Association of Salty and Sweet Taste Recognition with Food Reward and Subjective Control of Eating Behavior
Abstract
1. Introduction
2. Methods
2.1. Recruitment and Study Population
2.2. Study Design
2.3. Taste Recognition
2.4. Assessment of Food Reward and Control over Eating Behavior
2.4.1. The Leeds Food Preference Questionnaire
2.4.2. Power of Food Scale
2.4.3. Three-Factor Eating Questionnaire
2.5. Anthropometric Measures
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Association of Sweet and Salty Taste Recognition with Liking and Wanting (Food Reward)
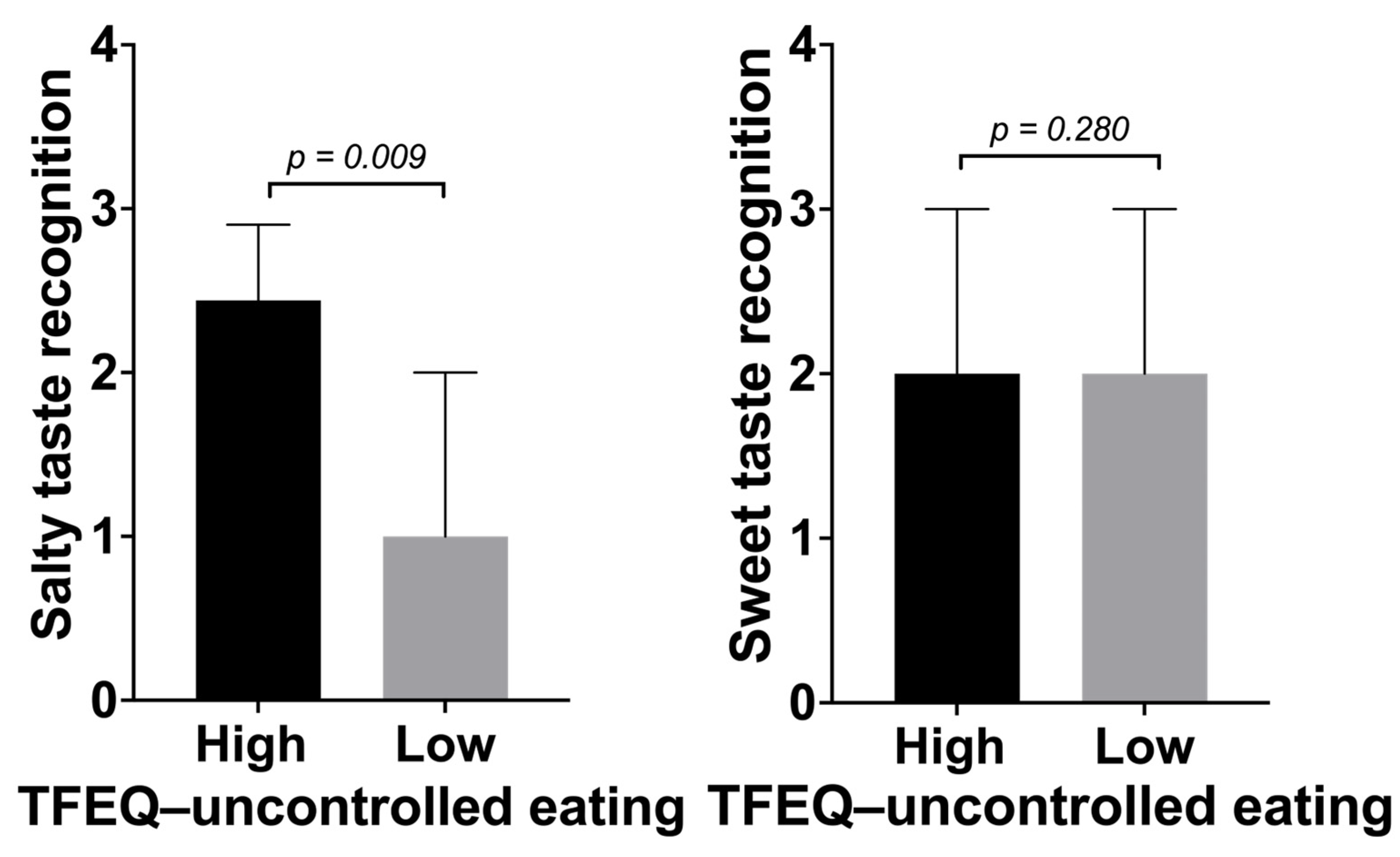
3.3. Association of Sweet and Salty Taste Recognition with Measures of Uncontrolled Eating
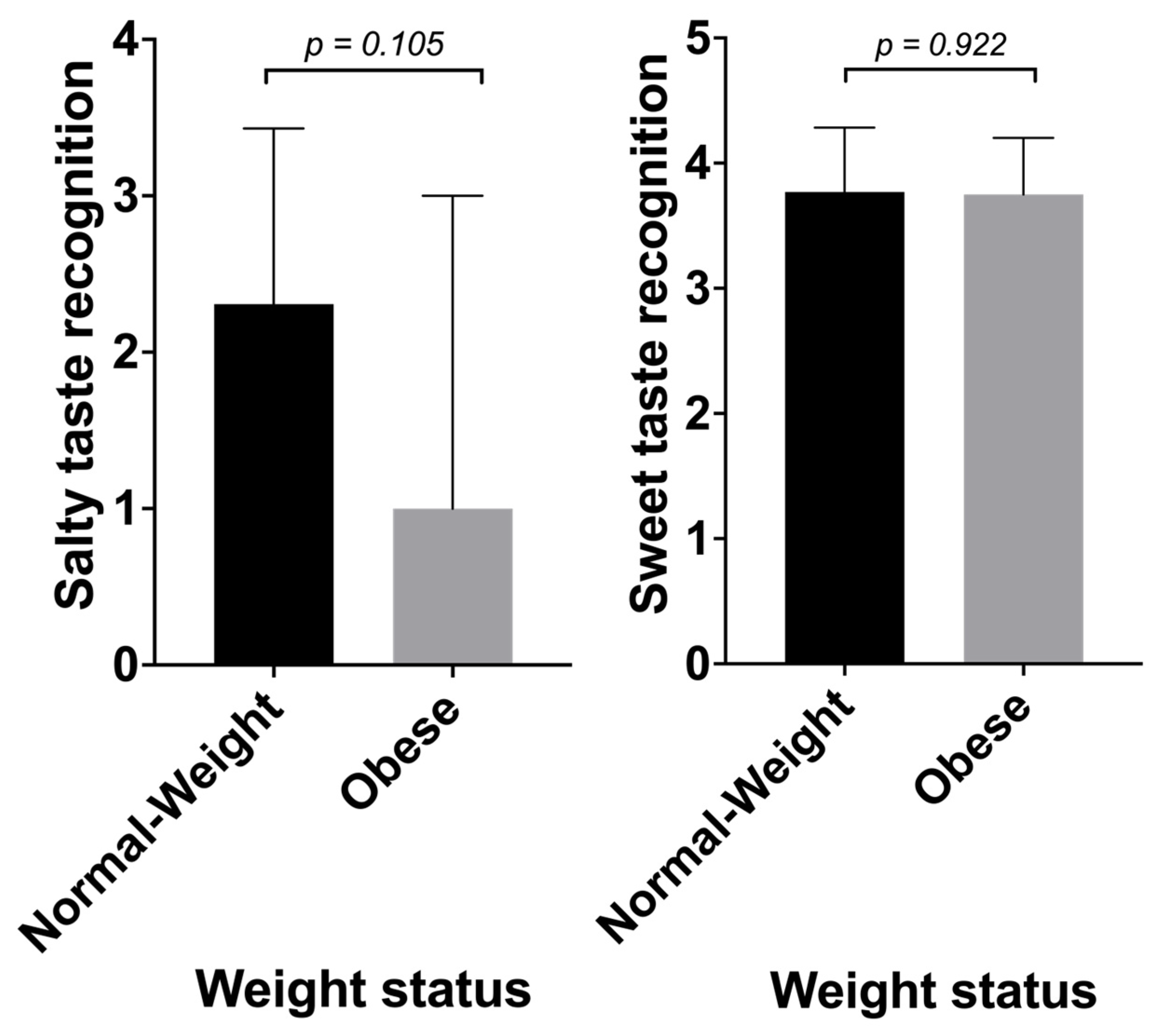
3.4. Taste Recognition and Obesity
4. Discussion
4.1. Association of Salty Taste Recognition with Food Reward and Uncontrolled Eating
4.2. Association of Sweet Taste Recognition and Food Reward and Uncontrolled Eating
4.3. Taste Recognition and Obesity
4.4. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Age | Sex | BMI | Smoking # | State Hunger ß | |
|---|---|---|---|---|---|
| (r/p) | (r/p) | (r/p) | (r/p) | (r/p) | |
| Sweet taste | −0.458/0.004 ** | −0.084/0.617 | −0.178/0.286 | 0.317/0.053 | −0.156/0.348 |
| Salty taste | −0.097/0.561 | 0.184/0.268 | −0.137/0.414 | −0.173/0.299 | −0.105/0.532 |
| Power of Food Scale | |||||
| Total score | −0.358/0.027 * | 0.091/0.589 | 0.134/0.422 | −0.125/0.453 | 0.042/0.803 |
| Availability | −0.314/0.055 | −0.070/0.676 | 0.194/0.244 | −0.218/0.188 | 0.136/0.416 |
| Taste | −0.170/0.308 | 0.132/0.428 | 0.224/0.175 | −0.056/0.737 | −0.068/0.687 |
| Present | −0.506/0.001 ** | 0.137/0.411 | −0.186/0.263 | 0.060/0.723 | 0.070/0.678 |
| Leeds Food Preference Qestionnaire-G | |||||
| EL (HFSA) | 0.105/0.531 | −0.075/0.655 | 0.144/0495 | −0.372/0.022 * | −0.051/0.760 |
| EL (LFSA) | −0.136/0.417 | 0.142/0.395 | 0.122/0.466 | −0.138/0.408 | −0.003/0.985 |
| EL (HFSW) | −0.298/0.070 | −0.046/0.782 | 0.117/0.483 | −0.020/0.906 | 0.207/0.213 |
| EL (LFSW) | −0.175/0.294 | −0.139/0.404 | −0.166/0.318 | 0.269/0.071 | 0.101/0.548 |
| EW (HFSA) | 0.133/0.499 | −0.096/0.568 | 0.154/0.357 | −0.395/0.014 * | −0.015/0.929 |
| EW (LFSA) | 0.082/0.624 | 0.046/0.782 | 0.119/0.475 | −0.211/0.204 | 0.080/0.634 |
| EW (HFSW) | −0.166/0.319 | −0.005/0.975 | 0.124/0.458 | −0.023/0.891 | 0.275/0.094 |
| EW (LFSW) | −0.128/0.444 | −0.067/0.689 | −0.128/0.444 | 0.306/0.062 | 0.096/0.565 |
| IW (HFSA) | 0.268/0.104 | 0.021/0.902 | 0.181/0.277 | −0.322/0.048 * | −0.120/0.474 |
| IW (LFSA) | 0.474/0.003 ** | 0.227/0.170 | 0.201/0.226 | −0.125/0.454 | −0.149/0.373 |
