.L.; Vander Veur, S.S.; Foster, G.D. The impact of self-monitoring of blood glucose on a behavioral weight loss intervention for patients with type 2 diabetes. Diabetes Educ. 2013, 39, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Thom, G.; McIntosh, A.; Messow, C.M.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; McCombie, L.; Malkova, D.; Al-Mrabeh, A.; Zhyzhneuskaya, S.; et al. Weight loss-induced increase in fasting ghrelin concentration is a predictor of weight regain: Evidence from the Diabetes Remission Clinical Trial (DiRECT). Diabetes Obes. Metab. 2021, 23, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tsintzas, K.; Macdonald, I.A.; Cordon, S.M.; Taylor, M.A. Effects of intermittent (5:2) or continuous energy restriction on basal and postprandial metabolism: A randomised study in normal-weight, young participants. Eur. J. Clin. Nutr. 2022, 76, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Chair, S.Y.; Cai, H.; Cao, X.; Qin, Y.; Cheng, H.Y.; Ng, M.T. Intermittent Fasting in Weight Loss and Cardiometabolic Risk Reduction: A Randomized Controlled Trial. J. Nurs. Res. 2022, 30, e185. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, K.; Sujka, M. Effect of Various Types of Intermittent Fasting (IF) on Weight Loss and Improvement of Diabetic Parameters in Human. Curr. Nutr. Rep. 2021, 10, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Xu, Y.; Gao, J.; Cao, Q.; Ding, Y.; Xin, Z.; Lu, M.; Li, X.; Song, H.; et al. Effect of 5:2 Regimens: Energy-Restricted Diet or Low-Volume High-Intensity Interval Training Combined with Resistance Exercise on Glycemic Control and Cardiometabolic Health in Adults with Overweight/Obesity and Type 2 Diabetes—A Three-Arm Randomized Controlled Trial. Diabetes Care 2024, 47, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.M.; da Cunha, F.M.; Caldeira da Silva, C.C.; Chausse, B.; Romano, R.L.; Garcia, C.C.; Colepicolo, P.; Medeiros, M.H.; Kowaltowski, A.J. Long-term intermittent feeding, but not caloric restriction, leads to redox imbalance, insulin receptor nitration, and glucose intolerance. Free Radic. Biol. Med. 2011, 51, 1454–1460. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Tinsley, G.M.; La Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience 2020, 42, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Tsitsou, S.; Zacharodimos, N.; Poulia, K.A.; Karatzi, K.; Dimitriadis, G.; Papakonstantinou, E. Effects of Time-Restricted Feeding and Ramadan Fasting on Body Weight, Body Composition, Glucose Responses, and Insulin Resistance: A Systematic Review of Randomized Controlled Trials. Nutrients 2022, 14, 4778. [Google Scholar] [CrossRef] [PubMed]
- Andriessen, C.; Fealy, C.E.; Veelen, A.; van Beek, S.M.M.; Roumans, K.H.M.; Connell, N.J.; Mevenkamp, J.; Moonen-Kornips, E.; Havekes, B.; Schrauwen-Hinderling, V.B.; et al. Three weeks of time-restricted eating improves glucose homeostasis in adults with type 2 diabetes but does not improve insulin sensitivity: A randomised crossover trial. Diabetologia 2022, 65, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Bandin, C.; Scheer, F.A.; Luque, A.J.; Avila-Gandia, V.; Zamora, S.; Madrid, J.A.; Gomez-Abellan, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gomez-Abellan, P.; Alburquerque-Bejar, J.J.; Lee, Y.C.; Ordovas, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef]
- Allison, K.C.; Hopkins, C.M.; Ruggieri, M.; Spaeth, A.M.; Ahima, R.S.; Zhang, Z.; Taylor, D.M.; Goel, N. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr. Biol. 2021, 31, 650–657.e3. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef]
| Types of Antidiabetic Drug(s) | Mode of Action | Effects on Glucose Parameters | Shortfall(s) |
|---|---|---|---|
| Insulin therapy | Direct glucose-lowering effect. Facilitation of glucose uptake by cells. Inhibition of hepatic glucose production. Promotion of glycogen synthesis. | Reduced FG, Reduced GT Reduced HbA1c | Weight gain |
| GLP-1RA | Slowing of gastric emptying Suppression of glucagon secretion Enhancement of glucose-dependent insulin secretion Improvement in Beta Cell Function Reduction of Appetite and Food Intake | Reduced FG, Reduced GT Reduced HbA1c | Gastrointestinal effects Gallbladder disease |
