Abstract
Sialyllactose (SL) is a functional human milk oligosaccharide essential for immune support, brain development, intestinal maturation, and antiviral defense. However, despite its established health benefits, the effect of SL on exercise performance and muscle mass in mice remains unknown. Here, we aimed to investigate, for the first time, the effects of 6′-SL on muscle functions. Seven-week-old male C57BL/6J mice were administered 100 mg/kg 6′-SL for 12 weeks, after which exhaustive treadmill performance was conducted. Moreover, muscle strength was examined by grip strength, and muscle phenotype characteristics such as muscle mass, muscle fiber size, and muscle protein expression were also examined. The administration of 6′-SL significantly improved exhaustive treadmill performance metrics, including distance and exhaustion time. Grip strength was also increased by 6′-SL administration. Additionally, 6′-SL increased muscle mass in both the gastrocnemius (GAS) and soleus. 6′-SL administration led to an increase in the minimum Feret’s diameter and the protein expression of total myosin heavy chain in the GAS muscle. In conclusion, 6′-SL administration in vivo led to increased running distance and time by increasing muscle mass and strength. These findings collectively indicate that 6′-SL is a potential agent for improving muscle health and exercise performance.
1. Introduction
Exercise performance is influenced by various factors, including muscle fiber composition, metabolic efficiency, muscle mass, muscle strength, and neuromuscular coordination [,,]. Many studies corroborate that increased muscle strength and mass facilitate the execution of common sports skills such as jumping, sprinting, and changing direction [,]. Strength training and dietary supplements are established strategies for increasing muscle mass, strength, and overall health []. Athletes and fitness enthusiasts commonly use various dietary supplements [,], such as creatine, protein powders, β-hydroxy β-methylbutyrate (β-HMB), chromium, vanadyl sulfate, boron, and leucine, all known to enhance muscle mass and strength [,]. However, long-term or high-dose usage of these supplements may lead to potential side effects, such as gastrointestinal disturbances and drug interactions []. Moreover, concerns about lack of efficacy, financial expense, lack of quality control, potential to produce significant toxicity, and little attention to dietary supplement regulation in public health have been raised [,]. Despite widespread consumption, thousands of dietary supplements have been produced and sold without strict regulation. Unlike food and drugs, dietary supplements do not have to be registered or approved by the FDA before they can be manufactured or sold [].
Although creatine supplementation is particularly popular for muscle growth, its effectiveness and safety remain debated []. Therefore, safe and accessible alternatives to address these concerns are imperative [].
Human milk oligosaccharides (HMOs), abundant in breast milk, play a pivotal role in several bioactive functions and significantly contribute to the nutritional value of breast milk []. Over 100 different HMOs, including sialylated and fucosylated oligosaccharides, have been identified to date []. Sialyllactose (SL) is the most abundant sialylated oligosaccharide, characterized by the linkage of N-acetylneuraminic acid to the galactosyl subunit of lactose. SL confers various health benefits and plays crucial roles in several physiological processes, such as gastrointestinal microbiota development, gut maturation, brain and cognitive development, and the enhancement of innate immunity as a decoy receptor for viruses, potential pathogens, and bacteria []. The predominant forms of SL are 3′-SL (with N-acetylneuraminic acid connected to the 3′ position of lactose) and 6′-SL (with N-acetylneuraminic acid connected to the 6′ position of lactose), of which 6′-SL exerts several therapeutic effects on diseases such as necrotizing enterocolitis, neuritogenesis, and benign prostatic hyperplasia [,,].
In relation to muscle health, 6′-SL has also been demonstrated to ameliorate myopathic phenotypes, such as muscle weight and locomotor activity, in symptomatic bifunctional UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) myopathy []. A recent study further demonstrated that 6′-SL could increase limb muscle power in patients with GNE myopathy []. Although ameliorative effects of 6′-SL on myopathy have been reported, the efficacy of 6′-SL on muscle function under normal conditions has not been evaluated. Hence, in the present study, we aimed to investigate the effects of 6′-SL on exercise performance capacity and muscle phenotype in normal young mice. This study will provide evidence for the use of 6′-SL as a supplement for athletes and sports enthusiasts.
