Adipokines and Myokines as Markers of Malnutrition and Sarcopenia in Patients Receiving Kidney Replacement Therapy: An Observational, Cross-Sectional Study
Abstract
1. Introduction
| Reference Values in Healthy Individuals | Reports on CKD Patients with Good Nutritional Status | Reports on CKD Patients with Poor Nutritional Status | Reports on Sarcopenia in CKD Patients | Key Functions | |
|---|---|---|---|---|---|
| Leptin | 0.33–19.85 ng/mL in men and 3.60–54.86 ng/mL in women [23] | ↑ | ↓ | ↓ | Regulates appetite, signals satiety, decreases food intake [16,24] |
| Adiponectin | 2–20 μg/mL [25] | ↑ | ↑ | Data lacking | Plays a role in energy homeostasis, anti-inflammatory effects [15,24] |
| IL-6 | <5.740 pg/mL [26] | ↑ | ↑ | ↑ | Pro-inflammatory effect; contributes to muscle protein breakdown and can impact appetite regulation [24,27] |
| Myostatin | 7–32 ng/mL [28] | ↑ | ↑↓ non-conclusive results | ↑↓ non-conclusive results | Muscle protein synthesis inhibition; contributes to muscle atrophy [29,30] |
| Irisin | 5.1–62.7 μg/mL [31] | ↓ | ↑↓ non-conclusive results | ↑↓ non-conclusive results | Associated with thermogenesis, involved in muscle protein synthesis [18,32] |
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.2.1. Standard Treatment
2.2.2. Validation of Reference Values—Control Group
2.3. Data Collection
2.4. Anthropometric Measurements
2.5. Body Composition
2.6. Assessment of Malnutrition and Malnutrition-Inflammation Complex Syndrome
2.7. Assessment of Biochemical Data
2.8. Statistical Analysis
2.8.1. Receiver Operating Characteristics—Biomarkers of Sarcopenia and Malnutrition
2.8.2. Models Predicting Malnutrition and Sarcopenia
3. Results
3.1. Baseline Data and Nutritional Status
3.2. Adipokines and Myokines
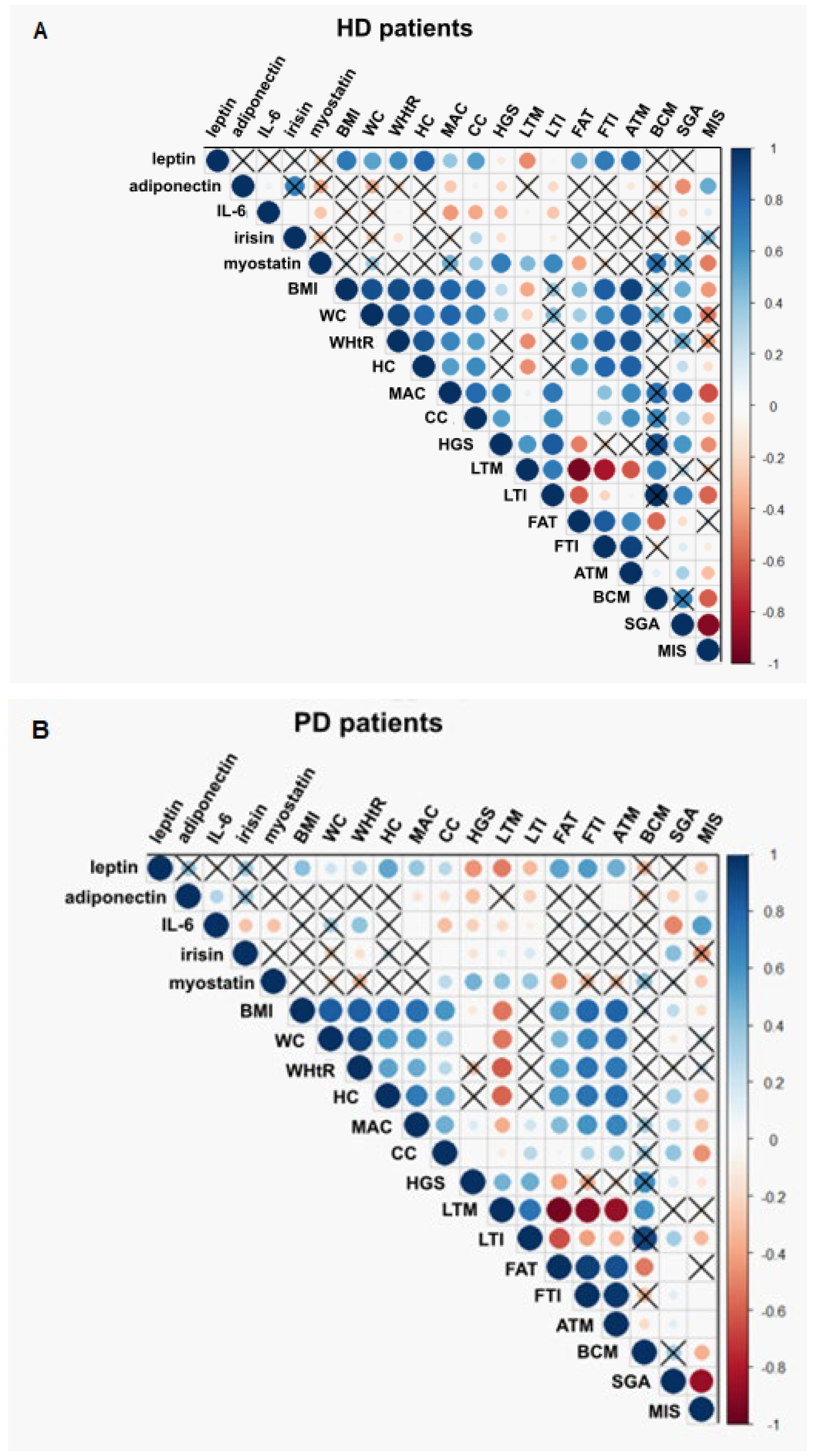
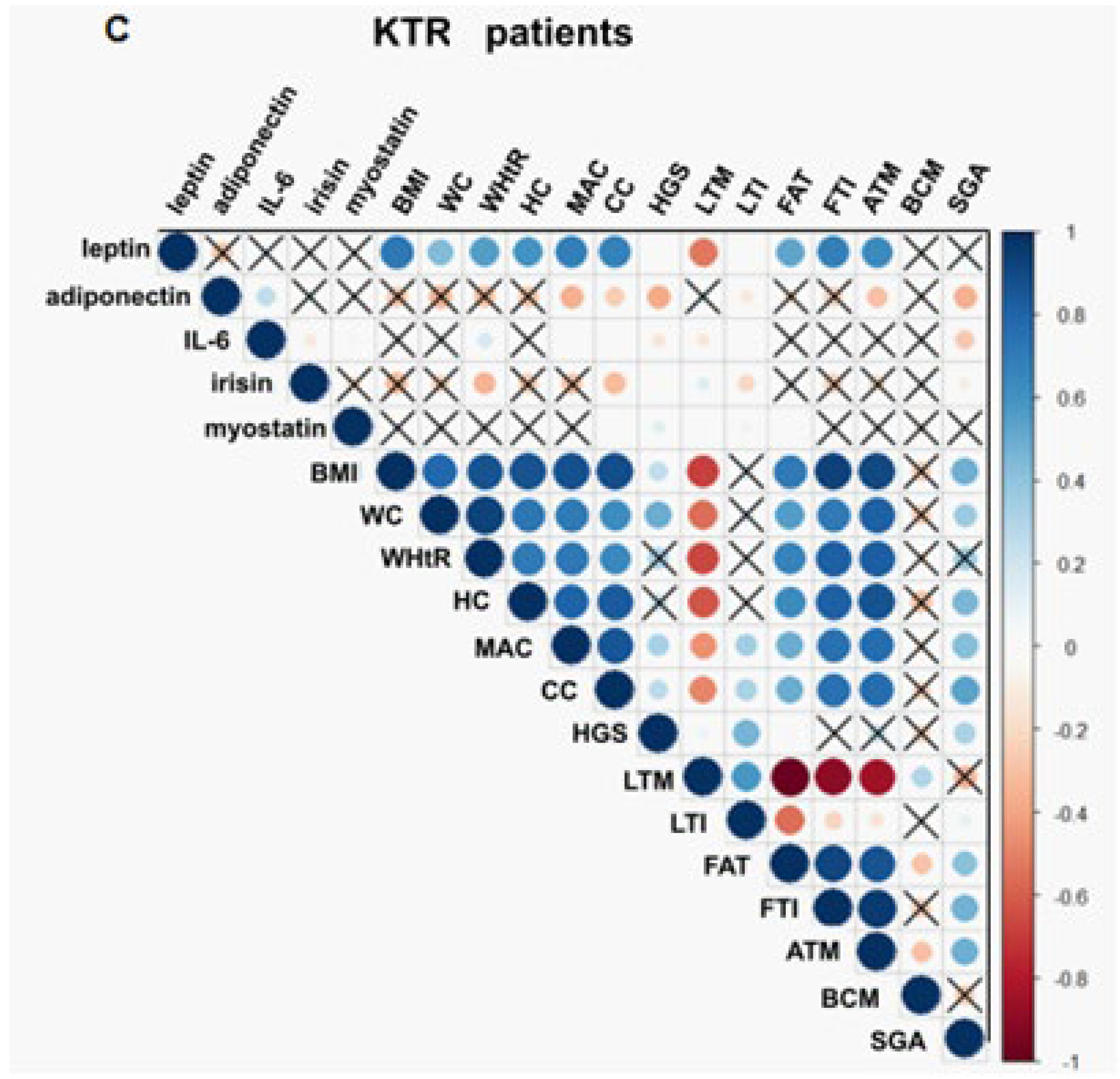
3.3. Association between Adipokines, Myokines, and Nutritional Parameters
3.4. Adipokines and Myokines as Markers of Malnutrition and Malnutrition-Inflammation Syndrome
3.5. Adipokines and Myokines as Markers of Sarcopenia
3.6. Regression Model to Predict Sarcopenia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef]
- Sobotka, L. (Ed.) Basics in Clinical Nutrition, 4th ed.; Publishinh House Galen: Prague, Czech Republic, 2012. [Google Scholar]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.P.; Almeida, L.S.; Neri, S.G.; Oliveira, J.S.; Wilkinson, T.J.; Ribeiro, H.S.; Lima, R.M. Prevalence of sarcopenia in patients with chronic kidney disease: A global systematic review and meta-analysis. J. Cachex-Sarcopenia Muscle 2024, 15, 501–512. [Google Scholar] [CrossRef]
