Maternal Fiber Intake and Perinatal Depression and Anxiety
Abstract
1. Introduction
1.1. Perinatal Depression and Anxiety (PDA)
1.2. Gut Microbiome and Mental Health
1.3. Diet, Microbiome and Mental Health
1.4. Microbiome in Pregnancy
1.5. Gestational Diet, Microbiome, and PDA
1.6. Study Objective
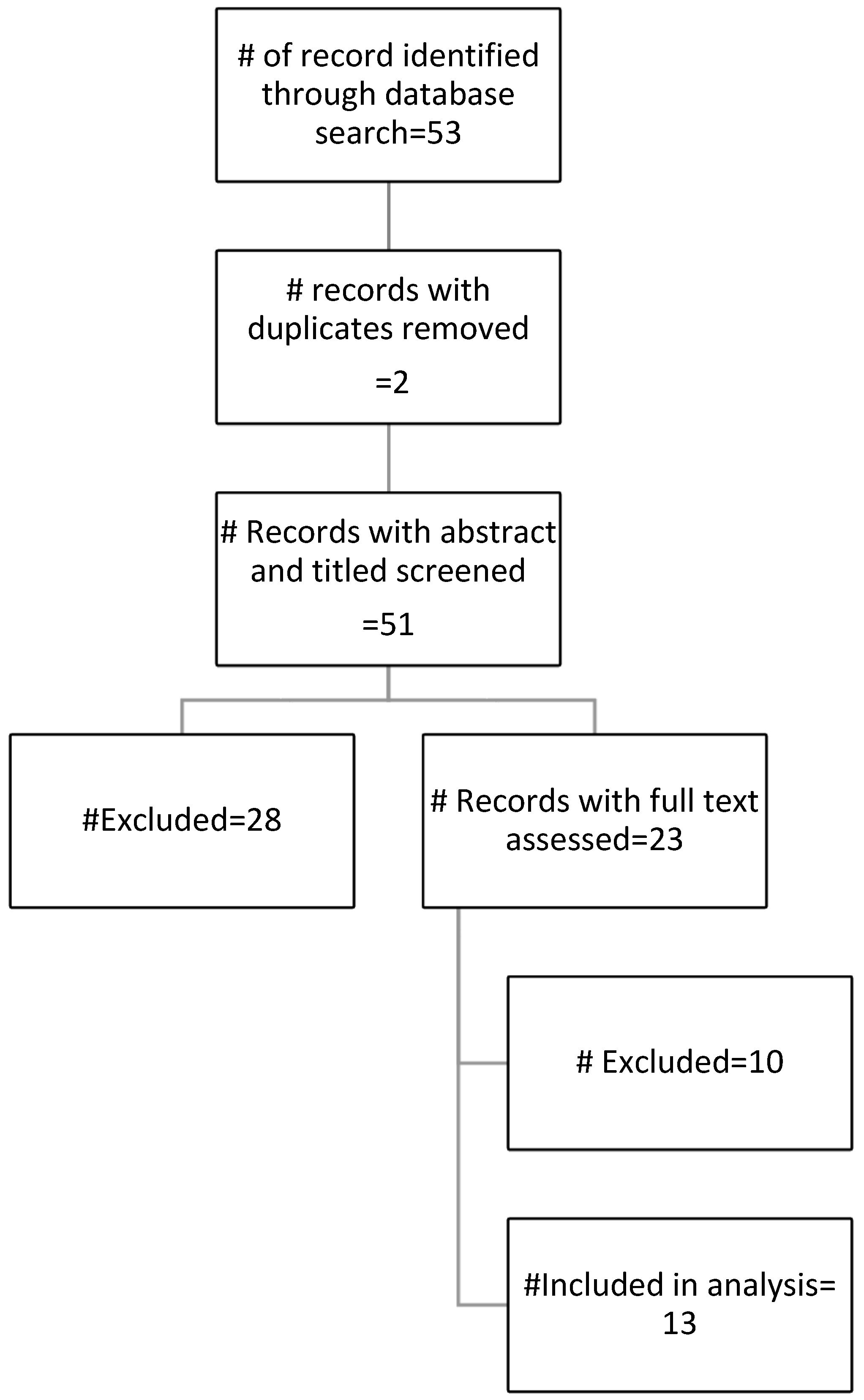
2. Materials and Methods
2.1. Determining Typical Serving Size (TSS)
2.2. Calculating Fiber Scores (FS)
2.3. Calculating Final Fiber Ranks (FFR)
2.4. Consumption Ranking of Highest Fiber FGs in Each DP
2.5. Simplifying Mental Health Outcomes
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Liu, J.; Shuai, H.; Cai, Z.; Fu, X.; Liu, Y.; Xiao, X.; Zhang, W.; Krabbendam, E.; Liu, S.; et al. Correction: Mapping global prevalence of depression among postpartum women. Transl. Psychiatry 2021, 11, 640. [Google Scholar] [CrossRef]
- Government of Canada SC. The Daily—Maternal Mental Health in Canada, 2018/2019. Published 24 June 2019. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/190624/dq190624b-eng.htm (accessed on 6 February 2024).