2. Materials and Methods
2.1. Animals and Treatments
Four-week-old male C57BL/6J mice, weighing 19–22 g, were obtained from Daehan Biolink (Seoul, Republic of Korea) and housed in a controlled environment (22–23 °C, 12/12 h light/dark cycle), with ad libitum access to water and a normal diet. This study was conducted at Pohang Technopark Biotechnology Center in accordance with the guidelines of the Pohang Technopark Animal Ethics Committee (ABCC 2022009, Pohang, Republic of Korea). After a 3-week acclimatization period, the mice (n = 20) were randomly divided by the investigators into two groups based on body weight. Group 1 (n = 10) was orally administered with water, whereas Group 2 (n = 10) was administered 100 mg/kg 6′-SL in water. We selected the dose by referring to other SL papers that showed efficacy. Both groups were treated once a day, 5 days per week, for 12 weeks. Animals’ health was monitored once daily, 5 days per week, through animal activity, panting, and fur condition. The following analyses were assessed: muscle weight measurement; exhaustive treadmill exercise test; forelimb grip strength test; dual-energy X-ray absorptiometry measurement; muscle fiber size measurement; and muscle protein expression. The 6′-SL used in this study was produced via enzyme synthesis by GeneChem, Inc. (Daejeon, Republic of Korea). All experimental procedures were approved by the Institutional Ethics Committee for the Care and Use of Animals.
2.2. Sample Collection
After 12 weeks of 6′-SL administration, mice were humanely euthanized, and gastrocnemius (GAS) and soleus (SOL) muscles were harvested. GAS and SOL muscle tissues were weighed, and GAS muscle tissues were either frozen in liquid nitrogen for protein extraction or fixed in 10% formalin for histological staining. Immediately after necropsy, analysis was performed.
2.3. Exercise Function Measurement
After 10 weeks of 6′-SL administration, an exhaustive treadmill exercise test was conducted. The test began at a speed of 10 m/min on a flat surface (0% slope) for 3 min. Subsequently, the speed was gradually increased to 20 m/min and maintained until the mice reached exhaustion, which was defined as the inability to continue running for 10 s []. A forelimb grip strength test was conducted at 3- and 10-weeks post-6′-SL administration using a maximal voluntary force testing system (BIO-G53; BIOSEB, Pinellas Park, FL, USA) [].
2.4. Dual-Energy X-ray Absorptiometry Measurement
After 12 weeks of 6′-SL administration, fat mass, bone mineral content (BMC), bone mineral density (BMD), bone area, and bone volume were measured using dual-energy X-ray absorptiometry (DEXA; InAlyzer; MEDIKORS, Seongnam-si, Republic of Korea).
2.5. Histological Tissue Staining
Formalin-fixed GAS muscles were embedded in paraffin and cut into 4 μm thick slices, stained with hematoxylin and eosin (H&E), and imaged using a Digital Fluorescence Slide Scanner (Axio Scan Z1, Carl Zeiss Microscopy GmbH, Jena, Germany). The minimum Feret’s diameter of muscle fibers and their percentage distribution were measured using ImageJ 1.53t [].
2.6. Western Blotting
Total protein was isolated from GAS muscles using the T-PER™ Tissue Protein Extraction Reagent (78510; Thermo Fisher, Rockford, IL, USA). Protein concentration was determined using the Quick Start™ Bradford Protein Assay (5000202; Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein were loaded onto 4–20% Mini-PROTEAN® TGX™ Precast Protein Gels (4561096; Bio-Rad Laboratories, Hercules, CA, USA). Subsequently, the resolved proteins were transferred onto a polyvinylidene difluoride membrane using the Trans-Blot Turbo Transfer System (1704156; Bio-Rad Laboratories, Hercules, CA, USA) and subjected to immunoblotting using anti-total myosin heavy chain (MHC) (sc-376157; Santa Cruz Biotechnology (SCBT), Dallas, Texas, USA) and anti-α-tubulin (sc-5286; Santa Cruz Biotechnology (SCBT)) overnight at 4 °C. Next, the membranes were incubated with the secondary antibodies (LF-SA8001; Abfrontier, Seoul, Republic of Korea) at room temperature for 1 h. After three washes with Tris-buffered saline containing 0.1% Tween, protein bands were detected using an enhanced chemiluminescence reagent (1705061; Bio-Rad Laboratories, Hercules, CA, USA).
