The Concentration of Salivary Extracellular Vesicles Is Related to Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Investigation of Study-Specific Outcome Variables
2.3. Isolation of Salivary EVs
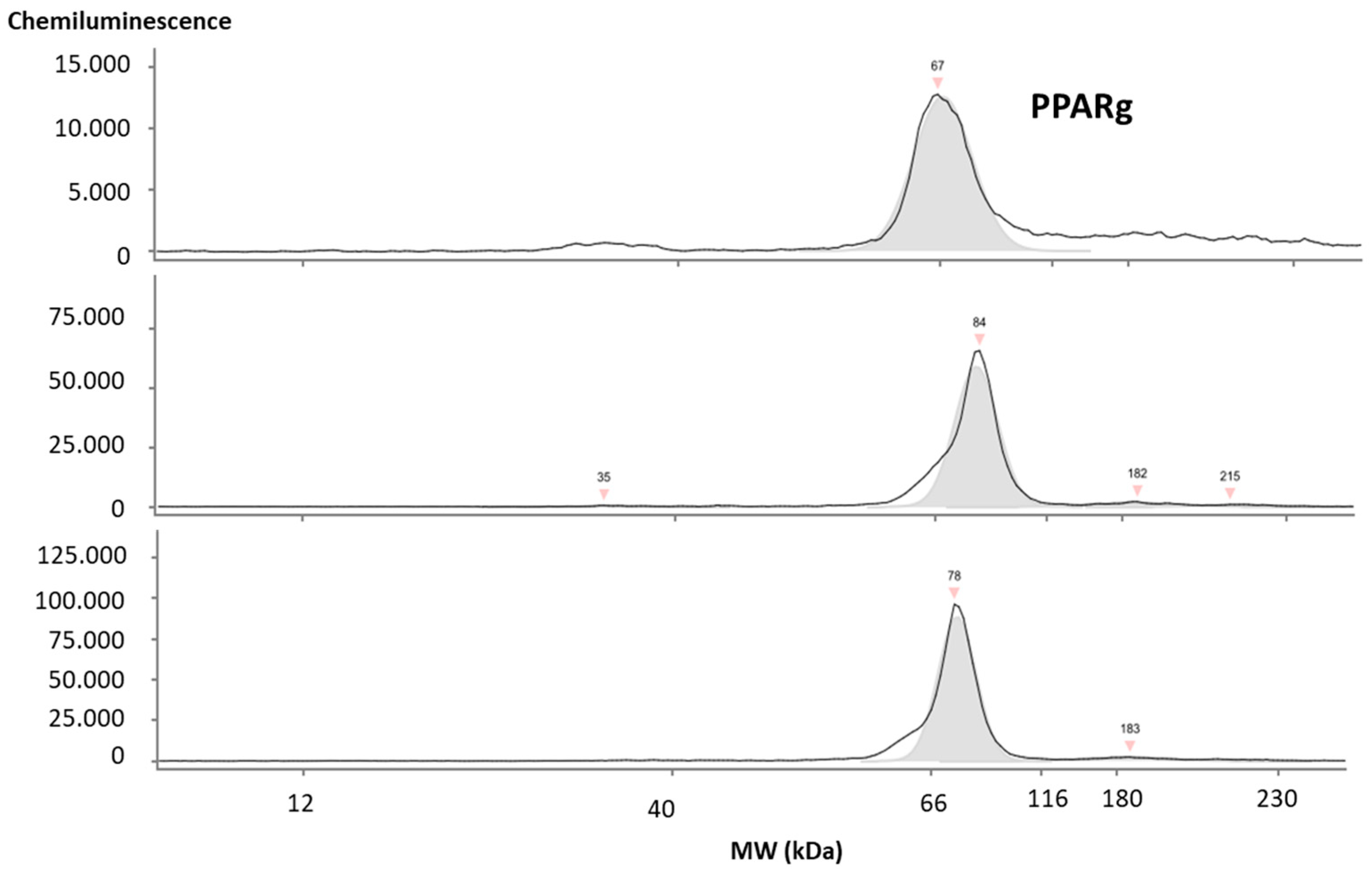
2.4. Characterization of Isolated Salivary EVs
2.4.1. Nanoparticle Tracking Analysis (NTA)
2.4.2. Western Blot and Simple Western Blot WB/JESS
2.5. ELISA
2.6. Statistics
3. Results
3.1. Characteristics of the Study Cohort
3.2. Characterization of Salivary EVs in the Total Cohort
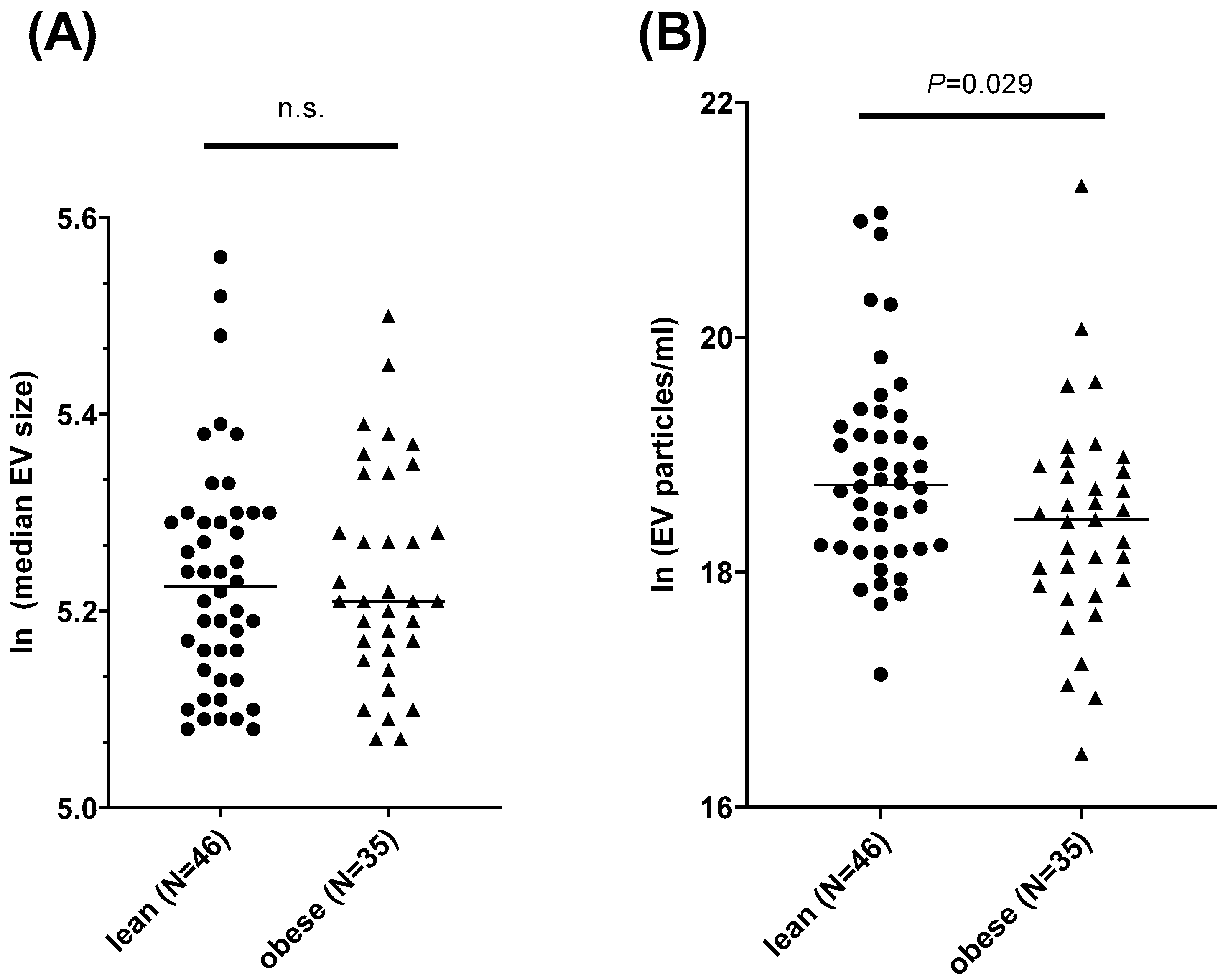
3.3. The Concentration of Salivary EVs Differs between People with Obesity and Normal Weight
3.4. The Concentration of Salivary EVs Is Correlated with Anthropometric Phenotypes
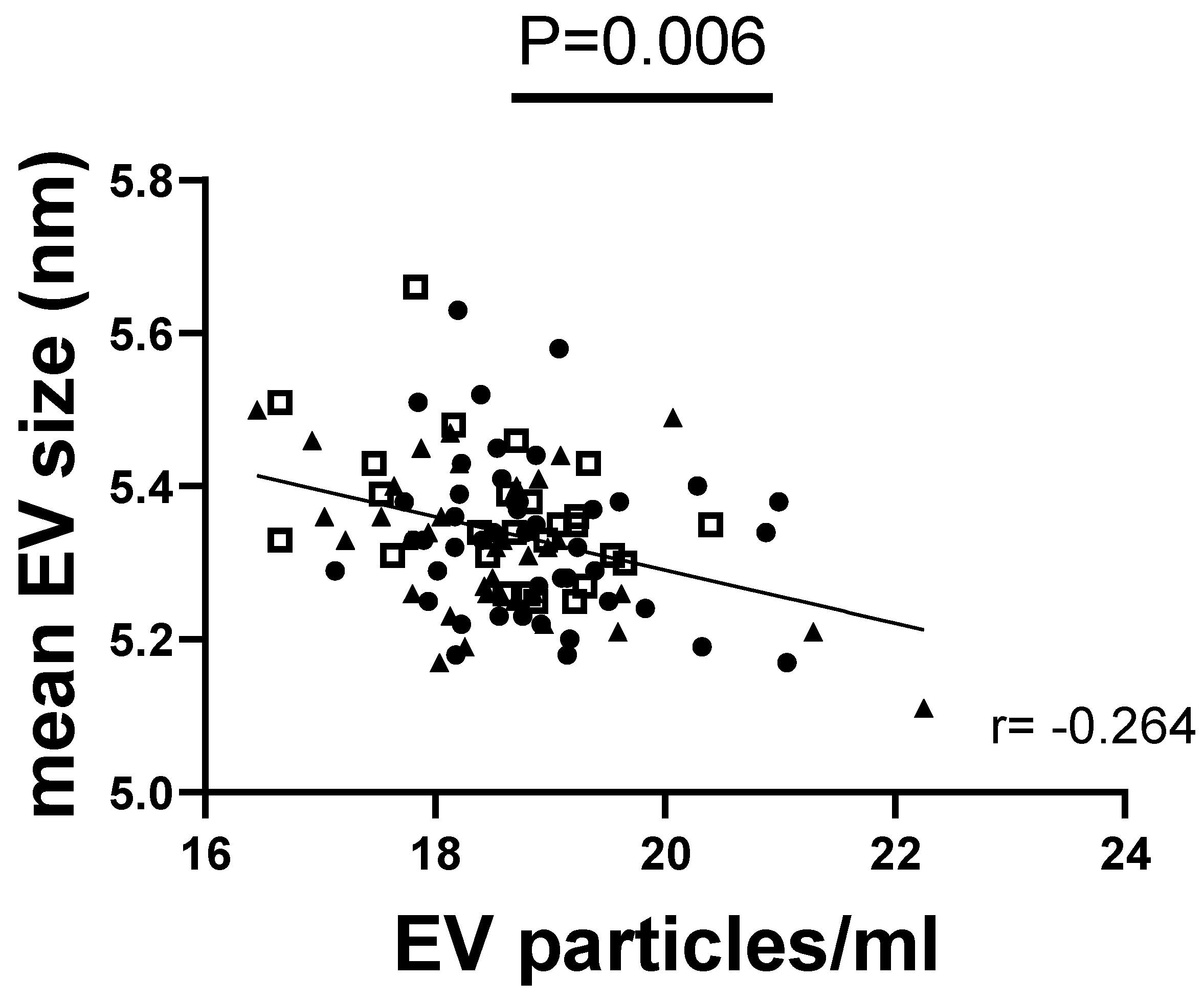
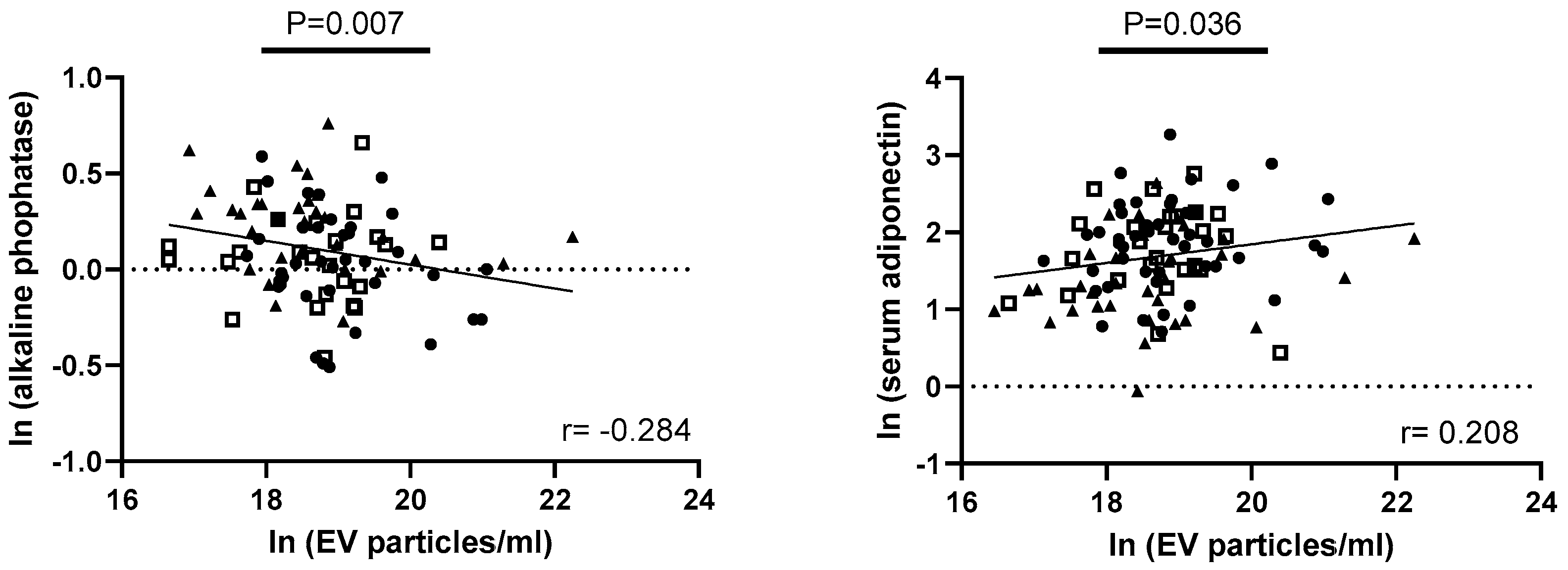
3.5. Exploring Correlations of EV Parameters with Metabolic Factors of Obesity, Taste Ability, and Lingual Taste Bud Density
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulz-Siegmund, M.; Aigner, A. Nucleic acid delivery with extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 173, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Sjoqvist, S.; Otake, K. Saliva and Saliva Extracellular Vesicles for Biomarker Candidate Identification-Assay Development and Pilot Study in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 5237. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, R.; Enciso-Martinez, A.; Bracht, J.W.P. Clinical applications and challenges in the field of extracellular vesicles. Med. Genet. 2023, 35, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Chen, M.; Xu, K.; Chen, B. The impact of obesity on adipocyte-derived extracellular vesicles. Cell. Mol. Life Sci. 2021, 78, 7275–7288. [Google Scholar] [CrossRef]