| DPP4i | Inhibition of DPP-4 enzyme Reduction in Blood Glucose Levels Prolongation of incretin hormone activity Weight management | Reduced FG Reduced GT Reduced HbA1c | Acute pancreatitis |
| SGL2i | Inhibition of SGLT2 in the kidneys Increased urinary glucose excretion. Reduction in blood glucose levels Caloric loss and weight reduction. Osmotic Diuresis. | Reduced FG, Reduced GT Reduced HbA1c | Urinary tract infection, Genital infection Lower limb amputation |
| Metformin | Enhanced peripheral glucose uptake. Inhibition of intestinal glucose transport. Improvement of lipid metabolism. | Reduced FG, Reduced GT Reduced HbA1c | Vitamin B12 deficiency Lactic acidosis |
| IF Regimen | Description | Positive Effects | Adverse Effects |
|---|---|---|---|
| 5:2 fasting diet [4,119,129] | Involves a 5-day non-fasting period and a 2-day fasting period. | Improvements in weight, HbA1c, lipids, fasting glucose, and quality of life | Fasting elevated the occurrence of hypoglycemia even with reduced medication. Fasting may lead to over-compensation during non-fasting days. |
| Alternate day fasting [110,111,130] | Involves alternating between a 24 h fasting period, during which individuals consume less than 25% of their usual energy needs, and a 24 h eating period, where they can eat normally. | Decrease in body weight, waist circumference, systolic blood pressure, and fasting plasma glucose. | Fatigue, Headaches |
| 16 h:8 h TRF [128,131] | Adhering to 16 h of abstinence from food and 8 h of food intake within 24 h | Heightens insulin sensitivity and fat oxidation and decreases body weight, fat profile, and inflammation | Hunger and irritability, Palpitations, dizziness, headache, abdominal pain, mood changes, vomiting, and hypoglycemia |
| 14 h:10 h TRF [131,132,133] | Adhering to 14 h of abstinence from food and 10 h of food intake within 24 h | Reduced body weight, improved HbA1c, enhanced body composition, lowered blood pressure, and decreased lipids associated with cardiovascular disease. | Disrupted Social Eating Patterns, Palpitations, dizziness, headache, abdominal pain, mood changes, vomiting, and hypoglycemia |
| IF Regimen | Description | Positive Effects | Adverse Effects |
|---|---|---|---|
| 5:2 fasting diet [165,180]. | Involves a 5-day non-fasting period and a 2-day fasting period. | Improvements in body weight, HbA1c, lipids, fasting glucose, and appetite score | Increased hunger |
| Alternate-day fasting [112,113,169] | Alternating 24 h fasting and 24 h eating period | Improved insulin sensitivity, decreased body weight, lower fasting insulin levels, improved IFG, reduced postprandial hyperglycemia, and decreased levels of HbA1c | Increased hunger, vomiting, redox imbalance, and glucose intolerance |
| Time-restricted feeding [175,176] | Fasting and consuming meals within a limited timeframe of 24 h. | Reduced FG, HbA1c, and postprandial glucose | Dizziness, hunger, nausea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msane, S.; Khathi, A.; Sosibo, A. Therapeutic Potential of Various Intermittent Fasting Regimens in Alleviating Type 2 Diabetes Mellitus and Prediabetes: A Narrative Review. Nutrients 2024, 16, 2692. https://doi.org/10.3390/nu16162692
Msane S, Khathi A, Sosibo A. Therapeutic Potential of Various Intermittent Fasting Regimens in Alleviating Type 2 Diabetes Mellitus and Prediabetes: A Narrative Review. Nutrients. 2024; 16(16):2692. https://doi.org/10.3390/nu16162692
Chicago/Turabian StyleMsane, Sthembiso, Andile Khathi, and Aubrey Sosibo. 2024. "Therapeutic Potential of Various Intermittent Fasting Regimens in Alleviating Type 2 Diabetes Mellitus and Prediabetes: A Narrative Review" Nutrients 16, no. 16: 2692. https://doi.org/10.3390/nu16162692
APA StyleMsane, S., Khathi, A., & Sosibo, A. (2024). Therapeutic Potential of Various Intermittent Fasting Regimens in Alleviating Type 2 Diabetes Mellitus and Prediabetes: A Narrative Review. Nutrients, 16(16), 2692. https://doi.org/10.3390/nu16162692