| IW (HFSW) | −0.241/0.145 | −0.077/0.644 | 0.073/0.665 | −0.007/0.969 | 0.057/0.733 |
| IW (LFSW) | −0.339/0.037 * | −0.062/0.712 | −0.391/0.015 * | 0.467/0.003 ** | 0.157/0.346 |
| Three-Factor Eating Questionnaire | |||||
| Disinhibition | 0.007/0.966 | −0.230/0.165 | 0.464/0.003 ** | −0.160/0.337 | −0.139/0.404 |
| Hunger | −0.194/0.234 | −0.138/0.410 | 0.116/0.488 | 0.023/0.890 | 0.112/0.503 |
| Restraint eating | 0.190/0.253 | 0.078/0.643 | 0.384/0.017 * | −0.122/0.467 | −0.019/0.908 |
| Cognitive restraint | 0.164/0.326 | 0.128/0.442 | 0.264/0.109 | −0.048/0.776 | 0.021/0.902 |
| Emotional eating | −0.024/0.886 | −0.489/0.002 ** | 0.236/0.155 | −0.266/0.106 | 0.099/0.553 |
| Uncontrolled eating | −0.144/0.387 | −0.068/0.685 | 0.199/0.231 | −0.031/0.851 | −0.193/0.245 |
References
- May, C.E.; Dus, M. Confection Confusion: Interplay Between Diet, Taste, and Nutrition. Trends Endocrinol. Metab. 2021, 32, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Mambrini, S.P.; Gilardini, L.; Scacchi, M.; Castelnuovo, G.; Pagliarini, E.; Bertoli, S. The phenomenon of abnormal eating and taste perception: What’s the link in subjects with obesity and eating disorders? Food Qual. Prefer. 2023, 104, 104744. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Jiang, P. Comparative biology of taste: Insights into mechanism and function. Flavour 2015, 4, 9. [Google Scholar] [CrossRef]
- Bigiani, A.; Shigemura, N.; Riso, P.; Behrens, M.; Rhyu, M.; Beckett, E.; Kolcic, I.; Cha, Y.-S. Salt Taste, Nutrition, and Health; MDPI: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Morris, M.J.; Na, E.S.; Johnson, A.K. Salt craving: The psychobiology of pathogenic sodium intake. Physiol. Behav. 2008, 94, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Lawrence, A.J. Salt Appetite, and the Influence of Opioids. Neurochem. Res. 2018, 43, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.A.; Stuber, G.D. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018, 27, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Crino, M.; Sacks, G.; Vandevijvere, S.; Swinburn, B.; Neal, B. The Influence on Population Weight Gain and Obesity of the Macronutrient Composition and Energy Density of the Food Supply. Curr. Obes. Rep. 2015, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fazzino, T.L.; Rohde, K.; Sullivan, D.K. Hyper-Palatable Foods: Development of a Quantitative Definition and Application to the US Food System Database. Obesity 2019, 27, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, D.P.; Newman, L.P.; Keast RS, J. Effects of salt and fat combinations on taste preference