- Bowen, A.; Muhajarine, N. Prevalence of Antenatal Depression in Women Enrolled in an Outreach Program in Canada. J. Obstet. Gynecol. Neonatal Nurs. 2006, 35, 491–498. [Google Scholar] [CrossRef]
- Gheorghe, M.; Varin, M.; Wong, S.L.; Baker, M.; Grywacheski, V.; Orpana, H. Symptoms of postpartum anxiety and depression among women in Canada: Findings from a national cross-sectional survey. Can. J. Public Health 2021, 112, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Falah-Hassani, K.; Shiri, R.; Dennis, C.-L. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol. Med. 2017, 47, 2041–2053. [Google Scholar] [CrossRef]
- Earls, M.F.; The Committee on Psychosocial Aspects of Child and Family Health. Incorporating Recognition and Management of Perinatal and Postpartum Depression Into Pediatric Practice. Pediatrics 2010, 126, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Slomian, J.; Honvo, G.; Emonts, P.; Reginster, J.-Y.; Bruyère, O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Women’s Health 2019, 15, 1745506519844044. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Tien, Y.; Bai, Y.-S.; Lin, C.-K.; Yin, C.-S.; Chung, C.-H.; Sun, C.-A.; Huang, S.-H.; Huang, Y.-C.; Chien, W.-C.; et al. Association of Postpartum Depression with Maternal Suicide: A Nationwide Population-Based Study. Int. J. Environ. Res. Public Health 2022, 19, 5118. [Google Scholar] [CrossRef] [PubMed]
- Leight, K.; Fitelson, E.; Kim, S.; Baker, A.S. Treatment of post-partum depression: A review of clinical, psychological and pharmacological options. Int. J. Women’s Health 2010, 3, 1–14. [Google Scholar] [CrossRef]
- Chabrol, H.; Teissedre, F.; Armitage, J.; Danel, M.; Walburg, V. Acceptability of psychotherapy and antidepressants for postnatal depression among newly delivered mothers. J. Reprod. Infant Psychol. 2004, 22, 5–12. [Google Scholar] [CrossRef]
- Grzeskowiak, L.E.; Saha, M.R.; Nordeng, H.; Ystrom, E.; Amir, L.H. Perinatal antidepressant use and breastfeeding outcomes: Findings from the Norwegian Mother, Father and Child Cohort Study. Acta Obstet. Gynecol. Scand. 2022, 101, 344–354. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Probiotic in Pregnancy Study Group. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Desai, V.; Kozyrskyj, A.L.; Lau, S.; Sanni, O.; Dennett, L.; Walter, J.; Ospina, M.B. Effectiveness of Probiotic, Prebiotic, and Synbiotic Supplementation to Improve Perinatal Mental Health in Mothers: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 622181. [Google Scholar] [CrossRef]
- Mörkl, S.; Wagner-Skacel, J.; Lahousen, T.; Lackner, S.; Holasek, S.J.; Bengesser, S.A.; Painold, A.; Holl, A.K.; Reininghaus, E. The Role of Nutrition and the Gut-Brain Axis in Psychiatry: A Review of the Literature. Neuropsychobiology 2020, 79, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Zhang, X.; Yu, Z.-H.; Zhang, Z.; Deng, M.; Zhao, J.-H.; Ruan, B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Cherak, S.J.; Fiest, K.M.; VanderSluis, L.; Basualdo-Hammond, C.; Lorenzetti, D.L.; Buhler, S.; Stadnyk, J.; Driedger, L.; Hards, L.; Gramlich, L.; et al. Nutrition interventions in populations with mental health conditions: A scoping review. Appl. Physiol. Nutr. Metab. 2020, 45, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Aucoin, M.; LaChance, L.; Naidoo, U.; Remy, D.; Shekdar, T.; Sayar, N.; Cardozo, V.; Rawana, T.; Chan, I.; Cooley, K. Diet and Anxiety: A Scoping Review. Nutrients 2021, 13, 4418. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef]