- Irmer, B.; Chandrabalan, S.; Maas, L.; Bleckmann, A.; Menck, K. Extracellular Vesicles in Liquid Biopsies as Biomarkers for Solid Tumors. Cancers 2023, 15, 1307. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, Q.; Wu, K.; Liu, L.; Zhao, M.; Yang, H.; Wang, X.; Wang, W. Extracellular vesicles as potential biomarkers and therapeutic approaches in autoimmune diseases. J. Transl. Med. 2020, 18, 432. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Shah, A.S.; Nakamura, T. Extracellular Vesicles: A Potential Novel Regulator of Obesity and Its Associated Complications. Children 2018, 5, 152. [Google Scholar] [CrossRef]
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.J.; Wang, Z.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A.; et al. Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatr. Res. 2015, 77, 447–454. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Luo, Z.; Jin, Z.; Ji, Y.; Ying, W. Adipose Tissue Macrophages Modulate Obesity-Associated β Cell Adaptations through Secreted miRNA-Containing Extracellular Vesicles. Cells 2021, 10, 2451. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Luo, Z.; Ji, Y.; Tang, K.; Jin, Z.; Ly, C.; Sears, D.D.; Mahata, S.; Ying, W. Accumulation of microbial DNAs promotes to islet inflammation and β cell abnormalities in obesity in mice. Nat. Commun. 2022, 13, 565. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, X.; Wang, Y.; Cao, Y.; Yao, D.; Sun, L.; Qin, L.; Qiu, H.; Zhan, X. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol. 2020, 228, e13339. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J. Food-Derived Extracellular Vesicles as Multi-Bioactive Complex and Their Versatile Health Effects. Antioxidants 2023, 12, 1862. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sundaram, K.; Mu, J.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.; Qiu, X.; Xu, F.; Yan, J.; et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat. Commun. 