and perception. Chem. Senses 2016, 41, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, H.; Alonso-Alonso, M.; Hill, J.O.; Kelley, M.; Khan, N.A. Obesity and the neurocognitive basis of food reward and the control of intake. Adv. Nutr. 2015, 6, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Feeney, E.L.; Allen, A.L. Do polymorphisms in chemosensory genes matter for human ingestive behavior? Food Qual. Prefer. 2013, 30, 202–216. [Google Scholar] [CrossRef]
- Ribeiro, G.; Oliveira-Maia, A.J. Sweet taste and obesity. Eur. J. Intern. Med. 2021, 92, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.; Schamarek, I.; Blüher, M. Consequences of obesity on the sense of taste: Taste buds as treatment targets? Diabetes Metab. J. 2020, 44, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.; Torres, S.; Fernandes, A.B.; Camacho, M.; Branco, T.L.; Martins, S.S.; Raimundo, A.; Oliveira-Maia, A.J.; Food Reward in Bariatric Surgery Portuguese Study Group. Enhanced sweet taste perception in obesity: Joint analysis of gustatory data from multiple studies. Front. Nutr. 2022, 9, 1028261. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; King, N.; Blundell, J.E. Liking vs. wanting food: Importance for human appetite control and weight regulation. Neurosci. Biobehav. Rev. 2007, 31, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Grol, M.; Cásedas, L.; Oomen, D.; Spronk, D.B.; Fox, E. Uncontrolled eating in healthy women has limited influence on food cue reactivity and food-related inhibitory control. Appetite 2022, 168, 105767. [Google Scholar] [CrossRef] [PubMed]
- Espel-Huynh, H.M.; Muratore, A.F.; Lowe, M.R. A narrative review of the construct of hedonic hunger and its measurement by the Power of Food Scale. Obes. Sci. Pract. 2018, 4, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Taruno, A.; Gordon, M.D. Molecular and Cellular Mechanisms of Salt Taste. Annu. Rev. Physiol. 2023, 85, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Sullivan, B.S.; Duffy, V.B. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol. Behav. 2010, 100, 369–380. [Google Scholar] [CrossRef]
- Institute of Medicine; Henney, J.E.; Taylor, C.L.; Boon, C.S. Taste and Flavor Roles of Sodium in Foods: A Unique Challenge to Reducing Sodium Intake. In Strategies to Reduce Sodium Intake in the United States; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Geerling, J.C.; Loewy, A.D. Central regulation of sodium appetite. Exp. Physiol. 2008, 93, 177–209. [Google Scholar] [CrossRef] [PubMed]
