The Effects of Caloric Restriction and Clinical Psychological Intervention on the Interplay of Gut Microbial Composition and Stress in Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Intervention Groups
2.2. Clinical Variables and Questionnaires
2.3. Biological Specimen Sampling
2.4. 16S rRNA Sequencing and Microbial Data Processing
2.5. Statistical Analysis
2.5.1. Description of Microbial Composition
2.5.2. Diversity Indices
2.5.3. Longitudinal Changes of Taxa
2.5.4. Multilevel Dimension-Reduction Analyses
3. Results
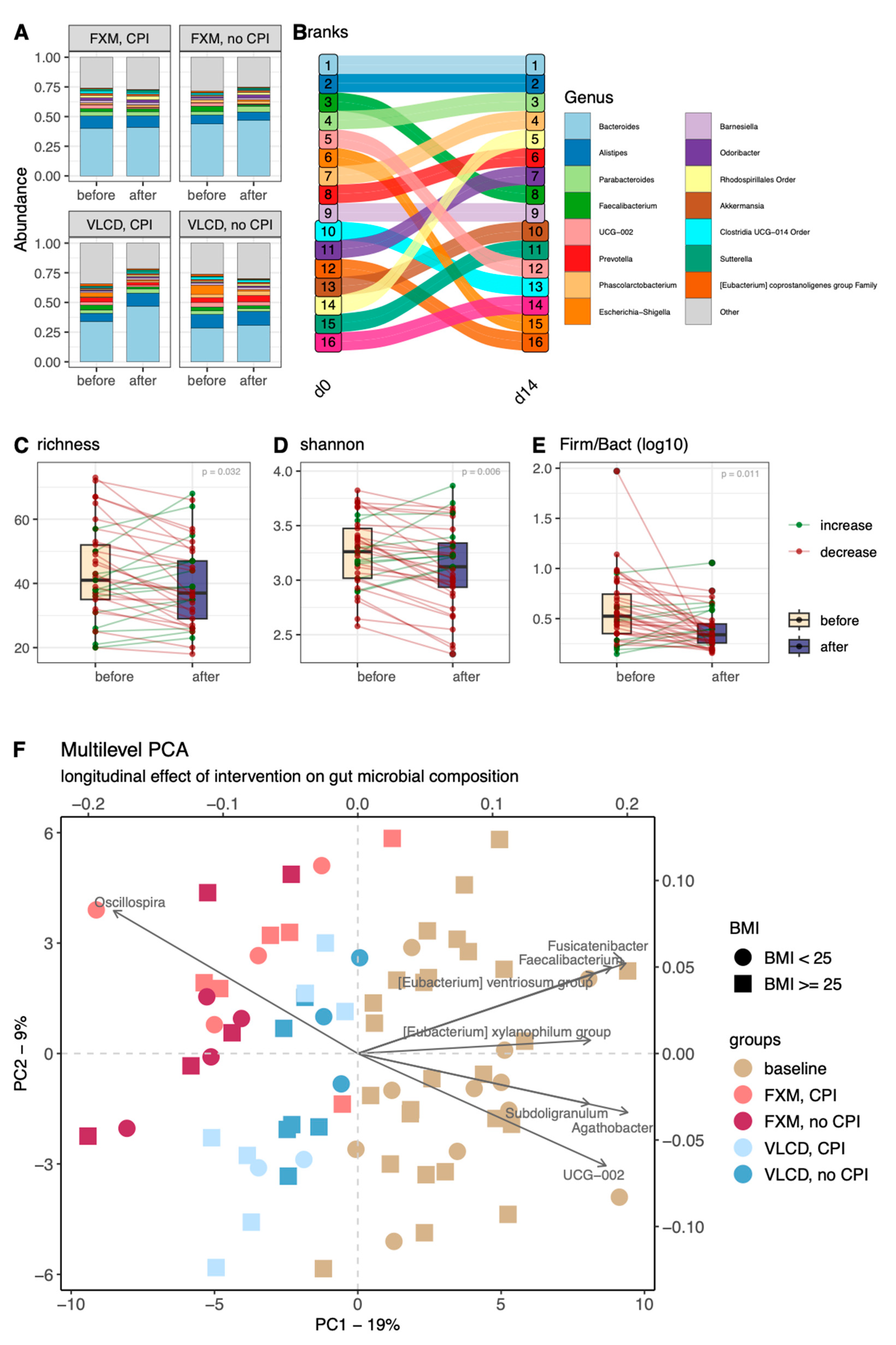
3.1. Microbial Composition
3.2. Diversity Indices
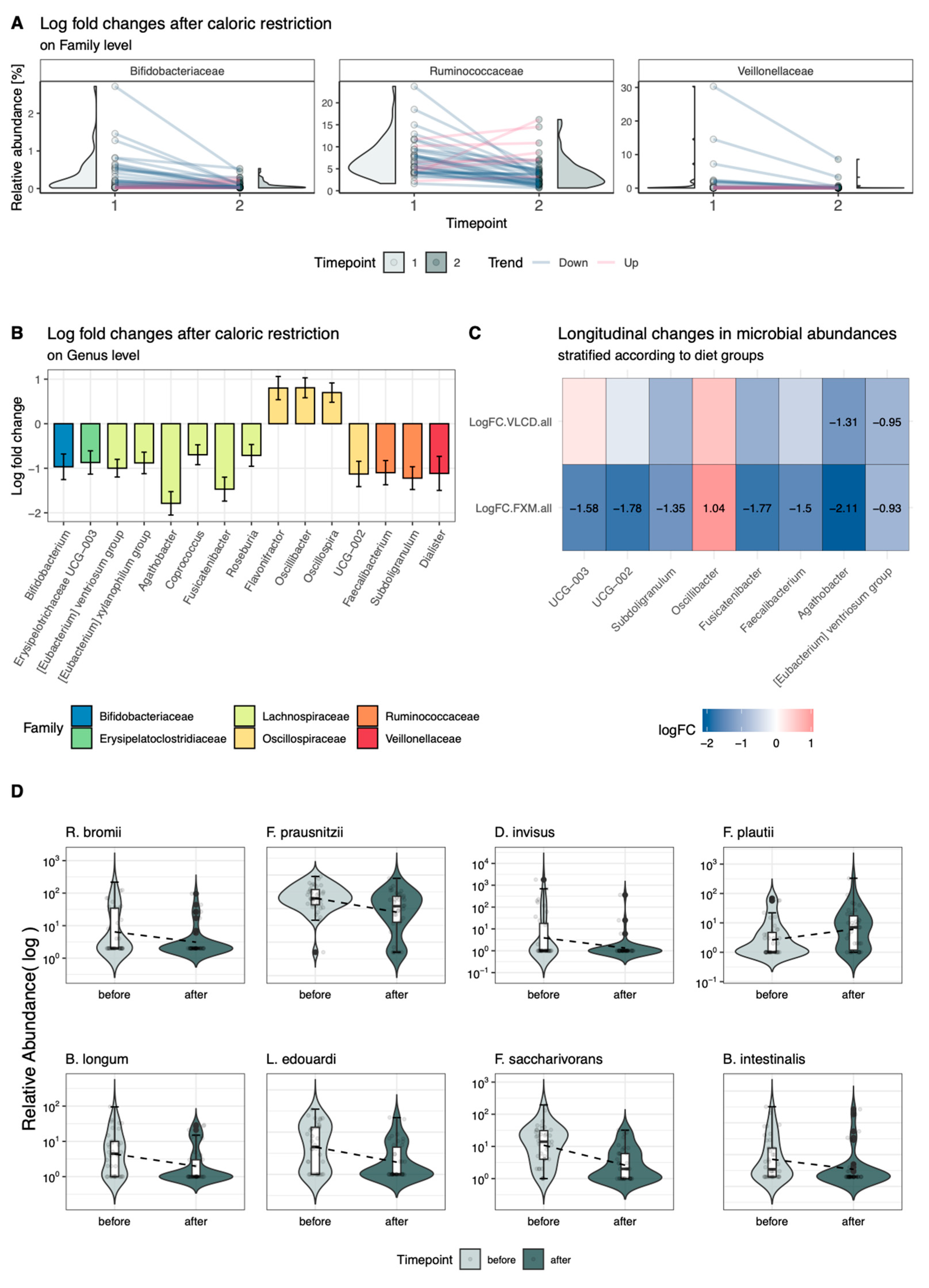
3.3. Longitudinal Changes in Relative Abundance
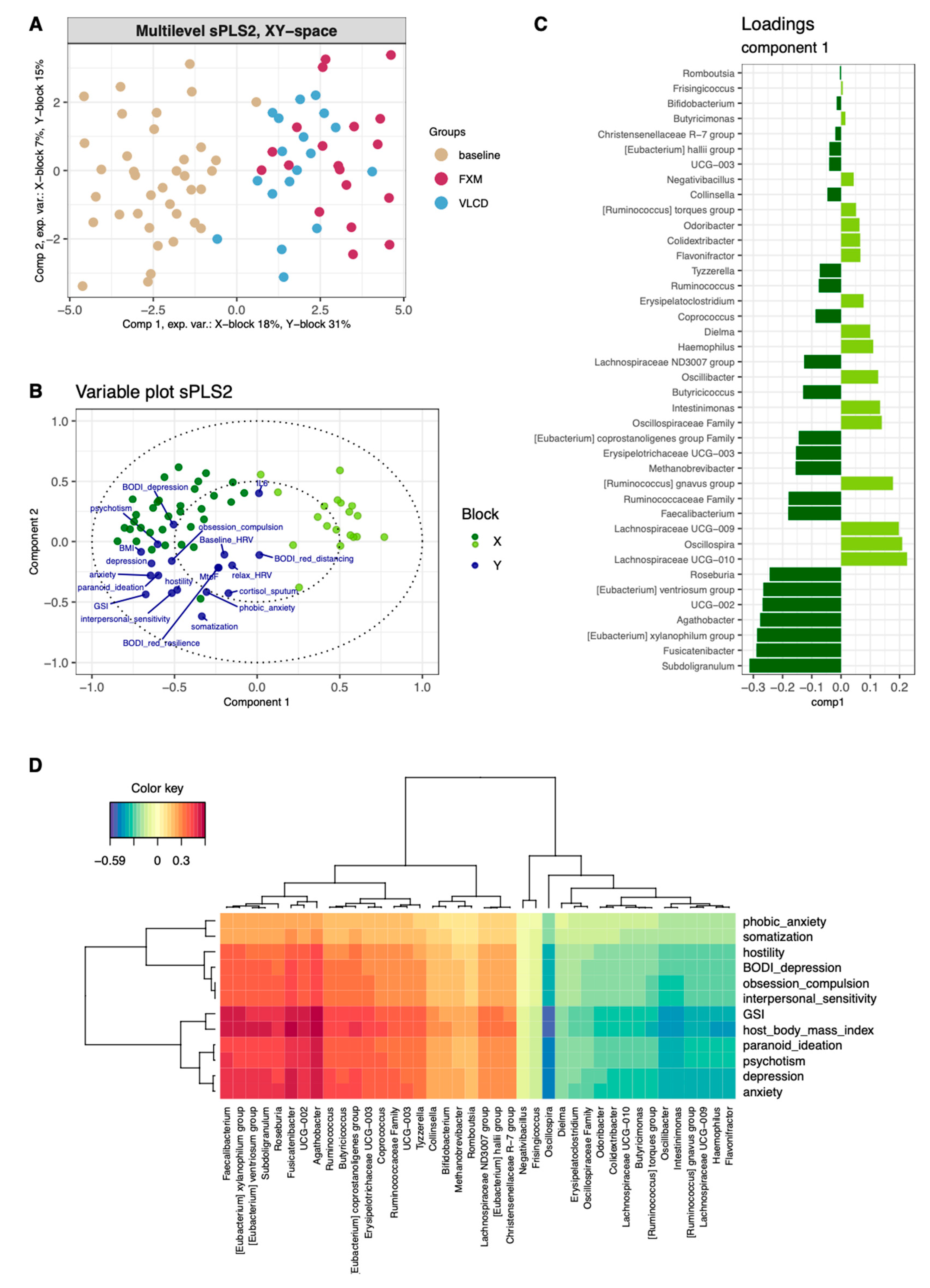
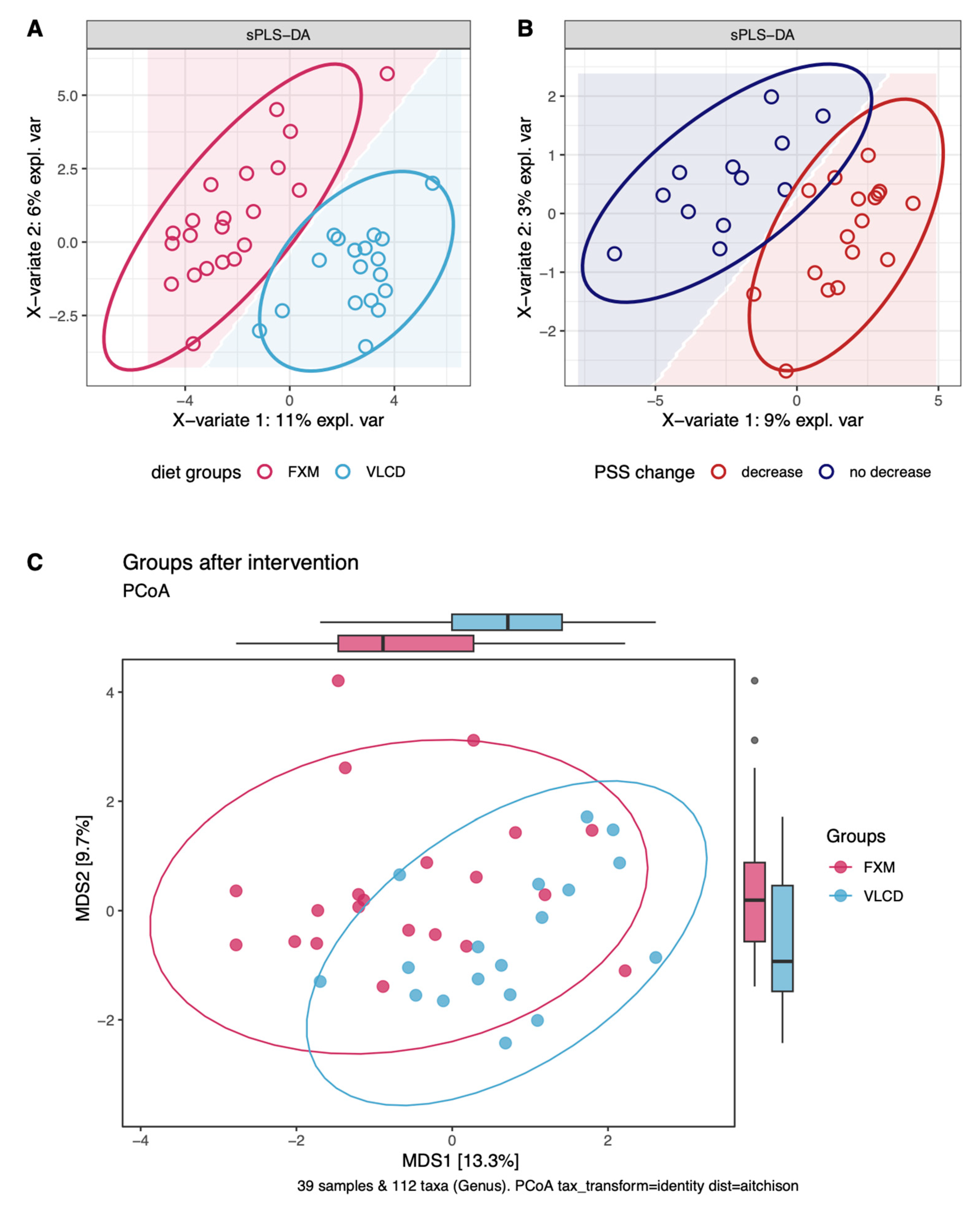
3.4. Multilevel Dimension-Reduction Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moulton, C.D.; Pickup, J.C.; Ismail, K. The link between depression and diabetes: The search for shared mechanisms. Lancet Diabetes Endocrinol. 2015, 3, 461–471. [Google Scholar] [CrossRef]
- Leutner, M.; Dervic, E.; Bellach, L.; Klimek, P.; Thurner, S.; Kautzky, A. Obesity as pleiotropic risk state for metabolic and mental health throughout life. Transl. Psychiatry 2023, 13, 175. [Google Scholar] [CrossRef]
- Naicker, K.; Johnson, J.A.; Skogen, J.C.; Manuel, D.; Øverland, S.; Sivertsen, B.; Colman, I. Type 2 Diabetes and Comorbid Symptoms of Depression and Anxiety: Longitudinal Associations with Mortality Risk. Diabetes Care 2017, 40, 352–358. [Google Scholar] [CrossRef]
- Serretti, A.; Mandelli, L. Antidepressants and body weight: A comprehensive review and meta-analysis. J. Clin. Psychiatry 2010, 71, 1259–1272. [Google Scholar] [CrossRef]
- Taheri, S.; Zaghloul, H.; Chagoury, O.; Elhadad, S.; Ahmed, S.H.; El Khatib, N.; Amona, R.A.; El Nahas, K.; Suleiman, N.; Alnaama, A.; et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): An open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 477–489. [Google Scholar] [CrossRef]