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Basilicata, M.; Pieri, M.; Marrone, G.; Nicolai, E.; Di Lauro, M.; Paolino, V.; Tomassetti, F.; Vivarini, I.; Bollero, P.; Bernardini, S.; et al. Saliva as Biomarker for Oral and Chronic Degenerative Non-Communicable Diseases. Metabolites 2023, 13, 889. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, K.; Choromańska, B.; Dąbrowska, E.; Bączek, W.; Myśliwiec, P.; Dadan, J.; Zalewska, A. Saliva of obese patients —Is it different? Postepy Hig. Med. Dosw. 2015, 69, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, L.; Gornowicz, A.; Pietraszewska, B.; Bielawski, K.; Bielawska, A. Which salivary components can differentiate metabolic obesity? PLoS ONE 2020, 15, e0235358. [Google Scholar] [CrossRef]
- Bombin, A.; Yan, S.; Bombin, S.; Mosley, J.D.; Ferguson, J.F. Obesity influences composition of salivary and fecal microbiota and impacts the interactions between bacterial taxa. Physiol. Rep. 2022, 10, e15254. [Google Scholar] [CrossRef]
- Roa, I.; Del Sol, M. Obesity, salivary glands and oral pathology. Colomb. Med. 2018, 49, 280–287. [Google Scholar] [CrossRef]
- López-Dávalos, P.C.; Requena, T.; Pozo-Bayón, M.Á.; Muñoz-González, C. Decreased retronasal olfaction and taste perception in obesity are related to saliva biochemical and microbiota composition. Food Res. Int. 2023, 167, 112660. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Espanca, R.; Costa, A.R.; Antunes, C.M.; Pomar, C.; Capela-Silva, F.; Pinheiro, C.C.; Domingues, P.; Amado, F.; Lamy, E. Comparison of salivary proteome of children with different sensitivities for bitter and sweet tastes: Association with body mass index. Int. J. Obes. 2019, 43, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Piombino, P.; Genovese, A.; Esposito, S.; Moio, L.; Cutolo, P.P.; Chambery, A.; Severino, V.; Moneta, E.; Smith, D.P.; Owens, S.M.; et al. Saliva from obese individuals suppresses the release of aroma compounds from wine. PLoS ONE 2014, 9, e85611. [Google Scholar] [CrossRef] [PubMed]