- Kanda, E.; Lopes, M.B.; Tsuruya, K.; Hirakata, H.; Iseki, K.; Karaboyas, A.; Bieber, B.; Jacobson, S.H.; Dasgupta, I.; Robinson, B.M. The combination of malnutrition-inflammation and functional status limitations is associated with mortality in hemodialysis patients. Sci. Rep. 2021, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Kopple, J.D.; Wolfson, M. Metabolic acidosis in maintenance dialysis patients: Clinical considerations. Kidney Int. 2003, 64, S13–S25. [Google Scholar] [CrossRef]
- Carrero, J.J.; Qureshi, A.R.; Axelsson, J.; Avesani, C.M.; E Suliman, M.; Kato, S.; Bárány, P.; Snaedal-Jonsdottir, S.; Alvestrand, A.; Heimbürger, O.; et al. Comparison of nutritional and inflammatory markers in dialysis patients with reduced appetite. Am. J. Clin. Nutr. 2007, 85, 695–701. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; McAllister, C.J.; Humphreys, M.H.; Kopple, J.D. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [CrossRef]
- Wathanavasin, W.; Banjongjit, A.; Avihingsanon, Y.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S.; Susantitaphong, P. Prevalence of Sarcopenia and Its Impact on Cardiovascular Events and Mortality among Dialysis Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4077. [Google Scholar] [CrossRef]
- Macedo, C.; Amaral, T.F.; Rodrigues, J.; Santin, F.; Avesani, C.M. Malnutrition and Sarcopenia Combined Increases the Risk for Mortality in Older Adults on Hemodialysis. Front. Nutr. 2021, 8, 721941. [Google Scholar] [CrossRef]
- Barretto, L.P.; Gomes, P.M.; Guidorizzi, N.R.; Neto, M.M.; Romao, E.A.; Chiarello, P.G. Post-transplant diabetes mellitus: Findings in nutritional status and body composition. Endocrinol. Diabetes Nutr. 2023, 70, 628–633. [Google Scholar] [CrossRef]
- Yang, M.; Luo, S.; Yang, J.; Chen, W.; He, L.; Liu, D.; Zhao, L.; Wang, X. Myokines: Novel therapeutic targets for diabetic nephropathy. Front. Endocrinol. 2022, 13, 1014581. [Google Scholar] [CrossRef] [PubMed]
- Czaja-Stolc, S.; Potrykus, M.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Pro-Inflammatory Profile of Adipokines in Obesity Contributes to Pathogenesis, Nutritional Disorders, and Cardiovascular Risk in Chronic Kidney Disease. Nutrients 2022, 14, 1457. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.; Rocha, S.; Valente, M.J.; Catarino, C.; Bronze-Da-Rocha, E.; Belo, L.; Santos-Silva, A. New Insights into Adiponectin and Leptin Roles in Chronic Kidney Disease. Biomedicines 2022, 10, 2642. [Google Scholar] [CrossRef] [PubMed]