- LaRose, J.G.; Leahey, T.M.; Lanoye, A.; Bean, M.K.; Fava, J.L.; Tate, D.F.; Evans, R.K.; Wickham, E.P.; Henderson, M.M. Effect of a Lifestyle Intervention on Cardiometabolic Health among Emerging Adults: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2231903. [Google Scholar] [CrossRef]
- Bourke, M.; Patten, R.K.; Dash, S.; Pascoe, M.; Craike, M.; Firth, J.; Bailey, A.; Jacka, F.; Parker, A.G. The Effect of Interventions That Target Multiple Modifiable Health Behaviors on Symptoms of Anxiety and Depression in Young People: A Meta-Analysis of Randomized Controlled Trials. J. Adolesc. Health 2022, 70, 208–219. [Google Scholar] [CrossRef]
- Velten, J.; Bieda, A.; Scholten, S.; Wannemüller, A.; Margraf, J. Lifestyle choices and mental health: A longitudinal survey with German and Chinese students. BMC Public Health 2018, 18, 632. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef]
- Aziz, T.; Khan, A.A.; Tzora, A.; Voidarou, C.C.; Skoufos, I. Dietary Implications of the Bidirectional Relationship between the Gut Microflora and Inflammatory Diseases with Special Emphasis on Irritable Bowel Disease: Current and Future Perspective. Nutrients 2023, 15, 2956. [Google Scholar] [CrossRef]
- Wu, W.L.; Adame, M.D.; Liou, C.W.; Barlow, J.T.; Lai, T.T.; Sharon, G.; Schretter, C.E.; Needham, B.D.; Wang, M.I.; Tang, W.; et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature 2021, 595, 409–414. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255-e119. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Djekic, D.; Shi, L.; Brolin, H.; Carlsson, F.; Särnqvist, C.; Savolainen, O.; Cao, Y.; Bäckhed, F.; Tremaroli, V.; Landberg, R.; et al. Effects of a Vegetarian Diet on Cardiometabolic Risk Factors, Gut Microbiota, and Plasma Metabolome in Subjects with Ischemic Heart Disease: A Randomized, Crossover Study. J. Am. Heart Assoc. 2020, 9, e016518. [Google Scholar] [CrossRef]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Anderson, E.M.; Rozowsky, J.M.; Fazzone, B.J.; Schmidt, E.A.; Stevens, B.R.; O’Malley, K.A.; Scali, S.T.; Berceli, S.A. Temporal Dynamics of the Intestinal Microbiome Following Short-Term Dietary Restriction. Nutrients 2022, 14, 2785. [Google Scholar] [CrossRef] [PubMed]
