Effects of Physical Exercise on the Microbiota in Irritable Bowel Syndrome
Abstract
1. Introduction
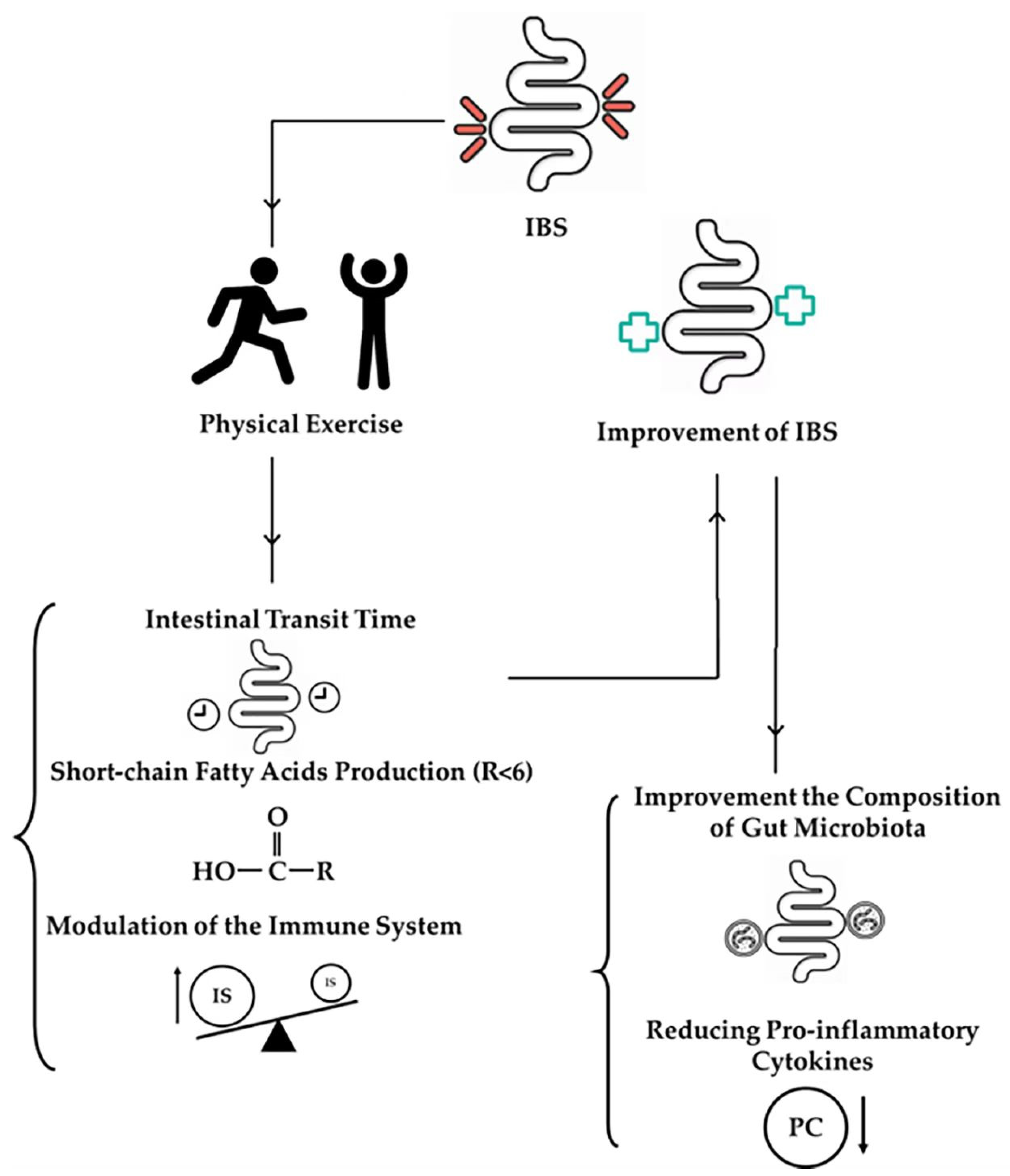
2. Impact of Exercise on the Gut Microbiota in IBS Patients
2.1. Aerobic Exercise
2.2. Resistance Exercise
2.3. Effects on Gut Microbiota Composition
2.4. Enhancement of Microbial Diversity
2.5. Increase in Beneficial Bacteria
2.6. Clinical Evidence in IBS Patients
2.7. Symptom Alleviation
2.8. Reduction in Pro-Inflammatory Cytokines
3. Mechanisms Underlying Exercise-Induced Changes in the Gut Microbiota
3.1. Intestinal Transit Time
3.2. SCFA Production
3.3. Modulation of the Immune System
4. Practical Implications, Recommendations, and Adaptive Strategies for IBS
4.1. Practical Implications for IBS Patients
4.2. Exercise Recommendations
4.3. Monitoring and Adaptation
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.; Rajilic-Stojanovic, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Ikechi, R.; Fischer, B.D.; DeSipio, J.; Phadtare, S. Irritable Bowel Syndrome: Clinical Manifestations, Dietary Influences, and Management. Healthcare 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Goodoory, V.C.; Ng, C.E.; Black, C.J.; Ford, A.C. Impact of Rome IV irritable bowel syndrome on work and activities of daily living. Aliment. Pharmacol. Ther. 2022, 56, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Hulisz, D. The burden of illness of irritable bowel syndrome: Current challenges and hope for the future. J. Manag. Care Pharm. 2004, 10, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hillestad, E.M.R.; van der Meeren, A.; Nagaraja, B.H.; Bjorsvik, B.R.; Haleem, N.; Benitez-Paez, A.; Sanz, Y.; Hausken, T.; Lied, G.A.; Lundervold, A.; et al. Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J. Gastroenterol. 2022, 28, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Porro, C.; Twfieg, M.E.; Benameur, N.; Panaro, M.A.; Filannino, F.M.; Hasan, A. Emerging Paradigms in Inflammatory Disease Management: Exploring Bioactive Compounds and the Gut Microbiota. Brain Sci. 2023, 13, 1226. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Shaikh, S.D.; Sun, N.; Canakis, A.; Park, W.Y.; Weber, H.C. Irritable Bowel Syndrome and the Gut Microbiome: A Comprehensive Review. J. Clin. Med. 2023, 12, 2558. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef]
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut Dysbiosis in Irritable Bowel Syndrome: A Narrative Review on Correlation with Disease Subtypes and Novel Therapeutic Implications. Microorganisms 2023, 11, 2369. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef]
- Chaudhry, T.S.; Senapati, S.G.; Gadam, S.; Mannam, H.; Voruganti, H.V.; Abbasi, Z.; Abhinav, T.; Challa, A.B.; Pallipamu, N.; Bheemisetty, N.; et al. The Impact of Microbiota on the Gut-Brain Axis: Examining the Complex Interplay and Implications. J. Clin. Med. 2023, 12, 5231. [Google Scholar] [CrossRef] [PubMed]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Management Options for Irritable Bowel Syndrome. Mayo Clin. Proc. 2018, 93, 1858–1872. [Google Scholar] [CrossRef] [PubMed]
- Paris, J. The mistreatment of major depressive disorder. Can. J. Psychiatry Rev. Can. Psychiatr. 2014, 59, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Masuy, I.; Pannemans, J.; Tack, J. Irritable bowel syndrome: Diagnosis and management. Minerva Gastroenterol. Dietol. 2020, 66, 136–150. [Google Scholar] [CrossRef]
- Eriksson, E.M.; Andren, K.I.; Kurlberg, G.K.; Eriksson, H.T. Aspects of the non-pharmacological treatment of irritable bowel syndrome. World J. Gastroenterol. 2015, 21, 11439–11449. [Google Scholar] [CrossRef]
- Nunan, D.; Cai, T.; Gardener, A.D.; Ordonez-Mena, J.M.; Roberts, N.W.; Thomas, E.T.; Mahtani, K.R. Physical activity for treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 2022, 6, CD011497. [Google Scholar] [CrossRef]