- Alix, P.M.; Guebre-Egziabher, F.; Soulage, C.O. Leptin as an uremic toxin: Deleterious role of leptin in chronic kidney disease. Biochimie 2014, 105, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Leal, D.V.; Ferreira, A.; Watson, E.L.; Wilund, K.R.; Viana, J.L. Muscle-Bone Crosstalk in Chronic Kidney Disease: The Potential Modulatory Effects of Exercise. Calcif. Tissue Int. 2021, 108, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Bataille, S.; Chauveau, P.; Fouque, D.; Aparicio, M.; Koppe, L. Myostatin and muscle atrophy during chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.-S.; Wang, C.-Y.; Lin, S.-L.; Hung, K.-C. Decrease in irisin in patients with chronic kidney disease. PLoS ONE 2013, 8, e64025. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, W.; Deng, Y.; Jia, L.; Lin, N.; Li, W.; Zhang, A. Lower serum irisin levels are associated with the increasing mortality of cardiovascular and cerebrovascular diseases in hemodialysis patients. Ann. Palliat. Med. 2021, 10, 6052–6061. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. 1), S1–S107. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Cheng, J.; Luo, Y.; Li, Y.; Zhang, F.; Zhang, X.; Zhou, X.; Ji, L. Sex- and body mass index-specific reference intervals for serum leptin: A population based study in China. Nutr. Metab. 2022, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Serrano, E.; Shenoy, P.; Cantarin, M.P.M. Adipose tissue metabolic changes in chronic kidney disease. Immunometabolism 2023, 5, e00023. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Choubey, M.; Tirumalasetty, M.B.; Arbee, S.; Mohib, M.M.; Wahiduzzaman; Mamun, M.A.; Uddin, M.B.; Mohiuddin, M.S. Adiponectin: A Promising Target for the Treatment of Diabetes and Its Complications. Life 2023, 13, 2213. [Google Scholar] [CrossRef] [PubMed]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.A.; Jeyaseelan, L. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.-D.; Bian, A.-L.; Hu, H.-Y.; Ma, Y.; Zhou, X.-Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-S.; Huang, C.-H.; Chen, S.-Y.; Yang, W.