- Ramezani, F.; Pourghazi, F.; Eslami, M.; Gholami, M.; Khonsari, N.M.; Ejtahed, H.-S.; Larijani, B.; Qorbani, M. Dietary fiber intake and all-cause and cause-specific mortality: An updated systematic review and meta-analysis of prospective cohort studies. Clin. Nutr. 2024, 43, 65–83. [Google Scholar] [CrossRef]
- Karlsson, F.; Tremaroli, V.; Nielsen, J.; Bäckhed, F. Assessing the Human Gut Microbiota in Metabolic Diseases. Diabetes 2013, 62, 3341–3349. [Google Scholar] [CrossRef]
- Gorczyca, K.; Obuchowska, A.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Changes in the Gut Microbiome and Pathologies in Pregnancy. Int. J. Environ. Res. Public Health 2022, 19, 9961. [Google Scholar] [CrossRef]
- Pantazi, A.C.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Lupu, V.V.; Kassim, M.A.K.; Mihai, L.; Frecus, C.E.; Chirila, S.I.; Lupu, A.; et al. Development of Gut Microbiota in the First 1000 Days after Birth and Potential Interventions. Nutrients 2023, 15, 3647. [Google Scholar] [CrossRef]
- Chia, A.-R.; Chen, L.-W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F.-F. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2019, 10, 685–695. [Google Scholar] [CrossRef]
- Sikorski, C.; Yang, S.; Stennett, R.; Miller, V.; Teo, K.; Anand, S.S.; Paré, G.; Yusuf, S.; Dehghan, M.; Mente, A. Changes in energy, macronutrient, and food consumption in 47 countries over the last 70 years (1950–2019): A systematic review and meta-analysis. Nutrition 2023, 108, 111941. [Google Scholar] [CrossRef]
- Canada, H. Nutrition Labelling: Nutrition Facts Table. Published 26 July 2004. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/nutrition-labelling/nutrition-facts-tables.html (accessed on 7 March 2024).
- Poutanen, K.S.; Fiszman, S.; Marsaux, C.F.M.; Pentikäinen, S.P.; E Steinert, R.; Mela, D.J. Recommendations for characterization and reporting of dietary fibers in nutrition research. Am. J. Clin. Nutr. 2018, 108, 437–444. [Google Scholar] [CrossRef]
- Smith, M.R.; Micha, R.; Golden, C.D.; Mozaffarian, D.; Myers, S.S. Global Expanded Nutrient Supply (GENuS) Model: A New Method for Estimating the Global Dietary Supply of Nutrients. PLoS ONE 2016, 11, e0146976. [Google Scholar] [CrossRef]
- Vilela, A.A.F.; Pinto, T.d.J.; Rebelo, F.; Benaim, C.; Lepsch, J.; Dias-Silva, C.H.; Castro, M.B.T.; Kac, G. Association of Prepregnancy Dietary Patterns and Anxiety Symptoms from Midpregnancy to Early Postpartum in a Prospective Cohort of Brazilian Women. J. Acad. Nutr. Diet. 2015, 115, 1626–1635. [Google Scholar] [CrossRef]
- Vilela, A.A.F.; Farias, D.R.; Eshriqui, I.; Vaz, J.d.S.; Franco-Sena, A.B.; Castro, M.B.T.; Olinto, M.T.A.; Machado, S.P.; da Silva, A.A.M.; Kac, G. Prepregnancy Healthy Dietary Pattern Is Inversely Associated with Depressive Symptoms among Pregnant Brazilian Women. J. Nutr. 2014, 144, 1612–1618. [Google Scholar] [CrossRef]
- Paskulin, J.T.; Drehmer, M.; Olinto, M.T.; Hoffmann, J.F.; Pinheiro, A.P.; Schmidt, M.I.; Nunes, M.A. Association between dietary patterns and mental disorders in pregnant women in Southern Brazil. Rev. Bras. Psiquiatr. 2017, 39, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Baskin, R.; Hill, B.; Jacka, F.N.; O’Neil, A.; Skouteris, H. Antenatal dietary patterns and depressive symptoms during pregnancy and early post-partum. Matern. Child Nutr. 2017, 13, e12218. [Google Scholar] [CrossRef]