- Kautzky, A.; Heneis, K.; Stengg, K.; Fröhlich, S.; Kautzky-Willer, A. Short Term Caloric Restriction and Biofeedback Enhance Psychological Wellbeing and Reduce Overweight in Healthy Women. J. Pers. Med. 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, M.A.; Frank, D.N.; Borengasser, S.J.; Ostendorf, D.M.; Ir, D.; Jambal, P.; Bing, K.; Wayland, L.; Siebert, J.C.; Bessesen, D.H.; et al. The Gut Microbiota during a Behavioral Weight Loss Intervention. Nutrients 2021, 13, 3248. [Google Scholar] [CrossRef] [PubMed]
- von Schwartzenberg, R.J.; Bisanz, J.E.; Lyalina, S.; Spanogiannopoulos, P.; Ang, Q.Y.; Cai, J.; Dickmann, S.; Friedrich, M.; Liu, S.Y.; Collins, S.L.; et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature 2021, 595, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Pyndt Jørgensen, B.; Winther, G.; Kihl, P.; Nielsen, D.S.; Wegener, G.; Hansen, A.K.; Sørensen, D.B. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015, 27, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Winther, G.; Pyndt Jørgensen, B.M.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sørensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Trapani, V.; Petito, V.; Reddel, S.; Pietropaolo, G.; Graziani, C.; Masi, L.; Gasbarrini, A.; Putignani, L.; Scaldaferri, F.; et al. Dietary Magnesium Alleviates Experimental Murine Colitis through Modulation of Gut Microbiota. Nutrients 2021, 13, 4188. [Google Scholar] [CrossRef] [PubMed]
- Xenaki, N.; Bacopoulou, F.; Kokkinos, A.; Nicolaides, N.C.; Chrousos, G.P.; Darviri, C. Impact of a stress management program on weight loss, mental health and lifestyle in adults with obesity: A randomized controlled trial. J. Mol. Biochem. 2018, 7, 78–84. [Google Scholar]
- Ilan, K.; Motro, Y.; Nemirovsky, A.; Schwartz, D.; Goren, G.; Sergienko, R.; Greenberg, D.; Slonim-Nevo, V.; Sarid, O.; Friger, M.; et al. Cognitive behavioral and mindfulness with daily exercise intervention is associated with changes in intestinal microbial taxa and systemic inflammation in patients with Crohn’s disease. Gut Microbes 2024, 16, 2337269. [Google Scholar] [CrossRef]