- Kersten, A.; Lorenz, A.; Nottmeier, C.; Schmidt, M.; Roesner, A.; Richter, F.C.; Röhrborn, K.; Witte, A.V.; Hahnel, S.; Koehne, T.; et al. The Obese Taste Bud study: Objectives and study design. Diabetes Obes. Metab. 2024, 26, 2054–2068. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Feron, G.; Canon, F. Main effects of human saliva on flavour perception and the potential contribution to food consumption. Proc. Nutr. Soc. 2018, 77, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, S.; Höchenberger, R.; Villringer, A.; Ohla, K. Higher sensitivity to sweet and salty taste in obese compared to lean individuals. Appetite 2017, 111, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.D.; Whittle, A. Obese individuals have higher preference and sensitivity to odor of chocolate. Chem. Senses 2015, 40, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Pîrsean, C.; Neguț, C.; Stefan-van Staden, R.-I.; Dinu-Pirvu, C.E.; Armean, P.; Udeanu, D.I. The salivary levels of leptin and interleukin-6 as potential inflammatory markers in children obesity. PLoS ONE 2019, 14, e0210288. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kita, S.; Tanaka, Y.; Fukuda, S.; Obata, Y.; Okita, T.; Nishida, H.; Takahashi, Y.; Kawachi, Y.; Tsugawa-Shimizu, Y.; et al. Adiponectin Stimulates Exosome Release to Enhance Mesenchymal Stem-Cell-Driven Therapy of Heart Failure in Mice. Mol. Ther. 2020, 28, 2203–2219. [Google Scholar] [CrossRef]
- Baquero, A.F.; Gilbertson, T.A. Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells. Am. J. Physiol. Cell Physiol. 2011, 300, C860–C871. [Google Scholar] [CrossRef] [PubMed]