- Kure Liu, C.; Joseph, P.V.; Feldman, D.E.; Kroll, D.S.; Burns, J.A.; Manza, P.; Volkow, N.D.; Wang, G.-J. Brain Imaging of Taste Perception in Obesity: A Review. Curr. Nutr. Rep. 2019, 8, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Verharen, J.P.H.; Roelofs, T.J.M.; Menting-Henry, S.; Luijendijk, M.C.M.; Vanderschuren, L.J.M.J.; Adan, R.A.H. Limbic control over the homeostatic need for sodium. Sci. Rep. 2019, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Hartley, I.E.; Liem, D.G.; Keast, R. Umami as an ‘Alimentary’ taste. A new perspective on taste classification. Nutrients 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Mohebbi, M.; Unrath, M.; Hummel, C.; Hummel, T. Different Neural Processing of Umami and Salty Taste Determined by Umami Identification Ability Independent of Repeated Umami Exposure. Neuroscience 2018, 383, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kersten, A.; Lorenz, A.; Nottmeier, C.; Schmidt, M.; Roesner, A.; Richter, F.C.; Röhrborn, K.; Witte, A.V.; Hahnel, S.; Koehne, T.; et al. The Obese Taste Bud Study: Objectives and Study design. Diabetes Obes. Metab. 2024, 26, 2054–2068. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C. LimeSurvey: An Open Source Survey Tool; LimeSurvey Project; Limesurvey GmbH: Hamburg, Germany, 2015. [Google Scholar]
- Schamarek, I.; Richter, F.; Tönjes, A.; Stumvoll, M.; Blüher, M.; Rohde-Zimmermann, K.; Finlayson, G. The German Leeds food preference Questionnaire (LFPQ-G): A validation study. Food Qual. Prefer. 2023, 112, 105035. [Google Scholar] [CrossRef]
- Finlayson, G.; King, N.; Blundell, J.E. Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiol. Behav. 2007, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Oustric, P.; Thivel, D.; Dalton, M.; Beaulieu, K.; Gibbons, C.; Hopkins, M.; Blundell, J.; Finlayson, G. Measuring food preference and reward: Application and cross-cultural adaptation of the Leeds Food Preference Questionnaire in human experimental research. Food Qual. Prefer. 2020, 80, 103824. [Google Scholar] [CrossRef]
- Lowe, M.R.; Butryn, M.L.; Didie, E.R.; Annunziato, R.A.; Thomas, J.G.; Crerand, C.E.; Ochner, C.N.; Coletta, M.C.; Bellace, D.; Wallaert, M.; et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite 2009, 53, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, E.; Neumann, M.; Nöhre, M.; Brähler, E.; Hilbert, A.; de Zwaan, M. Validation of the German Version of the Power of Food Scale in a General Population Sample. Obes. Facts 2019, 12, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Löffler, A.; Luck, T.; Then, F.S.; Sikorski, C.; Kovacs, P.; Böttcher, Y.; Breitfeld, J.; Tönjes, A.; Horstmann, A.; Löffler, M.; et al. Eating behaviour in the general population: An analysis of the factor structure of the German version of the three-factor-eating-questionnaire (TFEQ) and its association with the body mass index. PLoS ONE 2015, 10, e0133977. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Sotirelis, E.; Bojeh, R.; Maan, I.; Medalle, M.; Chik, X.S.F.; Keast, R.; Tucker, R.M. Is dietary intake associated with salt taste function and perception in adults? A systematic review. Food Qual. Prefer. 2021, 92, 104174. [Google Scholar] [CrossRef]
- Liem, D.G.; Miremadi, F.; Keast, R.S.J. Reducing sodium in foods: The effect on flavor. Nutrients 2011, 3, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Burger, K.S.; Yokum, S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am. J. Clin. Nutr. 2013, 98, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, S.A. Dietary salt and flavour: Mechanisms of taste perception and physiological controls. In Reducing Salt in Foods; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar] [CrossRef]
- Han, P.; Mohebbi, M.; Seo, H.S.; Hummel, T. Sensitivity to sweetness correlates to elevated reward brain responses to sweet and high-fat food odors in young healthy volunteers. Neuroimage 2020, 208, 116413. [Google Scholar] [CrossRef] [PubMed]
- Spetter, M.S.; Smeets, P.A.M.; de Graaf, C.; Viergever, M.A. Representation of sweet and salty taste intensity in the brain. Chem. Senses 2010, 35, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Peinado, B.R.R.; Frazão, D.R.; Bittencourt, L.O.; de Souza-Rodrigues, R.D.; Vidigal, M.T.C.; da Silva, D.T.; Paranhos, L.R.; Magno, M.B.; Fagundes, N.C.F.; Maia, L.C.; et al. Is obesity associated with taste alterations? a systematic review. Front. Endocrinol. 2023, 14, 1167119. [Google Scholar] [CrossRef] [PubMed]
- Zverev, Y.P. Effects of caloric deprivation and satiety on sensitivity of the gustatory system. BMC Neurosci. 2004, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Hanci, D.; Altun, H. Hunger state affects both olfactory abilities and gustatory sensitivity. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Ileri-Gurel, E.; Pehlivanoglu, B.; Dogan, M. Effect of acute stress on taste perception: In relation with baseline anxiety level and body weight. Chem. Senses 2013, 38, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.C.; Guimarães, R.d.F.; Namiranian, K.; Drapeau, V.; Mathieu, M.E. Effect of physical exercise on taste perceptions: A systematic review. Nutrients 2020, 12, 2741. [Google Scholar] [CrossRef]