2.7. Statistical Analysis
Data are presented as mean ± standard deviation. The sample size for each experiment was selected to calculate significant differences. Animals were excluded if they died prematurely, or tissue samples were lost during autopsy. The number of animals was adjusted between the control and 6′-SL treatment groups (muscle weight measurement, exhaustive treadmill exercise test, forelimb grip strength test, and dual-energy X-ray absorptiometry measurement, n = 8; muscle fiber size measurement and muscle protein expression, n = 3). All statistical analyses were performed using GraphPad Prism 10.1.0 (GraphPad Software, Inc., San Diego, CA, USA). Significant differences were determined using a two-tailed unpaired t-test followed by Dunnett’s multiple comparison test. Results with p < 0.05 were considered significant.
3. Results
3.1. 6′-SL Enhances Muscle Function
Exercise performance and grip strength were assessed to evaluate the effects of 6′-SL on muscle functions in mice. For exercise performance, mice were orally administered 100 mg/kg of 6′-SL for 10 weeks and subjected to an exhaustive treadmill exercise test where the maximum running distance, time to fatigue, and work performed were measured. Administration of 6′-SL led to significant increases in distance (control: 560.6 ± 76.1 m; 6′-SL: 674 ± 87.1 m), time (control: 33.5 ± 2.7 min; 6′-SL: 37.1 ± 2.6 min), and work (control: 18,502.2 ± 2400.2 m × kg; 6′-SL: 23,140.7 ± 2929.4 m × kg) compared with the values observed in the control group (Figure 1A).
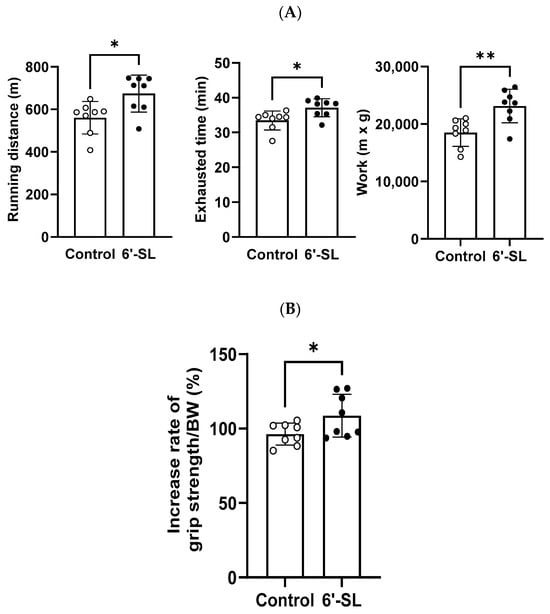
Figure 1.
Effect of 6′-SL on exercise performance and muscle strength. (A) Total running distance, exhaustion time, and work during exhaustive exercise were measured at 10 weeks. Work was calculated as running distance (m) × body weight (g). (B) Rate of increase in grip strength/body weight between weeks 3 and 10. Data are presented as mean ± standard deviation values for each group (n = 8). White dots mean control groups and black dots mean 6′-SL groups. A two-tailed unpaired t-test was used: * p < 0.05, ** p < 0.01 vs. control. Abbreviations: SL, sialyllactose; BW, body weight.
Grip strength was measured at weeks 3 and 10 of 6′-SL administration, and the rate of increase in grip strength between weeks 3 and 10 was calculated. The administration of 6′-SL led to a greater increase in grip strength than that observed in the control group (control: 96.3% ± 6.2%; 6′-SL: 108.6% ± 12%) (Figure 1B).
3.2. 6′-SL Increases the Volume and Size of GAS Muscles
Muscle weights and fiber diameter were measured to examine the effects of 6′-SL on muscle phenotype characteristics. The administration of 6′-SL increased the absolute weights of the GAS and SOL muscles (Figure 2A), which are key muscles of the calf used for walking, running, and jumping. To support the observed increase in muscle weights, we measured body weight, fat mass, and various bone mass parameters (BMC, BMD, bone area, and bone volume) (Table 1). An increase in body weight without corresponding fat and bone mass gain was observed, partly suggesting an increase in muscle mass. Additionally, 6′-SL administration increased the protein expression of total MHC, a late-stage differentiation marker (Figure 2B) []. H&E staining of the GAS muscle revealed larger muscle fiber diameters in the 6′-SL administration group than in the control group. This finding was confirmed by quantifying fiber diameter size using a percentage distribution curve, which showed a trend toward larger sizes, and by measuring the average minimum Feret’s diameter, which was significantly increased in the 6′-SL administration group (Figure 2C–E).