-S. Serum reference value of two potential doping candidates—Myostatin and insulin-like growth factor-I in the healthy young male. J. Int. Soc. Sports Nutr. 2017, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Tsuruya, K.; Yoshida, H.; Tokumoto, M.; Ueki, K.; Ooboshi, H.; Kitazono, T. Factors Associated with the Serum Myostatin Level in Patients Undergoing Peritoneal Dialysis: Potential Effects of Skeletal Muscle Mass and Vitamin D Receptor Activator Use. Calcif. Tissue Int. 2016, 99, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bataille, S.; Dou, L.; Bartoli, M.; Sallée, M.; Aniort, J.; Ferkak, B.; Chermiti, R.; McKay, N.; Da Silva, N.; Burtey, S.; et al. Mechanisms of myostatin and activin A accumulation in chronic kidney disease. Nephrol. Dial. Transplant. 2022, 37, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- González, D.A.; Rodríguez-Pérez, M.d.C.; Ferrer, M.F.; Fernández, F.J.C.; Rodríguez, I.M.; de León, A.C. Irisin, in women and men: Blood pressure, heart rate, obesity and insulin resistance. Front. Endocrinol. 2023, 14, 1193110. [Google Scholar] [CrossRef]
- Li, X.; Lindholm, B. The role of irisin in kidney diseases. Clin. Chim. Acta 2024, 554, 117756. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Watnick, S. Peritoneal Dialysis Adequacy; International Society for Peritoneal Dialysis: Los Altos, CA, USA, 2011. [Google Scholar]
- E Watson, P.; Watson, I.D.; Batt, R.D. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am. J. Clin. Nutr. 1980, 33, 27–39. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Mini Nutritional Assessment: A practical assessment tool for grading the nutritional state of elderly patients. Facts Res. Gerontol. 1994, 3 (Suppl. 2), 15–59. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Visser, R.; Dekker, F.W.; Boeschoten, E.W.; Stevens, P.; Krediet, R.T. Reliability of the 7-point subjective global assessment scale in assessing nutritional status of dialysis patients. Adv. Perit. Dialysis. Conf. Perit. Dial. 1999, 15, 222–225. [Google Scholar]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 May 2022).
- Zhang, J.; Shi, W.; Zou, M.; Zeng, Q.; Feng, Y.; Luo, Z.; Gan, H. Diagnosis, prevalence, and outcomes of sarcopenia in kidney transplantation recipients: A systematic review and meta-analysis. J. Cachex-Sarcopenia Muscle 2022, 14, 17–29. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2023. Available online: https://github.com/tidyverse/dplyr.https://dplyr.tidyverse.org (accessed on 26 February 2024).