- Mahindru, A.; Patil, P.; Agrawal, V. Role of Physical Activity on Mental Health and Well-Being: A Review. Cureus 2023, 15, e33475. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Bonilla, D.A.; Gomez-Miranda, L.M.; Calleja-Nunez, J.J.; Arias, N.; Martinez-Guardado, I. Examining the Interaction between Exercise, Gut Microbiota, and Neurodegeneration: Future Research Directions. Biomedicines 2023, 11, 2267. [Google Scholar] [CrossRef] [PubMed]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. (Auckl. N.Z.) 2022, 52, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.M.; Reimer, R.A. Unlocking a novel determinant of athletic performance: The role of the gut microbiota, short-chain fatty acids, and “biotics” in exercise. J. Sport Health Sci. 2023, 12, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Matei, B.; Winters-Stone, K.M.; Raber, J. Examining the Mechanisms behind Exercise’s Multifaceted Impacts on Body Composition, Cognition, and the Gut Microbiome in Cancer Survivors: Exploring the Links to Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Kapounkova, K.; Struhar, I. The relationship between the gut microbiome and resistance training: A rapid review. BMC Sports Sci. Med. Rehabil. 2024, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Xu, Y.; Lai, H.Y.; Yang, M.; Cheng, L.; Liu, X.; Sun, X.; Yang, Y.; Wang, J.; Lv, W.; et al. Effects of combined aerobic and resistance training on gut microbiota and cardiovascular risk factors in physically active elderly women: A randomized controlled trial. Front. Physiol. 2022, 13, 1004863. [Google Scholar] [CrossRef]
- Bianco, A.; Russo, F.; Franco, I.; Riezzo, G.; Donghia, R.; Curci, R.; Bonfiglio, C.; Prospero, L.; D’Attoma, B.; Ignazzi, A.; et al. Enhanced Physical Capacity and Gastrointestinal Symptom Improvement in Southern Italian IBS Patients following Three Months of Moderate Aerobic Exercise. J. Clin. Med. 2023, 12, 6786. [Google Scholar] [CrossRef]
- Kim, M.Y.; Choi, S.W. Dietary modulation of gut microbiota for the relief of irritable bowel syndrome. Nutr. Res. Pract. 2021, 15, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.C.; Bass, D.; Stentiford, G.D.; van der Giezen, M. Understanding the role of the shrimp gut microbiome in health and disease. J. Invertebr. Pathol. 2021, 186, 107387. [Google Scholar] [CrossRef] [PubMed]
- Geller, S.; Levy, S.; Avitsur, R. Psychological distress in individuals with irritable bowel syndrome: The roles of body image and self-criticism. Health Psychol. Behav. Med. 2024, 12, 2334466. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, H.; Qian, H.; Liu, C. Urolithin A Ameliorates Athletic Ability and Intestinal Microbiota in Sleep Deprivation from the Perspective of the Gut-Muscle Axis. Mol. Nutr. Food Res. 2024, 68, e2300599. [Google Scholar] [CrossRef] [PubMed]
- Floegel, T.A.; Giacobbi, P.R., Jr.; Dzierzewski, J.M.; Aiken-Morgan, A.T.; Roberts, B.; McCrae, C.S.; Marsiske, M.; Buman, M.P. Intervention markers of physical activity maintenance in older adults. Am. J. Health Behav. 2015, 39, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Sochacka, K.; Kotowska, A.; Lachowicz-Wisniewska, S. The Role of Gut Microbiota, Nutrition, and Physical Activity in Depression and Obesity-Interdependent Mechanisms/Co-Occurrence. Nutrients 2024, 16, 1039. [Google Scholar] [CrossRef] [PubMed]
- Pasta, A.; Formisano, E.; Calabrese, F.; Plaz Torres, M.C.; Bodini, G.; Marabotto, E.; Pisciotta, L.; Giannini, E.G.; Furnari, M. Food Intolerances, Food Allergies and IBS: Lights and Shadows. Nutrients 2024, 16, 265. [Google Scholar] [CrossRef]
- Wollny, T.; Daniluk, T.; Piktel, E.; Wnorowska, U.; Buklaha, A.; Gluszek, K.; Durnas, B.; Bucki, R. Targeting the Gut Microbiota to Relieve the Symptoms of Irritable Bowel Syndrome. Pathogens 2021, 10, 1545. [Google Scholar] [CrossRef]
- Zhao, H.; He, Z.; Yun, H.; Wang, R.; Liu, C. A Meta-Analysis of the Effects of Different Exercise Modes on Inflammatory Response in the Elderly. Int. J. Environ. Res. Public Health 2022, 19, 10451. [Google Scholar] [CrossRef]