- Fu, O.; Minokoshi, Y.; Nakajima, K.I. Recent Advances in Neural Circuits for Taste Perception in Hunger. Front. Neural Circuits 2021, 15, 609824. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Bertino, M.; Burke, D.; Engelman, K. Experimental sodium depletion and salt taste in normal human volunteers. Am. J. Clin. Nutr. 1990, 51, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Foster, S.; Winklebach, A.; Navarro, M.; Thomas, W.; Campbell, K.; Stowasser, M. Taste and Hypertension in Humans: Targeting Cardiovascular Disease. Curr. Pharm. Des. 2016, 22, 2290–2305. [Google Scholar] [CrossRef] [PubMed]

| Sample N = 38 | Normal-Weight N = 26 (68.4%) | ObeseN = 12 (31.6%) | p-Value * | |
|---|---|---|---|---|
| Body weight (kg) | 78.00 (34.03) | 68.45 (20.42) | 86.75 (38.35) | p < 0.001 |
| Height (cm) | 168.00 (15.00) | 175.00 (18.00) | 169.00 (15.00) | p = 0.053 |
| BMI (kg/m2) | 25.74 (9.78) | 22.25 (3.09) | 30.71 (15.26) | p < 0.001 |
| Age (years) | 33.50 (24.25) | 26.00 (27.5) | 41.00 (21.50) | p = 0.155 |
| Gender N = male/female (%) | 13/25 (34.2/65.8) | 10/16 (37.5/62.5) | 3/9 (27.3/72.7) | + p = 0.330 |
| Smoking # N = yes/no (%). | 6/32 (15.80/84.20) | 2/14 (12.50/87.50) | 4/18 (18.20/81.80) | + p < 0.001 |
| State hunger (mm) ß | 45.00 (50.00) | 50.00 (40.00) | 35.00 (60.00) | p = 0.510 |
| Sweet taste recognition | 4.00 (0.00) | 4.00 (0.00) | 4.00 (0.25) | p = 0.804 |
| Salty taste recognition | 2.00 (2.00) | 2.0 (2.00) | 2.00 (2.00) | p = 0.651 |
| PFS total | 2.43 (1.20) | 2.43 (0.95) | 2.43 (1.02) | p = 0.388 |
| POF availability | 1.80 (1.50) | 1.83 (0.79) | 1.83(1.50) | p = 0.492 |
| POF present | 2.50 (1.35) | 2.00 (1.37) | 2.88 (1.31) | p = 0.072 |
| POF taste | 2.80 (1.25) | 2.90 (1,25) | 2.70 (1.00) | p = 0.372 |
| Uncontrolled eating | 4.00 (5.00) | 3.00 (2.75) | 5.00 (5.25) | p = 0.073 |
| Restraint eating | 6.00 (6.00) | 4.50 (5.75) | 6.00 (5.00) | p = 0.529 |
| Cognitive restraint | 8.00 (6.25) | 6.50 (5.75) | 10.00 (7.00) | p = 0.781 |
| Emotional eating | 1.00 (2.00) | 0.00 (2.00) | 1.00 (2.00) | p = 0.298 |
| Hunger | 5.00 (4.00) | 4.50 (5.00) | 5.00 (4.50) | p = 0.455 |
| Disinhibition | 7.00 (6.00) | 5.50 (5.00) | 9.00 (4.25) | p = 0.013 |
| EW HFSA | 68.40 (35.57) | 64.50 (25.68) | 71.00 (27.82) | p = 0.284 |
| EW LFSA | 54.38 (20.75) | 53.13 (12.12) | 56.38 (34.49) | p = 0.529 |
| EW HFSW | 41.38 (41.32) | 40.0 (36.87) | 45.88 (47.82) | p = 0.693 |
| EW LFSW | 70.00 (24.82) | 73.75 (21.13) | 64.88 (33.25) | p = 0.438 |
| EL HFSA | 71.13 (22.50) | 67.63 (20.62) | 72.88 (26.31) | p = 0.510 |
| EL LFSA | 56.88 (24.56) | 53.13 (21) | 65.00 (27.38) | p = 0.312 |
| EL HFSW | 57.00 (39.65) | 56.25 (40.12) | 57.00 (41.94) | p = 0.693 |
| EL LFSW | 71.25 (28.61) | 74.75 (25.20) | 67.00 (36.00) | p = 0.258 |