- Meredith, T.L.; Corcoran, A.; Roper, S.D. Leptin’s effect on taste bud calcium responses and transmitter secretion. Chem. Senses 2015, 40, 217–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freire, C.; Ramsey, J.D.; Pho, H.; Kojima, R.; Zhao, Y.; Kim, L.; Anokye-Danso, F.; Berger, S.; Ahima, R.S.; Batrakova, E.V.; et al. Leptin-loaded Extracellular Vesicles Treat Sleep-disordered Breathing in Mice with Obesity. Am. J. Respir. Cell Mol. Biol. 2022, 67, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Calvo, S.S.-C.; Egan, J.M. The endocrinology of taste receptors. Nat. Rev. Endocrinol. 2015, 11, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.; Pruthi, N.; Seers, C.; Belobrov, S.; McCullough, M.; Celentano, A. Extracellular Vesicles in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 1197. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Wang, L.; Zhang, W.; Liu, B.; Wu, Y.; Pang, J.; Ma, C. The role of extracellular vesicles in periodontitis: Pathogenesis, diagnosis, and therapy. Front. Immunol. 2023, 14, 1151322. [Google Scholar] [CrossRef] [PubMed]
- Ferrant, J.; Pontis, A.; Zimmermann, F.; Dingli, F.; Poullet, P.; Loew, D.; Tarte, K.; Dumontet, E. Phenotypic and proteomic analysis of plasma extracellular vesicles highlights them as potential biomarkers of primary Sjögren syndrome. Front. Immunol. 2023, 14, 1207545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, S.; Xu, Q.; Zhang, Q.; Shanti, R.M.; Le, A.D. SIS-ECM Laden with GMSC-Derived Exosomes Promote Taste Bud Regeneration. J. Dent. Res. 2019, 98, 225–233. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Exosomes of pasteurized milk: Potential pathogens of Western diseases. J. Transl. Med. 2019, 17, 3. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- LimeSurvey GmbH. LimeSurvey: An Open Source Survey Tool; LimeSurvey GmBH: Hamburg, Germany, 2006–2024. Available online: http://www.limesurvey.org (accessed on 3 July 2024).