- Herbert, C.; Meixner, F.; Wiebking, C.; Gilg, V. Regular Physical Activity, Short-Term Exercise, Mental Health, and Well-Being Among University Students: The Results of an Online and a Laboratory Study. Front. Psychol. 2020, 11, 509. [Google Scholar] [CrossRef]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metab. Clin. Exp. 2019, 92, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xiao, H. Exercise for Mental Well-Being: Exploring Neurobiological Advances and Intervention Effects in Depression. Life 2023, 13, 1505. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Gerard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kosikowski, W.; Szczerbiński, M.; Gantzel, J.; Cukrowska, B. The Effectiveness of Synbiotic Preparation Containing Lactobacillus and Bifidobacterium Probiotic Strains and Short Chain Fructooligosaccharides in Patients with Diarrhea Predominant Irritable Bowel Syndrome-A Randomized Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 1999. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Su, W.; Zhao, H.; Li, H.; Wang, Z.; Cui, X.; Xi, C.; Gao, R.; Sun, Y.; Liu, C. Effects of different exercise modalities on lipid profile in the elderly population: A meta-analysis. Medicine 2023, 102, e33854. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, R.; Teng, J.; Song, G.; Huang, C.; Yuan, S.; Lu, Y.; Shen, S.; Liu, J.; Liu, C. A Meta-Analysis of the Effects of Different Training Modalities on the Inflammatory Response in Adolescents with Obesity. Int. J. Environ. Res. Public Health 2022, 19, 13224. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Camara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Yu, X.; Novak, P.; Gui, Q.; Yin, K. Enhancing intestinal barrier efficiency: A novel metabolic diseases therapy. Front. Nutr. 2023, 10, 1120168. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Ablitip, A.; Wang, R.; Luciana, T.; Wei, M.; Ma, X. Effects of Exercise on Gut Microbiota of Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1070. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L. A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Front. Nutr. 2019, 6, 191. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Fujie, S.; Yano, H.; Iemitsu, M. Aerobic exercise training-induced alteration of gut microbiota composition affects endurance capacity. J. Physiol. 2023, 601, 2329–2344. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.M.A.; Shahzad, S.; Dhillon, J. A systematic review on the effects of exercise on gut microbial diversity, taxonomic composition, and microbial metabolites: Identifying research gaps and future directions. Front. Physiol. 2023, 14, 1292673. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wu, X.; Liu, J.; Sun, J.; Wang, X.; Fan, G.; Meng, X.; Zhang, J.; Zhang, Y. The regulatory roles of dietary fibers on host health via gut microbiota-derived short chain fatty acids. Curr. Opin. Pharmacol. 2022, 62, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Morita, E.; Yokoyama, H.; Imai, D.; Takeda, R.; Ota, A.; Kawai, E.; Hisada, T.; Emoto, M.; Suzuki, Y.; Okazaki, K. Aerobic Exercise Training with Brisk Walking Increases Intestinal Bacteroides in Healthy Elderly Women. Nutrients 2019, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Mostamand, J.; Fani, M.; Chitsaz, N.; Feizi, A. The effect of aerobic exercises among women with mild and moderate irritable bowel syndrome: A pilot study. J. Bodyw. Mov. Ther. 2019, 23, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, G.; Prospero, L.; D’Attoma, B.; Ignazzi, A.; Bianco, A.; Franco, I.; Curci, R.; Campanella, A.; Bonfiglio, C.; Osella, A.R.; et al. The Impact of a Twelve-Week Moderate Aerobic Exercise Program on Gastrointestinal Symptom Profile and Psychological Well-Being of Irritable Bowel Syndrome Patients: Preliminary Data from a Southern Italy Cohort. J. Clin. Med. 2023, 12, 5359. [Google Scholar] [CrossRef] [PubMed]