- Kautzky, A.; Heneis, K.; Stengg, K.; Fröhlich, S.; Kautzky-Willer, A. Biological and Psychological Stress Correlates Are Linked to Glucose Metabolism, Obesity, and Gender Roles in Women. Neuroendocrinology 2022, 112, 130–142. [Google Scholar] [CrossRef]
- Sheldon Cohen, T.K.a.R.M. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Derogatis, L.R.; Spencer, P.M. Brief Symptom Inventory (BSI): Administration, Scoring and Procedures Manual; National Computer Systems: Minneapolis, MN, USA, 1993. [Google Scholar]
- Scheibenbogen, O.; Andorfer, U.; Kuderer, M.; Musalek, M. Prävalenz des Burnout-Syndroms in Österreich: Verlaufsformen und Relevante Präventions- und Behandlungsstrategien; Verfügbar unter: Vienna, Austria, 2017. [Google Scholar]
- Pjevac, P.; Hausmann, B.; Schwarz, J.; Kohl, G.; Herbold, C.W.; Loy, A.; Berry, D. An Economical and Flexible Dual Barcoding, Two-Step PCR Approach for Highly Multiplexed Amplicon Sequencing. Front. Microbiol. 2021, 12, 669776. [Google Scholar] [CrossRef]
- Gloor, G.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome Datasets Are Compositional: And This Is Not Optional. Front. Microbiol. 2017, 8, 2224. [Google Scholar] [CrossRef] [PubMed]
- Kim-Anh Lê Cao, Z.M.W. Multivariate Data Integration Using R: Methods and Applications with the mixOmics Package, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Manchishi, S.M.; Cui, R.J.; Zou, X.H.; Cheng, Z.Q.; Li, B.J. Effect of caloric restriction on depression. J. Cell. Mol. Med. 2018, 22, 2528–2535. [Google Scholar] [CrossRef] [PubMed]
- Tanca, A.; Abbondio, M.; Palomba, A.; Fraumene, C.; Marongiu, F.; Serra, M.; Pagnozzi, D.; Laconi, E.; Uzzau, S. Caloric restriction promotes functional changes involving short-chain fatty acid biosynthesis in the rat gut microbiota. Sci. Rep. 2018, 8, 14778. [Google Scholar] [CrossRef]
- Fabbiano, S.; Suárez-Zamorano, N.; Chevalier, C.; Lazarević, V.; Kieser, S.; Rigo, D.; Leo, S.; Veyrat-Durebex, C.; Gaïa, N.; Maresca, M.; et al. Functional Gut Microbiota Remodeling Contributes to the Caloric Restriction-Induced Metabolic Improvements. Cell Metab. 2018, 28, 907–921.e907. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Li, Q.; Song, C.; Han, N.; Yan, T.; Zhang, L.; Ren, D.; Zhao, Y.; Yang, X. Caloric Restriction, Friend or Foe: Effects on Metabolic Status in Association with the Intestinal Microbiome and Metabolome. J. Agric. Food Chem. 2022, 70, 14061–14072. [Google Scholar] [CrossRef]