- Han, Y.; Jia, L.; Zheng, Y.; Li, W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018, 14, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Gelsomino, L.; Barone, I.; Panza, S.; Augimeri, G.; Bonofiglio, D.; Rovito, D.; Naimo, G.D.; Leggio, A.; Catalano, S.; et al. Leptin Modulates Exosome Biogenesis in Breast Cancer Cells: An Additional Mechanism in Cell-to-Cell Communication. J. Clin. Med. 2019, 8, 1027. [Google Scholar] [CrossRef] [PubMed]
- Heiston, E.M.; Ballantyne, A.; Stewart, N.R.; La Salvia, S.; Musante, L.; Lanningan, J.; Erdbrügger, U.; Malin, S.K. Insulin infusion decreases medium-sized extracellular vesicles in adults with metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E378–E388. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Liu, Y.; Rudeski-Rohr, T.; Dahir, N.; Calder, A.; Gilbertson, T.A. Adiponectin Enhances Fatty Acid Signaling in Human Taste Cells by Increasing Surface Expression of CD36. Int. J. Mol. Sci. 2023, 24, 5801. [Google Scholar] [CrossRef] [PubMed]
- Desai, G.S.; Mathews, S.T. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J. Diabetes 2014, 5, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.M.; Kantarci, A.; Hartman, M.-L.; Denis, G.V.; Stephens, D.; Hasturk, H.; Yaskell, T.; Vargas, J.; Wang, X.; Cugini, M.; et al. Metabolic disease risk in children by salivary biomarker analysis. PLoS ONE 2014, 9, e98799. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.; Schamarek, I.; Blüher, M. Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets? Diabetes Metab. J. 2020, 44, 509–528. [Google Scholar] [CrossRef]
- Archer, N.; Shaw, J.; Cochet-Broch, M.; Bunch, R.; Poelman, A.; Barendse, W.; Duesing, K. Obesity is associated with altered gene expression in human tastebuds. Int. J. Obes. 2019, 43, 1475–1484. [Google Scholar] [CrossRef]
- Kaufman, A.; Kim, J.; Noel, C.; Dando, R. Taste loss with obesity in mice and men. Int. J. Obes. 2020, 44, 739–743. [Google Scholar] [CrossRef]
- Ahart, Z.C.; Martin, L.E.; Kemp, B.R.; Dutta Banik, D.; Roberts, S.G.E.; Torregrossa, A.-M.; Medler, K.F. Differential Effects of Diet and Weight on Taste Responses in Diet-Induced Obese Mice. Obesity 2020, 28, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Maliphol, A.B.; Garth, D.J.; Medler, K.F. Diet-induced obesity reduces the responsiveness of the peripheral taste receptor cells. PLoS ONE 2013, 8, e79403. [Google Scholar] [CrossRef]
- Ribeiro, G.; Oliveira-Maia, A.J. Sweet taste and obesity. Eur. J. Intern. Med. 2021, 92, 3–10. [Google Scholar] [CrossRef]
- Rurgo, S.; Cantone, E.; Pesce, M.; Efficie, E.; Musella, M.; Polese, B.; de Conno, B.; Pagliaro, M.; Seguella, L.; Guida, B.; et al. Sleeve Gastrectomy-Induced Body Mass Index Reduction Increases the Intensity of Taste Perception’s and Reduces Bitter-Induced Pleasantness in Severe Obesity. J. Clin. Med. 2022, 11, 3957. [Google Scholar] [CrossRef] [PubMed]
- Bond, S.T.; Calkin, A.C.; Drew, B.G. Adipose-Derived Extracellular Vesicles: Systemic Messengers and Metabolic Regulators in Health and Disease. Front. Physiol. 2022, 13, 837001. [Google Scholar] [CrossRef]
- Martin, P.J.; Haren, N.; Ghali, O.; Clabaut, A.; Chauveau, C.; Hardouin, P.; Broux, O. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol. 2015, 16, 10. [Google Scholar] [CrossRef]