- Bertuccioli, A.; Zonzini, G.B.; Cazzaniga, M.; Cardinali, M.; Di Pierro, F.; Gregoretti, A.; Zerbinati, N.; Guasti, L.; Matera, M.R.; Cavecchia, I.; et al. Sports-Related Gastrointestinal Disorders: From the Microbiota to the Possible Role of Nutraceuticals, a Narrative Analysis. Microorganisms 2024, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Lim, D.Y.; Yeo, W.S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Grundmann, O.; Yoon, S.L. Complementary and alternative medicines in irritable bowel syndrome: An integrative view. World J. Gastroenterol. 2014, 20, 346–362. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.Y.; Xu, D.Q.; Yue, S.J.; Fu, R.J.; Yang, J.; Xing, L.M.; Tang, Y.P. Action Mode of Gut Motility, Fluid and Electrolyte Transport in Chronic Constipation. Front. Pharmacol. 2021, 12, 630249. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, H.; Yan, Y.; Yang, W.; Chen, S.; Song, G.; Li, X.; Gu, Y.; Yun, H.; Li, Y. Synergistic Effect of Rhodiola rosea and Caffeine Supplementation on the Improvement of Muscle Strength and Muscular Endurance: A Pilot Study for Rats, Resistance Exercise-Untrained and -Trained Volunteers. Nutrients 2023, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic review with meta-analysis: The prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, S.M.; Al-Shreef, F.M. Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr. Health Sci. 2018, 18, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Brzozowski, B.; Mazur-Bialy, A.; Sliwowski, Z.; Brzozowski, T. The role of physical exercise in inflammatory bowel disease. BioMed Res. Int. 2014, 2014, 429031. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Donadio, J.L.S.; Fabi, J.P. Comparative analysis of pectin and prebiotics on human microbiota modulation in early life stages and adults. Food Funct. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef]
- Periard, J.D.; Eijsvogels, T.M.H.; Daanen, H.A.M. Exercise under heat stress: Thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. TEM 2022, 33, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Lyle, B.J.; Madsen, K.L.; Sonnenburg, J.; Verbeke, K.; Wu, G.D. Role for diet in normal gut barrier function: Developing guidance within the framework of food-labeling regulations. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G17–G39. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Fakharian, F.; Thirugnanam, S.; Welsh, D.A.; Kim, W.K.; Rappaport, J.; Bittinger, K.; Rout, N. The Role of Gut Dysbiosis in the Loss of Intestinal Immune Cell Functions and Viral Pathogenesis. Microorganisms 2023, 11, 1849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, L.; Zhang, G.; Dong, J.; Ren, Z.; Li, Z. The pathogenesis of gut microbiota in hepatic encephalopathy by the gut-liver-brain axis. Biosci. Rep. 2023, 43, BSR20222524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yu, B.; Sun, J.; Liu, Z.; Chen, H.; Ge, L.; Chen, D. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 61. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, J.; Zhu, S.; Xin, L.; Yu, C.; Shen, Z. The Role of Short Chain Fatty Acids in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2022, 28, 540–548. [Google Scholar] [CrossRef]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 2016, 7, 365–383. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, X.; Zhang, X. Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat Metabolic Disorders to Maintain Health. Nutrients 2023, 15, 1539. [Google Scholar] [CrossRef]
- Hughes, R.L.; Pindus, D.M.; Khan, N.A.; Burd, N.A.; Holscher, H.D. Associations between Accelerometer-Measured Physical Activity and Fecal Microbiota in Adults with Overweight and Obesity. Med. Sci. Sports Exerc. 2023, 55, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Holmes, Z.C.; Silverman, J.D.; Dressman, H.K.; Wei, Z.; Dallow, E.P.; Armstrong, S.C.; Seed, P.C.; Rawls, J.F.; David, L.A. Short-Chain Fatty Acid Production by Gut Microbiota from Children with Obesity Differs According to Prebiotic Choice and Bacterial Community Composition. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cai, Y.; Li, J.; Yau, S.Y.; Lu, W.; Stubbs, B.; Su, K.P.; Xu, G.; So, K.F.; Lin, K.; et al. Effects of aerobic exercise on gut microbiota in adolescents with subthreshold mood syndromes and healthy adolescents: A 12-week, randomized controlled trial. J. Affect. Disord. 2021, 293, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kopczynska, J.; Kowalczyk, M. The potential of short-chain fatty acid epigenetic regulation in chronic low-grade inflammation and obesity. Front. Immunol. 2024, 15, 1380476. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L. Nutrition, Physical Activity and Supplementation in Irritable Bowel Syndrome. Nutrients 2023, 15, 3662. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wei, Y.; Hashimoto, K. Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 785–793. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Thomas, A.; Thomas, A.; Butler-Sanchez, M. Dietary Modification for the Restoration of Gut Microbiome and Management of Symptoms in Irritable Bowel Syndrome. Am. J. Lifestyle Med. 2022, 16, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.U.; Yassine, H.M.; Sohail, A.; Thani, A.A.A. Impact of Physical Exercise on Gut Microbiome, Inflammation, and the Pathobiology of Metabolic Disorders. Rev. Diabet. Stud. RDS 2019, 15, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.C.; Gleeson, M. Acute and chronic effects of exercise on markers of mucosal immunity. Front. Biosci. (Landmark Ed.) 2009, 14, 4444–4456. [Google Scholar] [CrossRef]
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the Mucosal Immune System. BioMed Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef] [PubMed]
- Adammek, F.; Wences, T.; Walzik, D.; Trebing, S.; Belen, S.; Renpening, D.; Zimmer, P.; Joisten, N. Kinetics of immune cell mobilization during acute aerobic exercise in healthy adults. Int. J. Sports Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Schwartz, O.S.; Eliby, D.; Butler, C.A.; Huang, K.; O’Brien-Simpson, N.; Callaghan, B.L.; Dashper, S.G.; Gooley, P.R.; Whittle, S.; et al. Bugs and Brains, the Gut and Mental Health Study: A mixed-methods study investigating microbiota composition and function in anxiety, depression and irritable bowel syndrome. BMJ Open 2021, 11, e043221. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.L.; Correa, D.; Pak, S.C. Probiotics, prebiotics, and low FODMAP diet for irritable bowel syndrome—What is the current evidence? Complement. Ther. Med. 2019, 43, 73–80. [Google Scholar] [CrossRef]
- Burke, S.M.; Carron, A.V.; Eys, M.A. Physical activity context and university student’s propensity to meet the guidelines Centers for Disease Control and Prevention/American College of Sports Medicine. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2005, 11, Cr171–Cr176. [Google Scholar]
- Faulkner, M.S.; Michaliszyn, S.F. Exercise Adherence in Hispanic Adolescents with Obesity or Type 2 Diabetes. J. Pediatr. Nurs. 2021, 56, 7–12. [Google Scholar] [CrossRef]
- Wu, Y.R.; Tan, Z.B.; Lu, Y.; Liu, C.; Dong, W.G. Physical activity, sedentary behavior, and the risk of functional gastrointestinal disorders: A two-sample Mendelian randomization study. J. Dig. Dis. 2024, 25, 248–254. [Google Scholar] [CrossRef]
- Tolliver, B.A.; Herrera, J.L.; DiPalma, J.A. Evaluation of patients who meet clinical criteria for irritable bowel syndrome. Am. J. Gastroenterol. 1994, 89, 176–178. [Google Scholar] [PubMed]
- Shahabi, L.; Naliboff, B.D.; Shapiro, D. Self-regulation evaluation of therapeutic yoga and walking for patients with irritable bowel syndrome: A pilot study. Psychol. Health Med. 2016, 21, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Baart, A.M.; Mensink, M.; Witteman, B.J.M. The impact of running on gastrointestinal symptoms in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2024, 36, e14707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shen, F.; Wang, X.; Cheng, Y.; Guo, Y.; Qian, H.; Liu, Y. Reshaped Gut Microbial Composition and Functions Associated with the Antifatigue Effect of Salidroside in Exercise Mice. Mol. Nutr. Food Res. 2023, 67, e2300015. [Google Scholar] [CrossRef]