- Jie, Z.; Yu, X.; Liu, Y.; Sun, L.; Chen, P.; Ding, Q.; Gao, Y.; Zhang, X.; Yu, M.; Zhang, Y.; et al. The Baseline Gut Microbiota Directs Dieting-Induced Weight Loss Trajectories. Gastroenterology 2021, 160, 2029–2042.e2016. [Google Scholar] [CrossRef]
- Louis, S.; Tappu, R.M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N.; et al. A High Protein Calorie Restriction Diet Alters the Gut Microbiome in Obesity. Nutrients 2020, 12, 3221. [Google Scholar] [CrossRef]
- Heinsen, F.A.; Fangmann, D.; Müller, N.; Schulte, D.M.; Rühlemann, M.C.; Türk, K.; Settgast, U.; Lieb, W.; Baines, J.F.; Schreiber, S.; et al. Beneficial Effects of a Dietary Weight Loss Intervention on Human Gut Microbiome Diversity and Metabolism Are Not Sustained during Weight Maintenance. Obes. Facts 2016, 9, 379–391. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Büttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D.; et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef]
- Hu, X.; Xia, K.; Dai, M.; Han, X.; Yuan, P.; Liu, J.; Liu, S.; Jia, F.; Chen, J.; Jiang, F.; et al. Intermittent fasting modulates the intestinal microbiota and improves obesity and host energy metabolism. npj Biofilms Microbiomes 2023, 9, 19. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. A Beneficial Gut Organism from the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Li, C.; Stražar, M.; Mohamed, A.M.T.; Pacheco, J.A.; Walker, R.L.; Lebar, T.; Zhao, S.; Lockart, J.; Dame, A.; Thurimella, K.; et al. Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria. Cell 2024, 187, 1834–1852.e1819. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS ONE 2017, 12, e0179739. [Google Scholar] [CrossRef]
- Yuan, R.; Yang, L.; Yao, G.; Geng, S.; Ge, Q.; Bo, S.; Li, X. Features of gut microbiota in patients with anorexia nervosa. Chin. Med. J. 2022, 135, 1993–2002. [Google Scholar] [CrossRef]