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Sun, X.; Xu, W. Fast Implementation of DeLong’s Algorithm for Comparing the Areas under Correlated Receiver Operating Characteristic Curves. IEEE Signal Process. Lett. 2014, 21, 1389–1393. [Google Scholar] [CrossRef]
- Venkatraman, E.S.; Begg, C.B. A distribution-free procedure for comparing receiver operating characteristic curves for a paired experiment. Biometrika 1996, 83, 835–848. [Google Scholar] [CrossRef]
- Dunkler, D.; Plischke, M.; Leffondré, K.; Heinze, G. Augmented backward elimination: A pragmatic and purposeful way to develop statistical models. PLoS ONE 2014, 9, e113677. [Google Scholar] [CrossRef] [PubMed]
- Mori, K. Maintenance of Skeletal Muscle to Counteract Sarcopenia in Patients with Advanced Chronic Kidney Disease and Especially Those Undergoing Hemodialysis. Nutrients 2021, 13, 1538. [Google Scholar] [CrossRef] [PubMed]
- Gama-Axelsson, T.; Heimbürger, O.; Stenvinkel, P.; Bárány, P.; Lindholm, B.; Qureshi, A.R. Serum Albumin as Predictor of Nutritional Status in Patients with ESRD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1446–1453. [Google Scholar] [CrossRef]
- Yasar, E.; Tek, N.A.; Tekbudak, M.Y.; Yurtdaş, G.; Gülbahar, Ö.; Uyar, G.Ö.; Ural, Z.; Çelik, M.; Erten, Y. The Relationship Between Myostatin, Inflammatory Markers, and Sarcopenia in Patients with Chronic Kidney Disease. J. Ren. Nutr. 2022, 32, 677–684. [Google Scholar] [CrossRef]
- Asikin, M.D.; Widajanti, N.; Firdausi, H. Myostatin and Sarcopenia in Elderly among Haemodyalisis Patient. J. Nat. Sci. Biol. Med. 2021, 12, 290–299. [Google Scholar]
- Alexopoulos, T.; Vasilieva, L.; Kontogianni, M.D.; Tenta, R.; Georgiou, A.; Stroumpouli, E.; Mani, I.; Alexopoulou, A. Myostatin in combination with creatine phosphokinase or albumin may differentiate patients with cirrhosis and sarcopenia. Am. J. Physiol. Liver Physiol. 2021, 321, G543–G551. [Google Scholar] [CrossRef]
- Enoki, Y.; Watanabe, H.; Arake, R.; Sugimoto, R.; Imafuku, T.; Tominaga, Y.; Ishima, Y.; Kotani, S.; Nakajima, M.; Tanaka, M.; et al. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci. Rep. 2016, 6, 32084. [Google Scholar] [CrossRef]
- Hyun, Y.Y.; Lee, K.-B.; Oh, K.-H.; Ahn, C.; Park, S.K.; Chae, D.W.; Yoo, T.-H.; Cho, K.H.; Kim, Y.-S.; Hwang, Y.-H. Serum adiponectin and protein–energy wasting in predialysis chronic kidney disease. Nutrition 2016, 33, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Beberashvili, I.; Azar, A.; Khatib, A.; Abu Hamad, R.; Neheman, A.; Efrati, S.; Doenyas-Barak, K. Sarcopenic Obesity Versus Nonobese Sarcopenia in Hemodialysis Patients: Differences in Nutritional Status, Quality of Life, and Clinical Outcomes. J. Ren. Nutr. 2023, 33, 147–156. [Google Scholar] [CrossRef]