- Kim, A.M.; Keenan, B.T.; Jackson, N.; Chan, E.L.; Staley, B.; Poptani, H.; Torigian, D.A.; Pack, A.I.; Schwab, R.J. Tongue fat and its relationship to obstructive sleep apnea. Sleep 2014, 37, 1639–1648. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Polyzos, A.; Kapsogeorgou, E.K.; Thanos, D.; Manoussakis, M.N. Impaired anti-inflammatory activity of PPARγ in the salivary epithelia of Sjögren’s syndrome patients imposed by intrinsic NF-κB activation. J. Autoimmun. 2018, 86, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, X.; Shen, C. Peroxisome proliferator-activated receptor gamma in white and brown adipocyte regulation and differentiation. Physiol. Res. 2020, 69, 759–773. [Google Scholar] [CrossRef]
- Semple, R.K.; Chatterjee, V.K.K.; O’Rahilly, S. PPAR gamma and human metabolic disease. J. Clin. Investig. 2006, 116, 581–589. [Google Scholar] [CrossRef]
- Winck, F.V.; Prado Ribeiro, A.C.; Ramos Domingues, R.; Ling, L.Y.; Riaño-Pachón, D.M.; Rivera, C.; Brandão, T.B.; Gouvea, A.F.; Santos-Silva, A.R.; Coletta, R.D.; et al. Insights into immune responses in oral cancer through proteomic analysis of saliva and salivary extracellular vesicles. Sci. Rep. 2015, 5, 16305. [Google Scholar] [CrossRef] [PubMed]
- Haab, B.B.; Geierstanger, B.H.; Michailidis, G.; Vitzthum, F.; Forrester, S.; Okon, R.; Saviranta, P.; Brinker, A.; Sorette, M.; Perlee, L.; et al. Immunoassay and antibody microarray analysis of the HUPO Plasma Proteome Project reference specimens: Systematic variation between sample types and calibration of mass spectrometry data. Proteomics 2005, 5, 3278–3291. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Huo, N.; Wang, X.; Yang, S.; Wang, J.; Zhang, T. Exosomes from oral tissue stem cells: Biological effects and applications. Cell Biosci. 2020, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- El-Maradny, Y.A.; Rubio-Casillas, A.; Mohamed, K.I.; Uversky, V.N.; Redwan, E.M. Intrinsic factors behind long-COVID: II. SARS-CoV-2, extracellular vesicles, and neurological disorders. J. Cell. Biochem. 2023, 124, 1466–1485. [Google Scholar] [CrossRef] [PubMed]
- Blandin, A.; Amosse, J.; Froger, J.; Hilairet, G.; Durcin, M.; Fizanne, L.; Ghesquière, V.; Prieur, X.; Chaigneau, J.; Vergori, L.; et al. Extracellular vesicles are carriers of adiponectin with insulin-sensitizing and anti-inflammatory properties. Cell Rep. 2023, 42, 112866. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.E.; Fiori, J.L.; Gonzalez Mariscal, I.; Liu, Q.-R.; Goodstein, E.; Yang, H.; Shin, Y.-K.; Santa-Cruz Calvo, S.; Indig, F.E.; Egan, J.M. Insulin Is Transcribed and Translated in Mammalian Taste Bud Cells. Endocrinology 2018, 159, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Crosson, S.M.; Marques, A.; Dib, P.; Dotson, C.D.; Munger, S.D.; Zolotukhin, S. Taste Receptor Cells in Mice Express Receptors for the Hormone Adiponectin. Chem. Senses 2019, 44, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P.; Tuna, K.M.; Mestre, C.T.; Banwatt, E.S.; Alli, A.A. Activation of multiple receptors stimulates extracellular vesicle release from trophoblast cells. Physiol. Rep. 2020, 8, e14592. [Google Scholar] [CrossRef]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef]

| Clinical Parameter | N | Total Cohort | N | Lean | N | Obese | p-Value |
|---|---|---|---|---|---|---|---|
| sex (% female/male) | 119 | 62/38 | 49 | 64/37 | 44 | 66/34 | 0.791 |
| age (years) | 119 | 36 (26–49) | 49 | 30 (24–45) | 44 | 42 (30–54) | 0.006 |
| height (cm) | 119 | 1.72 (1.64–1.77) | 49 | 1.73 (1.65–1.78) | 44 | 1.71 (1.63–1.75) | 0.389 |