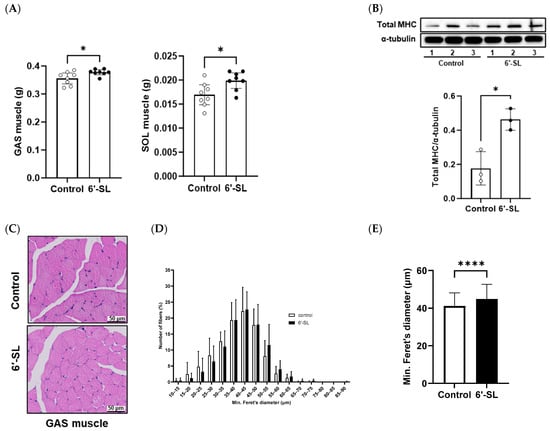
Figure 2.
Effect of 6′-SL on muscle mass and fiber formation in GAS and SOL muscles. (A) Absolute tissue weight (gastrocnemius (GAS) and soleus muscles) was measured at sacrifice. (B) Protein expression levels of the total myosin heavy chain of GAS muscle were measured using Western blotting. The relative band intensities of each protein were normalized to those of α-tubulin, and the quantified data are shown in the bar graphs. (C) Images of hematoxylin- and eosin-stained cross-sections of the GAS muscle; scale bar: 50 μm. (D) Percentage muscle fiber distribution based on min. Feret’s diameter of muscle fiber size of the GAS muscle. The number of muscle fibers measured in the control and 6′-SL groups was 1535 and 1697, respectively. (E) Mean of min. Feret’s diameter muscle fiber size of the GAS muscle. The number of muscle fibers measured in the control and 6′-SL groups was 591 and 633, respectively. Data are presented as mean ± standard deviation values for each group (n = 8 for (A), n = 3 for (B–E)). White dots mean control groups and black dots mean 6′-SL groups. A two-tailed unpaired t-test was used: * p < 0.05, **** p < 0.0001 vs. control. Abbreviations: SL, sialyllactose; GAS, gastrocnemius; SOL, soleus; MHC, myosin heavy chain.

Table 1.
Data of dual-energy X-ray absorptiometry measurements.
4. Discussion
This study demonstrated that 6′-SL, an HMO, affected exercise performance by altering muscle mass, fiber size, and MHC protein expression in C57BL/6J mice. Exercise performance is influenced by intrinsic factors, such as genetic constitution and athletic ability, as well as extrinsic factors, such as exercise training and proper nutrition [,].
Muscle mass plays a key role in exercise performance, influencing many aspects of strength and athletic ability []. Increased muscle mass improves endurance by enabling greater force exertion and improved storage of glycogen, the main fuel required for prolonged exercise []. Various nutritional supplements have been reported to increase muscle mass, strength, and exercise performance []. For example, DHEA, a precursor of sex steroid hormones, promotes protein synthesis and anabolism, resulting in increased muscle mass and strength []. Beta-methyl-hydroxy-beta-methylbutyrate has demonstrated efficacy in preserving muscle mass and strength in older individuals and promoting skeletal muscle hypertrophy in bodybuilders and strength/power athletes [,]. Creatine has also been reported to positively affect several aspects of exercise performance, including muscle mass and strength, glycogen synthesis, and aerobic capacity []. Additionally, amino acids, including branched-chain amino acids, glutamine, aspartates, and arginine, as well as protein supplements, such as whey protein and colostrum, have demonstrated efficacy in improving physical performance []. Despite the availability of numerous dietary supplements, many uncertainties persist regarding their safety and effectiveness.
MHC is crucial for muscle function by influencing muscle contraction, energy consumption, and structure []. Additionally, MHC is a marker protein commonly used to assess muscle fiber lengths, muscle mass, and strength []. According to previous studies, various materials such as α-lipoic acid, creatine, dihydromyricetin, ursolic acid, and leucine have been identified to increase MHC expression and improve muscle function. α-lipoic acid, a potent biological antioxidant, has been reported to promote MHC gene expression and maintain muscle mass in Otsuka Long–Evans Tokushima Fatty rats and reduce muscle degradation, promote muscle regeneration, and consequently maintain muscle mass in rats with type 2 diabetes mellitus []. Creatine supplementation increased muscle strength and size through increased MHC mRNA and protein levels []. Dihydromyricetin has been shown to upregulate MHC I expression through the AMPK signaling pathway, enhancing muscle performance []. Moreover, a previous study showed that ursolic acid and leucine significantly induced MHC protein expression and promoted C2C12 muscle cell differentiation [].