| IW HFSA | 10.97 (39.34) | 4.93 (48.05) | 24.39 (40.76) | p = 0.108 |
| IW LFSA | −17.87 (28.29) | −22.90 (30.78) | −14.25 (30.55) | p = 0.421 |
| IW HFSW | −32.97 (57.49) | −34.57 (47.36) | −27.19 (58.18) | p = 0.965 |
| IW LFSW | 24.82 (48.00) | 43.59 (49.47) | 18.60 (53.99) | p = 0.129 |
| Explicit Liking | Explicit Wanting | Implicit Wanting | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taste Quality | HFSA | LFSA | HFSW | LFSW | HFSA | LFSA | HFSW | LFSW | HFSA | LFSA | HFSW | LFSW |
| Salty | r = 0.267 (p = 0.105) | r = −0.066 (p = 0.695) | r = 0.004 (p = 0.980) | r = 0.118 (p = 0.481) | r = 0.297 (p = 0.070) | r = 0.124 (p = 0.458) | r = 0.029 (p = 0.865) | r = −0.192 (p = 0.249) | r = 0.349 (p = 0.032 *) | r = −0.222 (p = 0.181) | r = 0.046 (p = 0.784) | r = −0.178 (p = 0.284) |
| Sweet | r = −0.120 (p = 0.473) | r = 0.012 (p = 0.944) | r = 0.129 (p = 0.440) | r = 0.168 (p = 0.313) | r = 0.061 (p = 0.715) | r = 0.027 (p = 0.872) | r = 0.142 (p = 0.395) | r = 0.171 (p = 0.305) | r = − 0.195 (p = 0.242) | r = − 0.377 (p = 0.020 *) | r = 0.160 (p = 0.337) | r = 0.244 (p = 0.140) |
| Taste Quality | Total Score | Availability | Taste | Present |
|---|---|---|---|---|
| Salty | r = 0.371 (p = 0.022 *) | r = 0.181 (p = 0.276) | r = 0.284 (p = 0.084) | r = 0.442 (p = 0.005 **) |
| Sweet | r = 0.389 (p = 0.016 *) | r = 0.217 (p = 0.191) | r = 0.522 (p = 0.001 **) | r = 0.194 (p = 0.243) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schamarek, I.; Richter, F.C.; Finlayson, G.; Tönjes, A.; Stumvoll, M.; Blüher, M.; Rohde-Zimmermann, K. Association of Salty and Sweet Taste Recognition with Food Reward and Subjective Control of Eating Behavior. Nutrients 2024, 16, 2661. https://doi.org/10.3390/nu16162661
Schamarek I, Richter FC, Finlayson G, Tönjes A, Stumvoll M, Blüher M, Rohde-Zimmermann K. Association of Salty and Sweet Taste Recognition with Food Reward and Subjective Control of Eating Behavior. Nutrients. 2024; 16(16):2661. https://doi.org/10.3390/nu16162661
Chicago/Turabian StyleSchamarek, Imke, Florian Christoph Richter, Graham Finlayson, Anke Tönjes, Michael Stumvoll, Matthias Blüher, and Kerstin Rohde-Zimmermann. 2024. "Association of Salty and Sweet Taste Recognition with Food Reward and Subjective Control of Eating Behavior" Nutrients 16, no. 16: 2661. https://doi.org/10.3390/nu16162661
APA StyleSchamarek, I., Richter, F. C., Finlayson, G., Tönjes, A., Stumvoll, M., Blüher, M., & Rohde-Zimmermann, K. (2024). Association of Salty and Sweet Taste Recognition with Food Reward and Subjective Control of Eating Behavior. Nutrients, 16(16), 2661. https://doi.org/10.3390/nu16162661