- Shi, D.; Wang, G.-H.; Feng, W. Nutritional assessments in pregnancy and the risk of postpartum depression in Chinese women. A case-control study. Medicine 2020, 99, e21647. [Google Scholar] [CrossRef] [PubMed]
- Galbally, M.; Watson, S.J.; Boyce, P.; Anglin, R.; McKinnon, E.; Lewis, A.J. Maternal diet, depression and antidepressant treatment in pregnancy and across the first 12 months postpartum in the MPEWS pregnancy cohort study. J. Affect. Disord. 2021, 288, 74–82. [Google Scholar] [CrossRef]
- Cao, L.; Liu, Y.; Liang, X.; Zheng, Y.; Li, W.; Yan, J.; Huang, G. Association between dietary patterns during the third trimester and the risk of postpartum depression in China. J. Affect. Disord. 2020, 264, 370–375. [Google Scholar] [CrossRef]
- Huang, P.; Wei, D.; Xiao, W.; Yuan, M.; Chen, N.; Wei, X.; Xie, J.; Lu, J.; Xia, X.; Lu, M.; et al. Maternal dietary patterns and depressive symptoms during pregnancy: The Born in Guangzhou Cohort Study. Clin. Nutr. 2021, 40, 3485–3494. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Murakami, K.; Hirota, Y.; The Osaka Maternal and Child Health Study Group. Dietary patterns during pregnancy and the risk of postpartum depression in Japan: The Osaka Maternal and Child Health Study. Br. J. Nutr. 2011, 105, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Furukawa, S.; Arakawa, M. Dietary patterns and depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. J. Affect. Disord. 2018, 225, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.; Chia, A.-R.; Colega, M.T.; Chen, L.-W.; Fok, D.; Pang, W.W.; Godfrey, K.M.; Tan, K.H.; Yap, F.; Shek, L.P.-C.; et al. Prospective Associations of Maternal Dietary Patterns and Postpartum Mental Health in a Multi-Ethnic Asian Cohort: The Growing up in Singapore towards Healthy Outcomes (GUSTO) Study. Nutrients 2018, 10, 299. [Google Scholar] [CrossRef]
- Miura, K.; Takamori, A.; Hamazaki, K.; Tsuchida, A.; Tanaka, T.; Origasa, H.; Inadera, H.; The Japan Environment and Children’s Study Group. Dietary patterns during pregnancy and health-related quality of life: The Japan Environment and Children’s Study. PLoS ONE 2020, 15, e0236330. [Google Scholar] [CrossRef]
- Avalos, L.A.; Caan, B.; Nance, N.; Zhu, Y.; Li, D.-K.; Quesenberry, C.; Hyde, R.J.; Hedderson, M.M. Prenatal Depression and Diet Quality During Pregnancy. J. Acad. Nutr. Diet. 2020, 120, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Nakić Radoš, S. Anxiety During Pregnancy and Postpartum: Course, Predictors and Comorbidity with Postpartum Depression. Acta Clin. Croat. 2018, 57, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kee, M.Z.L.; Ponmudi, S.; Phua, D.Y.; Rifkin-Graboi, A.; Chong, Y.S.; Tan, K.H.; Chan, J.K.Y.; Broekman, B.F.; Chen, H.; Meaney, M.J. Preconception origins of perinatal maternal mental health. Arch. Women’s Ment. Health 2021, 24, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Yelverton, C.A.; Rafferty, A.A.; Moore, R.L.; Byrne, D.F.; Mehegan, J.; Cotter, P.D.; Van Sinderen, D.; Murphy, E.F.; Killeen, S.L.; McAuliffe, F.M. Diet and mental health in pregnancy: Nutrients of importance based on large observational cohort data. Nutrition 2022, 96, 111582. [Google Scholar] [CrossRef]
- Dubois, L.; Diasparra, M.; Bédard, B.; Colapinto, C.K.; Fontaine-Bisson, B.; Tremblay, R.E.; Fraser, W.D. Adequacy of nutritional intake during pregnancy in relation to prepregnancy BMI: Results from the 3D Cohort Study. Br. J. Nutr. 2018, 120, 335–344. [Google Scholar] [CrossRef]
| Fiber/Serving Size (grams) | Definition | Fiber Score |
|---|---|---|