- Thomann, A.K.; Wüstenberg, T.; Wirbel, J.; Knoedler, L.L.; Thomann, P.A.; Zeller, G.; Ebert, M.P.; Lis, S.; Reindl, W. Depression and fatigue in active IBD from a microbiome perspective-a Bayesian approach to faecal metagenomics. BMC Med. 2022, 20, 366. [Google Scholar] [CrossRef]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Huber-Ruano, I.; Calvo, E.; Mayneris-Perxachs, J.; Rodríguez-Peña, M.M.; Ceperuelo-Mallafré, V.; Cedó, L.; Núñez-Roa, C.; Miro-Blanch, J.; Arnoriaga-Rodríguez, M.; Balvay, A.; et al. Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate. Microbiome 2022, 10, 135. [Google Scholar] [CrossRef]
- Rong, H.; Xie, X.H.; Zhao, J.; Lai, W.T.; Wang, M.B.; Xu, D.; Liu, Y.H.; Guo, Y.Y.; Xu, S.X.; Deng, W.F.; et al. Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J. Psychiatr. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Halverson, T.; Alagiakrishnan, K. Gut microbes in neurocognitive and mental health disorders. Ann. Med. 2020, 52, 423–443. [Google Scholar] [CrossRef]
- Cutler, A.J.; Mattingly, G.W.; Maletic, V. Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder. Transl. Psychiatry 2023, 13, 228. [Google Scholar] [CrossRef]
- Gao, M.; Wang, J.; Liu, P.; Tu, H.; Zhang, R.; Zhang, Y.; Sun, N.; Zhang, K. Gut microbiota composition in depressive disorder: A systematic review, meta-analysis, and meta-regression. Transl. Psychiatry 2023, 13, 379. [Google Scholar] [CrossRef]
- Amin, N.; Liu, J.; Bonnechere, B.; MahmoudianDehkordi, S.; Arnold, M.; Batra, R.; Chiou, Y.J.; Fernandes, M.; Ikram, M.A.; Kraaij, R.; et al. Interplay of Metabolome and Gut Microbiome in Individuals with Major Depressive Disorder vs Control Individuals. JAMA Psychiatry 2023, 80, 597–609. [Google Scholar] [CrossRef]
- Zeamer, A.L.; Salive, M.C.; An, X.; Beaudoin, F.L.; House, S.L.; Stevens, J.S.; Zeng, D.; Neylan, T.C.; Clifford, G.D.; Linnstaedt, S.D.; et al. Association between microbiome and the development of adverse posttraumatic neuropsychiatric sequelae after traumatic stress exposure. Transl. Psychiatry 2023, 13, 354. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Maltz, R.M.; Keirsey, J.; Kim, S.C.; Mackos, A.R.; Gharaibeh, R.Z.; Moore, C.C.; Xu, J.; Somogyi, A.; Bailey, M.T. Social Stress Affects Colonic Inflammation, the Gut Microbiome, and Short-chain Fatty Acid Levels and Receptors. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 533–540. [Google Scholar] [CrossRef]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Chen, T.; Wang, X.; Zhang, X. Akkermansia muciniphila Improves Depressive-Like Symptoms by Modulating the Level of 5-HT Neurotransmitters in the Gut and Brain of Mice. Mol. Neurobiol. 2023, 61, 821–834. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- García-Legorreta, A.; Soriano-Pérez, L.A.; Flores-Buendía, A.M.; Medina-Campos, O.N.; Noriega, L.G.; Granados-Portillo, O.; Nambo-Venegas, R.; Tovar, A.R.; Mendoza-Vargas, A.; Barrera-Oviedo, D.; et al. Effect of Dietary Magnesium Content on Intestinal Microbiota of Rats. Nutrients 2020, 12, 2889. [Google Scholar] [CrossRef]
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.D.; Delzenne, N.M.; Muccioli, G.; Clément, K.; Cani, P.D. From correlation to causality: The case of Subdoligranulum. Gut Microbes 2020, 12, 1849998. [Google Scholar] [CrossRef]
| Variable | VLCD | FXM | p-Value |
|---|---|---|---|
| Age [years], mean ± SD | 53.5 ± 10.82 | 53.55 ± 13.48 | 0.99 a |
| Peri/postmenopause, n (%) | 14 (78%) | 14 (70%) | 0.11 d |
| BMI [kg/m2], mean ± SD | 28.69 ± 5.93 | 27.43 ± 5.93 | 0.47 b |
| CRP [mg/dL], mean ± SD | 0.51 ± 0.49 | 0.28 ± 0.36 | 0.06 b |
| Cortisol in sputum [µg/dL], mean ± SD | 0.46 ± 0.34 | 0.54 ± 0.32 | 0.23 b |
| IL6 [pg/mL], median ± IQR | 1.95 ± 3.32 | 1.87 ± 1.95 | 0.80 c |
| GSI, mean ± SD | 54.06 ± 9.84 | 57.5 ± 10.72 | 0.32 a |
| PSS, mean ± SD | 13.33 ± 3.73 | 16.94 ± 5.07 | 0.04 a |
| MtoF, mean ± SD | 1.11 ± 0.24 | 1.09 ± 0.24 | 0.86 b |
| BODI: dysfunctional compensation, median ± IQR | 62 ± 96.5 | 45 ± 78 | 0.48 c |
| BODI: reduced resilience, mean ± SD | 25.18 ± 15.3 | 35.95 ± 19.41 | 0.09 a |
| BODI: depression, median ± IQR | 33.2 ± 22.9 | 26.9 ± 27.7 | 0.30 c |
| BODI: reduced distancing, median ± IQR | 69.7 ± 35.73 | 53.8 ± 56.4 | 0.66 c |
| Baseline HRV [ms], mean ± SD | 12.21 ± 13.71 | 15.52 ± 13.59 | 0.36 b |
| Relaxed HRV [ms], mean ± SD | 16.07 ± 14.59 | 16.3 ± 9.74 | 0.62 b |