- Van Tellingen, A.; Grooteman, M.P.C.; Schoorl, M.; ter Wee, P.M.; Bartels, P.C.M.; van der Ploeg, T.; Nubé, M.J. Enhanced long-term reduction of plasma leptin concentrations by super-flux polysulfone dialysers. Nephrol. Dial. Transplant. 2004, 19, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Oh, K.; Chin, H.; Na, K.; Kim, Y.; Chae, D.-W.; Ahn, C.; Han, J.; Joo, K. Effective removal of leptin via hemodiafiltration with on-line endogenous reinfusion therapy. Clin. Nephrol. 2009, 72, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-W.; Yen, C.-J.; Chiang, H.-W.; Hung, K.-Y.; Tsai, T.-J.; Wu, K.-D. Adiponectin in peritoneal dialysis patients: A comparison with hemodialysis patients and subjects with normal renal function. Am. J. Kidney Dis. 2004, 43, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Murt, A.; Yalin, S.F.; Konukoglu, D.; Ronco, C.; Altiparmak, M.R. Fluctuations in Interleukin-6 Levels during Hemodialysis Sessions with Medium Cutoff Membranes: An Analysis on COVID-19 Case Series. Blood Purif. 2022, 51, 953–958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, T.; Kubota, M.; Nakamura, T.; Ebihara, I.; Koide, H. Interleukin-6 gene expression in peripheral blood mononuclear cells from patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Ren. Fail. 2000, 22, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Esposito, P.; Battaglia, Y.; La Porta, E.; Grignano, M.A.; Caramella, E.; Avella, A.; Peressini, S.; Sessa, N.; Albertini, R.; Di Natali, G.; et al. Significance of serum Myostatin in hemodialysis patients. BMC Nephrol. 2019, 20, 462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esposito, P.; La Porta, E.; Calatroni, M.; Grignano, M.A.; Milanesi, S.; Verzola, D.; Battaglia, Y.; Gregorini, M.; Libetta, C.; Garibotto, G.; et al. Modulation of Myostatin/Hepatocyte Growth Factor Balance by Different Hemodialysis Modalities. BioMed Res. Int. 2017, 2017, 7635459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maak, S.; Norheim, F.; A Drevon, C.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaynar, K.; Kural, B.V.; Ulusoy, S.; Cansiz, M.; Akcan, B.; Misir, N.; Yaman, S.; Kaya, N. Is there any interaction of resistin and adiponectin levels with protein-energy wasting among patients with chronic kidney disease. Hemodial. Int. 2013, 18, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Małgorzewicz, S.; Dębska-Slizień, A.; Czajka, B.; Rutkowski, B. Adipokines and Nutritional Status in Kidney Transplant Recipients. Transplant. Proc. 2014, 46, 2622–2626. [Google Scholar] [CrossRef] [PubMed]
- Feret, W.; Safranow, K.; Kwiatkowska, E.; Daniel, A.; Ciechanowski, K. Malnutrition and Erythropoietin Resistance among Patients with End-Stage Kidney Disease: Where Is the Perpetrator of Disaster? Nutrients 2022, 14, 5318. [Google Scholar] [CrossRef]
- Machiba, Y.; Inaba, M.; Mori, K.; Kurajoh, M.; Nishide, K.; Norimine, K.; Yamakawa, T.; Shoji, S.; Okuno, S. Paradoxical positive association of serum adiponectin with all-cause mortality based on body composition in Japanese haemodialysis patients. Sci. Rep. 2018, 8, 14699. [Google Scholar] [CrossRef]
- Lee, M.J.; A Lee, S.; Nam, B.Y.; Park, S.; Lee, S.-H.; Ryu, H.J.; Kwon, Y.E.; Kim, Y.L.; Park, K.S.; Oh, H.J.; et al. Irisin, a novel myokine is an independent predictor for sarcopenia and carotid atherosclerosis in dialysis patients. Atherosclerosis 2015, 242, 476–482. [Google Scholar] [CrossRef]