In this study, 6′-SL increased muscle weight (GAS and SOL), GAS muscle fiber size, and MHC protein expression in the GAS muscle. These results suggest that 6′-SL could enhance endurance exercise performance, including exhaustion time, total distance, and work output, by increasing muscle mass and strength in C57BL/6J mice.
Additionally, the safety of 6′-SL has been previously reported in piglets, healthy adults, and infant formulas [,,].
Proposed mechanisms for improving muscle health include enhanced anti-inflammatory and antioxidant properties, increased expression of the mammalian target of the rapamycin (mTOR) signaling pathway, reduced protein degradation, and improved mitochondrial function [,]. Whey protein has been reported to induce the mTOR pathway in resistance-exercising young men [,]. Ginseng, which has antioxidant and anti-inflammatory properties, improves muscle regeneration post-exercise in healthy adults []. Branched-chain amino acids and vitamin D supplementation improved mitochondrial function and enhanced strength and performance in atrophic muscle []. Notably, the precise mechanism by which 6′-SL enhances exercise performance was not determined in this study. However, 6′-SL has been demonstrated to inhibit lipopolysaccharide-induced inflammatory symptoms in intestinally inflamed suckling mice []. Furthermore, in Caenorhabditis elegans, 6′-SL improved endurance exercise performance by increasing glycogenolysis and affecting mitochondrial function []. Based on these previous reports, we intend to conduct more comprehensive studies to elucidate the mechanisms by which 6′-SL affects muscle phenotype and functions.
Previous studies on the pathology of GNE myopathy have shown that 6′-SL has ameliorative effects in both mice and humans [,]. Based on these reports, 6′-SL may have clinical relevance for individuals in normal conditions, such as athletes and sports enthusiasts.
5. Conclusions
In conclusion, this study demonstrated that 6′-SL increased muscle mass and strength, thereby enhancing exercise performance in young C57BL/6J mice. These findings suggest that 6′-SL is both beneficial and safe for enhancing exercise performance. To develop 6′-SL as a dietary supplement to improve human exercise performance, testing in clinical settings including subjects such as athletes and sports enthusiasts will be required.
Author Contributions
Conceptualization and methodology, E.-J.P., L.-L.K. and Y.-A.K.; investigation, H.-R.G. and Y.-A.K.; writing—original draft preparation, H.-R.G.; writing—review and editing, E.-J.P., J.-O.L. and H.-Y.L.; formal analysis, H.-R.G.; visualization and validation, H.-R.G. and Y.-A.K.; project administration, H.-R.G.; supervision, Y.-A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bio-industry Core Technology Development Business of the Ministry of Trade, Industry, and Energy (20014827); the Technology Development Program of the Ministry of SMEs and Startups (Korea) (S3122086); and the National Research Foundation of Korea (NRF) (NRF-2020M3H1A107531414). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The experimental procedures were conducted following the guidelines of the NIH for the Care and Use of Laboratory Animals and were approved by the Pohang Technopark Animal Ethics Committee (ABCC 2022009; Pohang, Republic of Korea, 11 August 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
Jie-Oh Lee, Hayyoung Lee, and Yong-An Kim from POSTECH declare no conflicts of interest. Hiroe Go, Eun-Jung Park, and Lila Kim were employed by GeneChem Inc. Lila Kim holds stock in GeneChem Inc. GeneChem Inc. provided support in the form of salaries for authors Hiroe Go, Eun-Jung Park, and Lila Kim. GeneChem Inc. is promoting HMOs for use as dietary supplements and will market them soon.