- Smith, K.A.; Kisiolek, J.N.; Willingham, B.D.; Morrissey, M.C.; Leyh, S.M.; Saracino, P.G.; Baur, D.A.; Cook, M.D.; Ormsbee, M.J. Ultra-endurance triathlon performance and markers of whole-body and gut-specific inflammation. Eur. J. Appl. Physiol. 2020, 120, 349–357. [Google Scholar] [CrossRef]
- Zia, J.K.; Barney, P.; Cain, K.C.; Jarrett, M.E.; Heitkemper, M.M. A Comprehensive Self-Management Irritable Bowel Syndrome Program Produces Sustainable Changes in Behavior After 1 Year. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 212–219.e2. [Google Scholar] [CrossRef]
- Zhou, H.; Li, D.; Cheng, G.; Fan, J.; Lu, H. An epidemiologic study of irritable bowel syndrome in adolescents and children in South China: A school-based study. Child Care Health Dev. 2010, 36, 781–786. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, D.; Shi, L.; Liang, L.; Xu, T.; Chang, M.; Chen, W.; Wu, D.; Zhang, F.; Fang, X. Intestinal symptoms and psychological factors jointly affect quality of life of patients with irritable bowel syndrome with diarrhea. Health Qual. Life Outcomes 2015, 13, 49. [Google Scholar] [CrossRef]
- Warnock, J.K.; Clayton, A.H. Chronic episodic disorders in women. Psychiatr. Clin. N. Am. 2003, 26, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Chiarioni, G.; Whitehead, W.E. The role of biofeedback in the treatment of gastrointestinal disorders. Nat. Clin. Pr. Gastroenterol. Hepatol. 2008, 5, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Weaver-Toedtman, K.R.; Walch, M.; Kiracofe, L.; Bedingfield, A.; Cook, L.; Resnick, B.; Renn, C.L.; Dorsey, S.G. Feasibility and Acceptability of an Online Yoga Study Among Individuals with Irritable Bowel Syndrome (IBS). Int. J. Yoga Ther. 2023, 33, 8. [Google Scholar] [CrossRef] [PubMed]
- van Dulmen, A.M.; Fennis, J.F.; Mokkink, H.G.; van der Velden, H.G.; Bleijenberg, G. Doctors’ perception of patients’ cognitions and complaints in irritable bowel syndrome at an out-patient clinic. J. Psychosom. Res. 1994, 38, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Barclay, Y.; Harper, L.; Marchant, C.; Seamark, L.; Hickson, M. Feasibility, acceptability and cost efficiency of using webinars to deliver first-line patient education for people with Irritable Bowel Syndrome as part of a dietetic-led gastroenterology service in primary care. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2020, 33, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, W.E.; Crowell, M.D. Psychologic considerations in the irritable bowel syndrome. Gastroenterol. Clin. N. Am. 1991, 20, 249–267. [Google Scholar] [CrossRef]
- Zhong, B.; Liu, Q. Medical Insights from Posts About Irritable Bowel Syndrome by Adolescent Patients and Their Parents: Topic Modeling and Social Network Analysis. J. Med. Internet Res. 2021, 23, e26867. [Google Scholar] [CrossRef] [PubMed]
- Van Malderen, K.; De Man, J.G.; De Winter, B.Y.; De Schepper, H.U. Epidemiological characteristics of a population visiting a patient-centered informative website about irritable bowel syndrome. Acta Gastroenterol. Belg. 2023, 86, 17–25. [Google Scholar] [CrossRef]
- Zhu, S.; Min, L.; Guo, Q.; Li, H.; Yu, Y.; Zong, Y.; Wang, L.; Li, P.; Gu, J.; Zhang, S. Transcriptome and methylome profiling in a rat model of irritable bowel syndrome induced by stress. Int. J. Mol. Med. 2018, 42, 2641–2649. [Google Scholar] [CrossRef]
- Zijdenbos, I.L.; de Wit, N.J.; van der Heijden, G.J.; Rubin, G.; Quartero, A.O. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database Syst. Rev. 2009, Cd006442. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, E.; Li, Y.; Jia, Y.; Li, F. Exercise therapy of patients with irritable bowel syndrome: A systematic review of randomized controlled trials. Neurogastroenterol. Motil. 2019, 31, e13461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.R.; Ni, X.M.; Zhang, X.A.; Tian, H. Effect of cognitive behavior therapy combined with exercise intervention on the cognitive bias and coping styles of diarrhea-predominant irritable bowel syndrome patients. World J. Clin. Cases 2019, 7, 3446–3462. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, F.; Fan, F.; Sedas, A.C.; Wang, J. Mind-Body Interventions for Irritable Bowel Syndrome Patients in the Chinese Population: A Systematic Review and Meta-Analysis. Int. J. Behav. Med. 2017, 24, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.S.; Wang, J.J.; Zou, L.; Chen, J.Y.; Li, K.W.; Liao, L.M.; Ruan, J.R.; Li, N.; Chu, H.R. Anti-inflammation effect of moxibustion for rats with diarrhea-predominant irritable bowel syndrome based on multiple miRNAs regulating NF-κB signal pathway. Zhongguo Zhen Jiu = Chin. Acupunct. Moxibustion 2022, 42, 654–662. [Google Scholar] [CrossRef]
- Zloof, Y.; Peretz, L.; Braun, M.; Simchoni, M.; Tsur, A.M.; Tzur, D.; Derazne, E.; Ben-Tov, A.; Pinhas-Hamiel, O.; Amarilyo, G.; et al. Hypermobility spectrum disorders and irritable bowel syndrome: A nationwide study of 1.6 million adolescents. J. Gastroenterol. Hepatol. 2023, 38, 2076–2082. [Google Scholar] [CrossRef]
- Wade, U.; Pascual-Figal, D.A.; Rabbani, F.; Ernst, M.; Albert, A.; Janssens, I.; Dierckxsens, Y.; Iqtadar, S.; Khokhar, N.A.; Kanwal, A.; et al. The Possible Synergistic Pharmacological Effect of an Oral Berberine (BBR) and Curcumin (CUR) Complementary Therapy Alleviates Symptoms of Irritable Bowel Syndrome (IBS): Results from a Real-Life, Routine Clinical Practice Settings-Based Study. Nutrients 2024, 16, 1204. [Google Scholar] [CrossRef]
- Mora-Gonzalez, J.; Migueles, J.H.; Esteban-Cornejo, I.; Cadenas-Sanchez, C.; Pastor-Villaescusa, B.; Molina-García, P.; Rodriguez-Ayllon, M.; Rico, M.C.; Gil, A.; Aguilera, C.M.; et al. Sedentarism, Physical Activity, Steps, and Neurotrophic Factors in Obese Children. Med. Sci. Sports Exerc. 2019, 51, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Staller, K.; Paz, M.; Rones, R.; Macklin, E.A.; Garcia-Fischer, I.; Murray, H.B.; Kuo, B. Virtual Tai Chi program for patients with irritable bowel syndrome with constipation: Proof-of-concept feasibility trial. Neurogastroenterol. Motil. 2022, 34, e14429. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. The human gut phageome: Composition, development, and alterations in disease. Front. Microbiol. 2023, 14, 1213625. [Google Scholar] [CrossRef]
- Tonkin-Crine, S.; Bishop, F.L.; Ellis, M.; Moss-Morris, R.; Everitt, H. Exploring patients’ views of a cognitive behavioral therapy-based website for the self-management of irritable bowel syndrome symptoms. J. Med. Internet Res. 2013, 15, e190. [Google Scholar] [CrossRef]
- Üçüncü, M.Z.; Çoruh Akyol, B.; Toprak, D. The early diagnosis of fibromyalgia in irritable bowel syndrome patients. Med. Hypotheses 2020, 143, 110119. [Google Scholar] [CrossRef] [PubMed]
- Zweig, A.; Schindler, V.; Becker, A.S.; van Maren, A.; Pohl, D. Higher prevalence of joint hypermobility in constipation predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13353. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Furukawa, S.; Miyake, T.; Watanabe, J.; Kato, A.; Kusumoto, K.; Takeshita, E.; Ikeda, Y.; Yamamoto, N.; Saeki, Y.; et al. Exercise habits that include exercise partners and irritable bowel syndrome in a young Japanese population: A cross-sectional study. Eur. J. Gastroenterol. Hepatol. 2023, 35, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Zomorrodi, S.; Rasoulzadeh Tabatabaie, S.K.; Azadfallah, P.; Ebrahimidaryani, N.; Arbabi, M. Long Term Effects of Mindfulness on Quality of life in Irritable Bowel Syndrome. Iran. J. Psychiatry 2015, 10, 100–105. [Google Scholar] [PubMed]
- Zyoud, S.H.; Smale, S.; Waring, W.S.; Sweileh, W.; Al-Jabi, S.W. Global research trends in the microbiome related to irritable bowel syndrome: A bibliometric and visualized study. World J. Gastroenterol. 2021, 27, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.L.; Kamp, K.J.; Burr, R.L.; Tang, H.J.; Dobra, A.; Shulman, R.J.; Heitkemper, M.M. Age Differences in Core Symptoms and Symptom Relationships in Patients With Irritable Bowel Syndrome: A Network Analysis. Am. J. Gastroenterol. 2023, 118, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Zwolińska-Wcisło, M.; Galicka-Latała, D.; Rozpondek, P.; Rudnicka-Sosin, L.; Mach, T. Frequency of celiac disease and irritable bowel syndrome coexistance and its influence on the disease course. Przegl. Lek. 2009, 66, 126–129. [Google Scholar] [PubMed]