- He, W.-Y.; Wu, F.; Pang, X.-X.; Chen, G.-J.; A, L.-T.; He, L.; Wang, S.; Tang, C.-S.; Zhang, A.-H. Irisin is Associated with Urotensin II and Protein Energy Wasting in Hemodialysis Patients. Kidney Blood Press. Res. 2016, 41, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kałużna, M.; Hoppe, K.; Schwermer, K.; Ibrahim, A.Y.; Pawlaczyk, K.; Ziemnicka, K. Adropin and irisin levels in relation to nutrition, body composition, and insulin resistance in patients with end-stage renal disease on chronic hemodialysis and peritoneal dialysis. Pol. Arch. Intern. Med. 2016, 126, 474–482. [Google Scholar] [CrossRef][Green Version]
- Yilmaz, H.; Cakmak, M.; Darcin, T.; Inan, O.; Sahiner, E.; Demir, C.; Aktas, A.; Bilgic, M.A.; Akcay, A. Circulating irisin levels reflect visceral adiposity in non-diabetic patients undergoing hemodialysis. Ren. Fail. 2016, 38, 914–919. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, W.; Wang, X.; Han, Q.; Bai, Q.; Zhang, A. Relationship between Serum Irisin Level, All-Cause Mortality, and Cardiovascular Mortality in Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2023, 49, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Demir, C.; Dursun, A.D.; Sarıyıldız, G.T.; Arslan, A.I. Serum irisin levels and osteoporosis in patients with advanced chronic kidney disease and renal transplant recipients. Int. Urol. Nephrol. 2023, 55, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Liu, Y.-M.; Liao, M.-T.; Zheng, C.-M.; Lu, C.-L.; Liu, W.-C.; Hung, K.-C.; Lin, S.-M.; Lu, K.-C. Indoxyl sulfate mediates low handgrip strength and is predictive of high hospitalization rates in patients with end-stage renal disease. Front. Med. 2023, 10, 1023383. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, S.E.; Lee, J.Y.; Jeong, H.J.; Son, Y.K.; An, W.S. Serum myostatin levels are associated with abdominal aortic calcification in dialysis patients. Kidney Res. Clin. Pr. 2019, 38, 481–489. [Google Scholar] [CrossRef]
- Delanaye, P.; Bataille, S.; Quinonez, K.; Buckinx, F.; Warling, X.; Krzesinski, J.-M.; Pottel, H.; Burtey, S.; Bruyère, O.; Cavalier, E. Myostatin and Insulin-Like Growth Factor 1 Are Biomarkers of Muscle Strength, Muscle Mass, and Mortality in Patients on Hemodialysis. J. Ren. Nutr. 2019, 29, 511–520. [Google Scholar] [CrossRef]



| Baseline Data | |||||
|---|---|---|---|---|---|
| All | HD | PD | KTR | p-Values | |
| N | 180 | 84 | 44 | 52 | |
| Females/Males (n) (%) | 80/100 (44.4%/55.6) | 36/48 (42.9/57.1) | 21/23 (47.7/52.3) | 23/29 (44.2/55.8) | 0.860 |
| Age (in years) | 56.1 ± 16.3 | 61.7 ± 16.4 | 52.2 ± 17.8 | 50.4 ± 11.6 | 0.002 |
| Dialysis vintage (in months) | 19 (9–48) | 28.5 (9–72) | 14 (7–29) | 18 (10–36) before KT | 0.040 |
| Kt/V | - | 1.7 (1.5–1.9) per session | 2.3 (1.9–3.2) weekly | - | - |
| Ultrafiltration (mL) | - | 2200 (1200–3000) per session | 1000 (550–1220) per day | - | - |
| Anthropometric and physical data | |||||
| All | HD | PD | KTR | p-values | |
| BMI (kg/m2) | 25.9 (22.6–28.7) | 24.6 (22.4–27.7) | 27.2 (23.6–29.4) | 26.2 (22.8–29.6) | 0.220 |
| BMI ≥ 30 (%) | 17.8 | 14.3 | 18.2 | 23.1 | 0.430 |
| BMI < 23 (%) | 29.4 | 35.2 | 20.5 | 28.8 | 0.250 |
| MAC (cm) | 28 (25–30) | 26 (23–29) | 28 (27–31) | 29 (27–31) | <0.001 |
| MAC < 22 cm (%) | 5.6 | 8.3 | 4.5 | 1.9 | 0.260 |
| CC (cm) | 35 (32–38) | 33 (30–35) | 38 (36–41) | 36 (33–38.8) | <0.001 |
| CC < 31 cm (%) | 18.3 | 32.1 | 4.5 | 7.7 | <0.001 |