| <1 g | Very Low | 1 |
| 1 to <2 g | Low | 2 |
| 2 to <5 g | Moderate | 3 |
| 5+ g | Very High | 4 |
| Food Group Items | Fiber/100 g | TSS (g) | Fiber/TSS | Fiber Score |
|---|---|---|---|---|
| Pineapple | 1.4 g | 129 g | 1.8 g | 2 |
| Banana | 1.7 g | 118 g | 2.01 g | 3 |
| Orange | 2.5 g | 131 g | 3.27 g | 3 |
| Fruits Food Group | 2.36 g | 3 | ||
| FGs | Fiber/100 g | Fiber/100 g Rank | FS | FS Rank | Sum of Ranks | FFR |
|---|---|---|---|---|---|---|
| Fruits | 1.87 | 4 | 3 | 2 | 6 | 3 |
| Legumes | 3.5 | 1 | 4 | 1 | 2 | 1 |
| Nuts | 3 | 2 | 2 | 3 | 5 | 2 |
| Cereals and Grains | 2.7 | 3 | 2 | 3 | 6 | 3 |
| Consumption Ranking | % Consumption Ranking | Dietary Pattern-1 | Dietary Pattern-2 | Dietary Pattern 3 |
|---|---|---|---|---|
| 1 | 14.2 | Seafood | Legumes * | Soda |
| 2 | 28.6 | Fruits | Fruits | Cereals and Grains |
| 3 | 42.9 | Meats and Poultry | Nuts * | Fruits |
| 4 | 57.1 | Sodas | Seafood | Seafood |
| 5 | 71.4 | Legumes * | Cereals and Grains | Nuts * |
| 6 | 85.7 | Cereals and Grains | Meats and Poultry | Meats and Poultry |
| 7 | 100 | Nuts * | Sodas | Legumes * |
| Confidence Intervals of Spearman’s Rho | ||||
|---|---|---|---|---|
| Top 3 FG | Spearman’s Rho | 95% Confidence Intervals (2-Tailed) a,b | p-Value c | |
| Lower | Upper | |||
| 1st Ranked | −0.407 | −0.664 | −0.064 | 0.019 |
| 2nd Ranked | −0.063 | −0.407 | 0.296 | 0.727 |
| 3rd Ranked | −0.455 | −0.696 | −0.122 | 0.008 |
| Average of 1st, 2nd, and 3rd | −0.419 | −0.672 | −0.078 | 0.015 |
| 1st % Ranking | −0.501 | −0.726 | −0.181 | 0.003 |
| 2nd % Ranking | −0.095 | −0.433 | 0.267 | 0.599 |
| 3rd % Ranking | −0.454 | −0.695 | −0.120 | 0.008 |
| Average of 1st, 2nd, and 3rd %s | −0.556 | −0.760 | −0.253 | 0.001 |
| Depressed | Non-Depressed | Antidepressant Treated | |||
|---|---|---|---|---|---|
| Study # | # of Food Groups/Items | Top 3 Fiber FGs | Top 3 Rank (z-Score) | Top 3 Rank (z-Score) | Top 3 Rank (z-Score) |
| Galbally, 2021 [36] | 9 | Cereals | 5 (−0.80) | 6 (−0.85) | 6 (−0.79) |
| Fruit | 3 (0.51) | 3 (0.79) | 3 (0.66) | ||
| Bread | 4 (0.00) | 4 (−0.05) | 4 (−0.05) | ||
| Avalos, 2020 [43] | 12 | Whole Grains | 4 (0.44) | 4 (0.63) | |
| Fatty Acids | 5 (0.05) | 5 (0.17) | |||
| Greens and Beans ** | 12 (−1.30) | 11 (−1.15) | |||
| Total Fruits ** | 10 (−0.96) | 9 (−0.83) | |||
| Shi, 2020 [35] | 14 | Staple Foods-Wheat | 11 (−1.25) | 11 (−1.21) | |
| Other Vegetables ** | 1 (3.28) | 1 (3.89) | |||
| Light Vegetables ** | 4 (0.98) | 4 (1.34) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi, N.; Turner, T.; Gallant, F.; Chandrakumar, A.; Kohli, R.; Lester, R.; Forte, V.; Cooley, K. Maternal Fiber Intake and Perinatal Depression and Anxiety. Nutrients 2024, 16, 2484. https://doi.org/10.3390/nu16152484
Ebrahimi N, Turner T, Gallant F, Chandrakumar A, Kohli R, Lester R, Forte V, Cooley K. Maternal Fiber Intake and Perinatal Depression and Anxiety. Nutrients. 2024; 16(15):2484. https://doi.org/10.3390/nu16152484
Chicago/Turabian StyleEbrahimi, Neda, Tiffany Turner, Faith Gallant, Abinaa Chandrakumar, Roshni Kohli, Rebecca Lester, Victoria Forte, and Kieran Cooley. 2024. "Maternal Fiber Intake and Perinatal Depression and Anxiety" Nutrients 16, no. 15: 2484. https://doi.org/10.3390/nu16152484
APA StyleEbrahimi, N., Turner, T., Gallant, F., Chandrakumar, A., Kohli, R., Lester, R., Forte, V., & Cooley, K. (2024). Maternal Fiber Intake and Perinatal Depression and Anxiety. Nutrients, 16(15), 2484. https://doi.org/10.3390/nu16152484