References
- Zierath, J.R.; Hawley, J.A. Skeletal muscle fiber type: Influence on contractile and metabolic properties. PLoS Biol. 2004, 2, e348. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C. Effects of physical activity and inactivity on muscle fatigue. Front. Physiol. 2012, 3, 142. [Google Scholar] [CrossRef]
- Akbar, S.; Soh, K.G.; Jazaily Mohd Nasiruddin, N.; Bashir, M.; Cao, S.; Soh, K.L. Effects of neuromuscular training on athletes physical fitness in sports: A systematic review. Front. Physiol. 2022, 13, 939042. [Google Scholar] [CrossRef]
- Suchomel, T.J.; Nimphius, S.; Stone, M.H. The Importance of Muscular Strength in Athletic Performance. Sports Med. 2016, 46, 1419–1449. [Google Scholar] [CrossRef]
- Nakai, Y.; Usumoto, Y.; Takeshita, Y. The Effects of Regional Muscle Strength and Mass on Standing Long Jump Performance. Muscles 2024, 3, 60–70. [Google Scholar] [CrossRef]
- Samadi, M.; Khosravy, T.; Azadbakht, L.; Rezaei, M.; Mosafaghadir, M.; Kamari, N.; Bagheri, A.; Pasdar, Y.; Najafi, F.; Hamze, B.; et al. Major dietary patterns in relation to muscle strength status among middle-aged people: A cross-sectional study within the RaNCD cohort. Food Sci. Nutr. 2021, 9, 6672–6682. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.; Depiesse, F.; Geyer, H. The use of dietary supplements by athletes. J. Sports Sci. 2007, 25 (Suppl. 1), S103–S113. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef]
- Kreider, R.B. Dietary supplements and the promotion of muscle growth with resistance exercise. Sports Med. 1999, 27, 97–110. [Google Scholar] [CrossRef]
- Beaudart, C.; Rabenda, V.; Simmons, M.; Geerinck, A.; Araujo de Carvalho, I.; Reginster, J.Y.; Amuthavalli Thiyagarajan, J.; Bruyère, O. Effects of Protein, Essential Amino Acids, B-Hydroxy B-Methylbutyrate, Creatine, Dehydroepiandrosterone and Fatty Acid Supplementation on Muscle Mass, Muscle Strength and Physical Performance in Older People Aged 60 Years and Over. A Systematic Review of the Literature. J. Nutr. Health Aging 2018, 22, 117–130. [Google Scholar]
- Beck, K.L.; Thomson, J.S.; Swift, R.J.; von Hurst, P.R. Role of nutrition in performance enhancement and postexercise recovery. Open Access J. Sports Med. 2015, 6, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Starr, R.R. Too Little, Too Late: Ineffective Regulation of Dietary Supplements in the United States. Am. J. Public Health 2015, 105, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Ten Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, X.; Xu, L.; Xiao, M.; Lu, L. Enzymatic Synthesis of 6′-Sialyllactose, a Dominant Sialylated Human Milk Oligosaccharide, by a Novel exo-alpha-Sialidase from Bacteroides fragilis NCTC9343. Appl. Environ. Microbiol. 2018, 84, e00071-18. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Jin, B.R.; Chung, T.W.; Bae, S.J.; Park, H.; Ryu, D.; Jin, L.; An, H.J.; Ha, K.T. 6-sialyllactose ameliorates dihydrotestosterone-induced benign prostatic hyperplasia through suppressing VEGF-mediated angiogenesis. BMB Rep. 2019, 52, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; He, J.H.; Mi, Y.J.; Shen, H.F.; Schachner, M.; Zhao, W.J. A mimetic peptide of alpha2,6-sialyllactose promotes neuritogenesis. Neural Regen. Res. 2020, 15, 1058–1065. [Google Scholar]
- Sodhi, C.P.; Wipf, O.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Niño, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. The human milk oligosaccharides 2′-fucosyllactose and 6′-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 2021, 89, 91–101. [Google Scholar] [CrossRef]
- Yonekawa, T.; Malicdan, M.C.; Cho, A.; Hayashi, Y.K.; Nonaka, I.; Mine, T.; Yamamoto, T.; Nishino, I.; Noguchi, S. Sialyllactose ameliorates myopathic phenotypes in symptomatic GNE myopathy model mice. Brain 2014, 137, 2670–2679. [Google Scholar] [CrossRef]