| Stressed HRV [ms], mean ± SD | 16.35 ± 16.12 | 17.03 ± 11 | 0.71 b |
| BSI: obsession-compulsion, mean ± SD | 52.76 ± 9.09 | 55.85 ± 11.76 | 0.38 a |
| BSI: phobic anxiety, median ± IQR | 45 ± 10 | 55 ± 16 | 0.03 c |
| BSI: interpersonal sensitivity, mean ± SD | 53.47 ± 9.64 | 55.35 ± 9.54 | 0.56 a |
| BSI: psychotism, median ± IQR | 54 ± 10 | 54 ± 2.5 | 0.58 c |
| BSI: hostility, mean ± SD | 53.94 ± 11.7 | 52.7 ± 10.56 | 0.74 a |
| BSI: paranoid ideation, mean ± SD | 51.24 ± 8.91 | 56.1 ± 8.23 | 0.10 a |
| BSI: anxiety, mean ± SD | 54.53 ± 8.95 | 55.15 ± 12.42 | 0.86 a |
| BSI: depression, median ± IQR | 55 ± 17 | 55 ± 8.75 | 0.33 c |
| BSI: somatization, mean ± SD | 54.18 ± 9.93 | 57.5 ± 10.56 | 0.33 a |
| Model | R2 | p.adj |
|---|---|---|
| time | 0.031 | <0.05 |
| time * CPI | 0.047 | n.s. |
| time * diet | 0.079 | <0.05 |
| time * diet + CPI | 0.09 | <0.05 |
| time * diet + CPI + age | 0.104 | <0.05 |
| time * diet + CPI + age + BMI | 0.12 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellach, L.; Kautzky-Willer, A.; Heneis, K.; Leutner, M.; Kautzky, A. The Effects of Caloric Restriction and Clinical Psychological Intervention on the Interplay of Gut Microbial Composition and Stress in Women. Nutrients 2024, 16, 2584. https://doi.org/10.3390/nu16162584
Bellach L, Kautzky-Willer A, Heneis K, Leutner M, Kautzky A. The Effects of Caloric Restriction and Clinical Psychological Intervention on the Interplay of Gut Microbial Composition and Stress in Women. Nutrients. 2024; 16(16):2584. https://doi.org/10.3390/nu16162584
Chicago/Turabian StyleBellach, Luise, Alexandra Kautzky-Willer, Kathrin Heneis, Michael Leutner, and Alexander Kautzky. 2024. "The Effects of Caloric Restriction and Clinical Psychological Intervention on the Interplay of Gut Microbial Composition and Stress in Women" Nutrients 16, no. 16: 2584. https://doi.org/10.3390/nu16162584
APA StyleBellach, L., Kautzky-Willer, A., Heneis, K., Leutner, M., & Kautzky, A. (2024). The Effects of Caloric Restriction and Clinical Psychological Intervention on the Interplay of Gut Microbial Composition and Stress in Women. Nutrients, 16(16), 2584. https://doi.org/10.3390/nu16162584