- Zugasti Murillo, A.; Estremera Arévalo, F.; Petrina Jáuregui, E. Diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) in the treatment of irritable bowel syndrome: Indications and design. Endocrinol. Nutr. 2016, 63, 132–138. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, S.; Li, H.; Zhang, Z.; Zhang, Q.; Chen, L.; Zhao, Y.; Chen, Y.; Gu, J.; Min, L.; et al. Identification of Gut Microbiota and Metabolites Signature in Patients With Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Soffer, E.E.; Scalabrini, P.; Pope, C.E., 2nd; Wingate, D.L. Effect of stress on oesophageal motor function in normal subjects and in patients with the irritable bowel syndrome. Gut 1988, 29, 1591–1594. [Google Scholar] [CrossRef]
- Sperber, A.D. Review article: Epidemiology of IBS and other bowel disorders of gut-brain interaction (DGBI). Aliment. Pharmacol. Ther. 2021, 54 (Suppl. S1), S1–S11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, X.; Wang, X.; Xi, F.; Luo, X.; Yao, L.; Tang, H. Pharmacological and non-pharmacological treatments for irritable bowel syndrome: Protocol for a systematic review and network meta-analysis. Medicine 2019, 98, e16446. [Google Scholar] [CrossRef] [PubMed]
- von dem Knesebeck, O.; Barbek, R.; Makowski, A.C. Social inequalities in aggravating factors of somatic symptom persistence (SOMA.SOC): Study protocol for a mixed-method observational study focusing on irritable bowel syndrome and fatigue. BMJ Open 2023, 13, e070635. [Google Scholar] [CrossRef] [PubMed]
- Zuzek, R.; Potter, M.; Talley, N.J.; Agréus, L.; Andreasson, A.; Veits, L.; Vieth, M.; Walker, M.M. Prevalence of Histological Gastritis in a Community Population and Association with Epigastric Pain. Dig. Dis. Sci. 2024, 69, 528–537. [Google Scholar] [CrossRef] [PubMed]
- González-Revuelta, M.E.; Novas, N.; Gázquez, J.A.; Rodríguez-Maresca, M.; García-Torrecillas, J.M. User Perception of New E-Health Challenges: Implications for the Care Process. Int. J. Environ. Res. Public Health 2022, 19, 3875. [Google Scholar] [CrossRef]
- Yan, J.; Miao, Z.W.; Lu, J.; Ge, F.; Yu, L.H.; Shang, W.B.; Liu, L.N.; Sun, Z.G. Acupuncture plus Chinese Herbal Medicine for Irritable Bowel Syndrome with Diarrhea: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med 2019, 2019, 7680963. [Google Scholar] [CrossRef] [PubMed]
- Zoller, V.; Laguna, A.L.; Prazeres Da Costa, O.; Buch, T.; Göke, B.; Storr, M. Fecal microbiota transfer (FMT) in a patient with refractory irritable bowel syndrome. Dtsch. Med. Wochenschr. 2015, 140, 1232–1236. [Google Scholar] [CrossRef]
- Zia, J.; Chung, C.F.; Xu, K.; Dong, Y.; Schenk, J.M.; Cain, K.; Munson, S.; Heitkemper, M.M. Inter-Rater Reliability of Provider Interpretations of Irritable Bowel Syndrome Food and Symptom Journals. J. Clin. Med. 2017, 6, 105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, J.; Zhou, Q.; Wang, C.; Hu, J.; Liu, C. Effects of Physical Exercise on the Microbiota in Irritable Bowel Syndrome. Nutrients 2024, 16, 2657. https://doi.org/10.3390/nu16162657
Li C, Li J, Zhou Q, Wang C, Hu J, Liu C. Effects of Physical Exercise on the Microbiota in Irritable Bowel Syndrome. Nutrients. 2024; 16(16):2657. https://doi.org/10.3390/nu16162657
Chicago/Turabian StyleLi, Chunpeng, Jianmin Li, Qiaorui Zhou, Can Wang, Jiahui Hu, and Chang Liu. 2024. "Effects of Physical Exercise on the Microbiota in Irritable Bowel Syndrome" Nutrients 16, no. 16: 2657. https://doi.org/10.3390/nu16162657
APA StyleLi, C., Li, J., Zhou, Q., Wang, C., Hu, J., & Liu, C. (2024). Effects of Physical Exercise on the Microbiota in Irritable Bowel Syndrome. Nutrients, 16(16), 2657. https://doi.org/10.3390/nu16162657