- Park, Y.E.; Park, E.; Choi, J.; Go, H.; Park, D.B.; Kim, M.Y.; Sung, N.J.; Kim, L.; Shin, J.H. Pharmacokinetics and clinical efficacy of 6′-sialyllactose in patients with GNE myopathy: Randomized pilot trial. Biomed. Pharmacother. 2023, 168, 115689. [Google Scholar] [CrossRef]
- Kim, Y.A.; Oh, S.H.; Lee, G.H.; Hoa, P.T.; Jin, S.W.; Chung, Y.C.; Lee, Y.C.; Jeong, H.G. Platycodon grandiflorum-derived saponin attenuates the eccentric exercise-induced muscle damage. Food Chem. Toxicol. 2018, 112, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Menalled, L.B.; Patry, M.; Ragland, N.; Lowden, P.A.; Goodman, J.; Minnich, J.; Zahasky, B.; Park, L.; Leeds, J.; Howland, D.; et al. Comprehensive behavioral testing in the R6/2 mouse model of Huntington’s disease shows no benefit from CoQ10 or minocycline. PLoS ONE 2010, 5, e9793. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, N.; Soibam, B.; Li, L.; Wang, J.; Byers, L.A.; Liu, Y.; Schwartz, R.J.; Stewart, M.D. HIRA deficiency in muscle fibers causes hypertrophy and susceptibility to oxidative stress. J. Cell Sci. 2017, 130, 2551–2563. [Google Scholar] [CrossRef] [PubMed]
- Murru, C.; Duvert, L.; Magdinier, F.; Casanova, A.; Alloncle, A.-P.; Testa, S.; Al-Kattan, A. Assessment of laser-synthesized Si nanoparticle effects on myoblast motility, proliferation and differentiation: Towards potential tissue engineering applications. Nanoscale Adv. 2024, 6, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Kuok Ho, D.T. A Review of the Association between Environmental Factors and Athletic Performance. Sport Sci. 2021, 1, 21–30. [Google Scholar]
- Spagnolo, A.; Klug, S.; Schenkl, C.; Schwarzer, M. Links between Exercise Capacity, Exercise Training, and Metabolism. Compr. Physiol. 2023, 13, 5115–5155. [Google Scholar] [PubMed]
- Pourreza, S.; Shahinfar, H.; Bazshahi, E.; Gholami, F.; Djafarian, K.; Shab-Bidar, S. Association of the Mediterranean Dietary Quality Index with handgrip strength and muscle endurance: A cross-sectional study. Food Sci. Nutr. 2022, 10, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Schytz, C.T.; Ørtenblad, N.; Birkholm, T.A.; Plomgaard, P.; Nybo, L.; Kolnes, K.J.; Andersen, O.E.; Lundby, C.; Nielsen, J.; Gejl, K.D. Lowered muscle glycogen reduces body mass with no effect on short-term exercise performance in men. Scand. J. Med. Sci. Sports 2023, 33, 1054–1071. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Rawson, E.S. Nutritional supplements to increase muscle mass. Crit. Rev. Food Sci. Nutr. 1999, 39, 317–328. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M. The Role of Dehydroepiandrosterone (DHEA) in Skeletal Muscle. Vitam. Horm. 2018, 108, 205–221. [Google Scholar]
- Wilson, G.J.; Wilson, J.M.; Manninen, A.H. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review. Nutr. Metab. 2008, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of β-hydroxy-β-methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wax, B.; Kerksick, C.M.; Jagim, A.R.; Mayo, J.J.; Lyons, B.C.; Kreider, R.B. Creatine for Exercise and Sports Performance, with Recovery Considerations for Healthy Populations. Nutrients 2021, 13, 1915. [Google Scholar] [CrossRef]
- Williams, M. Dietary supplements and sports performance: Amino acids. J. Int. Soc. Sports Nutr. 2005, 2, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.T.A.; Ochala, J. Myosin Heavy Chain as a Novel Key Modulator of Striated Muscle Resting State. Physiology 2023, 38, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Wu, C.H.; Huang, W.J.; Lo, Y.M.; Lin, S.X.; Wu, J.S.; Huang, W.C.; Shen, S.C. Alleviative effects of α-lipoic acid on muscle atrophy via the modulation of TNF-α/JNK and PI3K/AKT pathways in high-fat diet and streptozotocin-induced type 2 diabetic rats. Food Sci. Nutr. 2023, 11, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; Rosene, J. Effects of oral creatine and resistance training on myosin heavy chain expression. Med. Sci. Sports Exerc. 2001, 33, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, X.; Chen, D.; Yu, B.; He, J.; Zheng, P.; Luo, Y.; Chen, H.; Yan, H.; Huang, Z. Dihydromyricetin alters myosin heavy chain expression via AMPK signaling pathway in porcine myotubes. Food Funct. 2022, 13, 10525–10534. [Google Scholar] [CrossRef]