| weight (kg) | 119 | 80 (65–101.8) | 49 | 63.2 (58.25–74.15) | 44 | 106.05 (91.73–124.5) | <0.001 |
| BMI (kg/m²) | 119 | 26.66 (22.52–34.01) | 49 | 22.26 (20.62–23.53) | 44 | 35.87 (31.73–44.11) | <0.001 |
| waist (cm) | 119 | 89 (75–104) | 49 | 74 (70.5–81) | 44 | 112.5 (100.38–123.5) | <0.001 |
| hip (cm) | 119 | 102 (91.5–114) | 49 | 91 (87–95.5) | 44 | 122 (109.88–135) | <0.001 |
| WHR | 119 | 0.86 (0.8–0.93) | 49 | 0.82 (0.77–0.87) | 44 | 0.91 (0.84–0.96) | <0.001 |
| fat mass (%) | 98 | 29.5 (21.95–40.4) | 45 | 23.83 (18.94–27.18) | 33 | 43.10 (39.79–54.45) | <0.001 |
| systolic BP (mmHg) | 70 | 120 (111–130.5) | 21 | 112 (105–120.5) | 28 | 125.5 (117–139.75) | 0.004 |
| diastolic BP (mmHg) | 70 | 78 (73–84.25) | 21 | 75 (68.5–81) | 28 | 80 (76–88.5) | 0.008 |
| ALP (µkat/L) | 100 | 1.13 (0.95–1.35) | 40 | 1.04 (0.90–1.24) | 36 | 1.32 (1.07–1.49) | <0.001 |
| total cholesterol (mmol/L) | 102 | 4.75 (4.2–5.58) | 41 | 4.56 (4.02–5.19) | 37 | 4.81 (4.31–5.49) | 0.152 |
| non-HDL cholesterol (mmol/L) | 117 | 3.2 (2.48–3.94) | 48 | 2.77 (2.26–3.54) | 43 | 3.52 (2.8–4.03) | <0.001 |
| HDL cholesterol (mmol/L) | 102 | 2.89 (2.29–3.71) | 41 | 2.6 (1.98–3.18) | 37 | 3.1 (2.64–3.71) | 0.013 |
| LDL cholesterol (mmol/L) | 102 | 1.5 (1.25–1.93) | 41 | 1.81 (1.49–2.14) | 37 | 1.3 (1.06–1.51) | <0.001 |
| triglycerides (mmol/L) | 102 | 1 (0.76–1.57) | 41 | 0.77 (0.66–1) | 37 | 1.28 (0.98–2.01) | <0.001 |
| FFA (mmol/L) | 102 | 0.61 (0.45–0.78) | 41 | 0.61 (0.47–0.78) | 37 | 0.66 (0.55–0.82) | 0.37 |
| HbA1c (%) | 114 | 5.4 (5.2–5.8) | 45 | 5.3 (5.1–5.7) | 43 | 5.6 (5.2–6.1) | 0.011 |
| fasting glucose (mmol/L) | 116 | 4.74 (4.44–5.13) | 47 | 4.46 (4.28–4.7) | 43 | 5.12 (4.79–5.68) | <0.001 |
| fasting insulin serum (pmol/L) | 100 | 38.4 (28.15–82.4) | 41 | 28.3 (22.7–38.9) | 36 | 88.1 (69.95–125.68) | <0.001 |
| insulin saliva (pmol/L) | 107 | 10.25 (4.32–19.8) | 48 | 7.4 (3.21–11.31) | 36 | 18.87 (9.24–30.05) | <0.001 |
| adiponectin serum (µg/mL) | 114 | 5.33 (3.47–8.21) | 47 | 6.75 (4.48–9.7) | 42 | 3.91 (2.62–5.88) | <0.001 |
| adiponectin saliva (ng/mL) | 104 | 6.4 (2.53–16.68) | 47 | 6.0 (2.20–17.10) | 34 | 6.15 (2.80–15.92) | 0.886 |
| leptin serum (ng/mL) | 110 | 11.6 (2.87–21.95) | 46 | 3.84 (1.46–11.82) | 40 | 21.58 (11.93–40.66) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röhrborn, K.; Krueger, M.; Kalusa, M.; Fietz, S.A.; Ewe, A.; Aigner, A.; Stumvoll, M.; Kovacs, P.; Blüher, M.; Schamarek, I.; et al. The Concentration of Salivary Extracellular Vesicles Is Related to Obesity. Nutrients 2024, 16, 2633. https://doi.org/10.3390/nu16162633
Röhrborn K, Krueger M, Kalusa M, Fietz SA, Ewe A, Aigner A, Stumvoll M, Kovacs P, Blüher M, Schamarek I, et al. The Concentration of Salivary Extracellular Vesicles Is Related to Obesity. Nutrients. 2024; 16(16):2633. https://doi.org/10.3390/nu16162633
Chicago/Turabian StyleRöhrborn, Kristin, Martin Krueger, Mirjam Kalusa, Simone A. Fietz, Alexander Ewe, Achim Aigner, Michael Stumvoll, Peter Kovacs, Matthias Blüher, Imke Schamarek, and et al. 2024. "The Concentration of Salivary Extracellular Vesicles Is Related to Obesity" Nutrients 16, no. 16: 2633. https://doi.org/10.3390/nu16162633
APA StyleRöhrborn, K., Krueger, M., Kalusa, M., Fietz, S. A., Ewe, A., Aigner, A., Stumvoll, M., Kovacs, P., Blüher, M., Schamarek, I., & Rohde-Zimmermann, K. (2024). The Concentration of Salivary Extracellular Vesicles Is Related to Obesity. Nutrients, 16(16), 2633. https://doi.org/10.3390/nu16162633