- Kim, M.; Sung, B.; Kang, Y.J.; Kim, D.H.; Lee, Y.; Hwang, S.Y.; Yoon, J.H.; Yoo, M.A.; Kim, C.M.; Chung, H.Y.; et al. The combination of ursolic acid and leucine potentiates the differentiation of C2C12 murine myoblasts through the mTOR signaling pathway. Int. J. Mol. Med. 2015, 35, 755–762. [Google Scholar] [CrossRef]
- Phipps, K.R.; Baldwin, N.J.; Lynch, B.; Stannard, D.R.; Šoltésová, A.; Gilby, B.; Mikš, M.H.; Röhrig, C.H. Toxicological safety evaluation of the human-identical milk oligosaccharide 6′-sialyllactose sodium salt. J. Appl. Toxicol. 2019, 39, 1444–1461. [Google Scholar] [CrossRef]
- Kim, J.H.; Yong, S.Y.; Kim, S.H.; Baek, A.; Go, T.H.; Kang, D.R. Randomized, triple-blind, placebo-controlled study to evaluate the safety of 6′-Sialyllactose in healthy adults. Regul. Toxicol. Pharmacol. 2022, 129, 105110. [Google Scholar] [CrossRef] [PubMed]
- Golden, R.; Sutkus, L.; Bauer, L.; Donovan, S.; Dilger, R. Determining the safety and efficacy of dietary supplementation with 3′-sialyllactose or 6′-sialyllactose on growth, tolerance, and brain sialic acid concentrations. Front. Nutr. 2023, 10, 1278804. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, D.; Negro, M.; Arcelli, E.; Marzatico, F. Anti-inflammatory Dietary Interventions and Supplements to Improve Performance during Athletic Training. J. Am. Coll. Nutr. 2015, 34 (Suppl. 1), 62–67. [Google Scholar] [CrossRef] [PubMed]
- Therdyothin, A.; Phiphopthatsanee, N.; Isanejad, M. The Effect of Omega-3 Fatty Acids on Sarcopenia: Mechanism of Action and Potential Efficacy. Mar. Drugs 2023, 21, 399. [Google Scholar] [CrossRef] [PubMed]
- Farnfield, M.M.; Carey, K.A.; Gran, P.; Trenerry, M.K.; Cameron-Smith, D. Whey protein ingestion activates mTOR-dependent signalling after resistance exercise in young men: A double-blinded randomized controlled trial. Nutrients 2009, 1, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Yoshida, N.; Maekawa, D.; Kitakaze, T.; Kobayashi, Y.; Kitano, T.; Fujita, T.; Okuwa-Hayashi, H.; Harada, N.; Nakano, Y.; et al. 5-Hydroxy-7-methoxyflavone derivatives from Kaempferia parviflora induce skeletal muscle hypertrophy. Food Sci. Nutr. 2018, 7, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castellanos, B.; Martínez-López, P.; Bailón-Moreno, R.; Esquius, L. Effect of Ginseng Intake on Muscle Damage Induced by Exercise in Healthy Adults. Nutrients 2024, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Cochet, C.; Belloni, G.; Buondonno, I.; Chiara, F.; D’Amelio, P. The Role of Nutrition in the Treatment of Sarcopenia in Old Patients: From Restoration of Mitochondrial Activity to Improvement of Muscle Performance, a Systematic Review. Nutrients 2023, 15, 3730. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, S.; Kim, G.; Shin, H.J.; Lee, E.J.; Lee, C.S.; Yoon, S.; Lee, E.; Lim, A.; Kim, S.H. Ameliorating effect of 2′-Fucosyllactose and 6′-Sialyllactose on lipopolysaccharide-induced intestinal inflammation. J. Dairy. Sci. 2024, 107, 4147–4160. [Google Scholar] [CrossRef]
- Arellano Spadaro, J.; Hishida, Y.; Matsunaga, Y.; van Es-Remers, M.; Korthout, H.; Kim, H.K.; Poppelaars, E.; Keizer, H.; Iliopoulou, E.; van Duijn, B.; et al. 3′sialyllactose and 6′sialyllactose enhance performance in endurance-type exercise through metabolic adaptation. Food Sci. Nutr. 2023, 11, 6199–6212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).