| HGS (kg) | 27.4± 10.8 | 23.2 ± 10.5 | 28.6 ± 10.9 | 33.1 ± 8 | <0.001 |
| Body composition assessed with BIA | |||||
| All | HD | PD | KTR | p-values | |
| OH (L) | 0.9 (−0.2–1.9) | 0.7 (−0.8–1.9) | 1.35 (0.4–3.6) | 0.8 (−0.1–1.7) | 0.009 |
| TBW (L) | 33.3 (29–39) | 32 (27.9–35.3) | 37.1 (31.1–41.8) | 34.1 (30.9–40.5) | 0.006 |
| ECW (L) | 15.7 (13.9–19.3) | 14.9 (13.2–17.1) | 18.3 (14.4–20.3) | 15.8 (14.6–19.1) | 0.005 |
| ICW (L) | 17.7 (14.9–20.3) | 16.6 (14.2–19.2) | 17.9 (15.9–21) | 18.6 (15.8–20.4) | 0.010 |
| LTM (%) | 48.3± 12.6 | 48 ± 13.5 | 50.3 ± 12.5 | 47 ± 11.2 | 0.430 |
| LTI (kg/m2) | 12.1 ± 2.5 | 11.6 ± 2.6 | 13.1 ± 2.4 | 11.9 ± 2 | 0.007 |
| FAT (%) | 36.4 ± 9.9 | 36.6 ± 10.8 | 34.1 ± 9.8 | 38 ± 8.4 | 0.180 |
| FTI (kg/m2) | 12.6 (9.6–16.8) | 12.1 (9.5–15.3) | 12.8 (9.6–15.5) | 12.9 (9.8–17.5) | 0.500 |
| ATM (kg) | 37.8± 15.5 | 36.3 ± 16 | 36.5 ± 15 | 41 ± 15.2 | 0.160 |
| BCM (kg) | 18 (14.4–22.8) | 15.9 (12.9–21.6) | 19.2 (15.8–23.9) | 18.8 (15–22.7) | 0.030 |
| 7-Point SGA, n (%) | |||||
| All | HD | PD | KTR | p-values | |
| 7 | 36 (20) | 7 (8.3) | 13 (29.5) | 16 (30.8) | 0.003 |
| 6 | 85 (47.2) | 46 (54.8) | 15 (34.1) | 24 (46.2) | |
| 5 | 38 (21.1) | 21 (25) | 10 (22.7) | 7 (13.5) | |
| 4 | 15 (8.3) | 9 (10.7) | 2 (4.5) | 4 (7.7) | |
| 3 | 3 (1.7) | 0 (0) | 3 (6.8) | 0 (0) | |
| 2 | 2 (1.1) | 1 (1.2) | 0 (0) | 1 (1.9) | |
| 1 | 1 (0.6) | 0 (0) | 1 (2.3) | 0 (0) | |
| Well-nourished (%) | 67.2 | 63.1 | 63.6457 | 76.9 | 0.210 |
| Malnourished (%) | 32.8 | 36.9 | 36.4 | 23.1 | |
| Cause of CKD, n (%) | |||||
| All | HD | PD | KTR | p-value | |
| Glomerulonephritis | 50 (27.8) | 19 (22.6) | 13 (29.5) | 18 (34.6) | 0.720 |
| Diabetic nephropathy | 32 (17.8) | 15 (17.9) | 10 (22.7) | 7 (13.5) | |
| Hypertensive nephropathy | 14 (7.8) | 4 (4.8) | 8 (18.2) | 2 (3.8) | |
| ADPKD | 23 (12.8) | 12 (14.3) | 3 (6.8) | 8 (15.4) | |
| Other | 54 (30) | 31 (36.9) | 7 (15.9) | 16 (30.8) | |
| Basic Biochemical Data | ||||||
|---|---|---|---|---|---|---|
| Parameters | References Value | All | HD Patients | PD Patients | KTR | p-Values |
| N | 180 | 84 | 44 | 52 | ||
| Creatinine (mg/dL) | 0.7–1.2 | 5.3 (2.2–8.4) | 7.2 (5.0–9.1) | 7.7 (5.2–10.1) | 1.3 (1.04–1.9) | <0.001 |
| eGFR CKD-EPI (mL/min/1.73 m2) | >90 | - | - | - | 53.5 (39–69.5) | - |
| BUN (mg/dL) | 8.4–25.7 | 47.7± 16.3 | 52.3 ± 14.3 | 49.4 ± 14.2 | 31.4 ± 15 | <0.001 |
| Calcium (mg/dL) | 8.9–10 | 9 (8.4–9.5) | 8.8 (8.3–9.3) | 8.8 (8.4–9.2) | 9.7 (9.4–10) | <0.001 |
| Phosphorus (mg/dL) | 2.3–4.7 | 5 (3.7–6.3) | 5.2 (3.9–6.5) | 5.8 (4.8–6.9) | 2.9 (2.5–3.3) | <0.001 |
| Sodium (mmol/L) | 135–145 | 139 (137–141) | 138 (135–141) | 140 (138–141.5) | 140 (139–141.5) | 0.004 |
| Potassium (mmol/L) | 3.5–5.1 | 4.8 (4.3–5.4) | 5.3 (4.7–5.7) | 4.5 (4.1–5.3) | 4.3 (4.1–4.8) | <0.001 |
| Hemoglobin (g/dL) | 12–15 for F, 13–17 for M | 11.1 (10.1–13.1) | 10.3 (9.7–11.1) | 11.1 (10.3–12.2) | 14.1 (12.7–15) | <0.001 |
| Biochemical markers of nutritional status | ||||||
| References value | All | HD | PD | KTR | p-values | |
| Albumin (g/dL) | 3.8–5.2 | 3.5 (3.2–4) | 3.4 (3.1–3.6) | 3.35 (2.95–3.7) | 4.1 (4–4.35) | <0.001 |
| Albumin level <3.8 (%) | 63.9 | 86.9 | 86.4 | 7.7 | <0.001 | |
| Albumin level <3.5 (%) | 42.8 | 61.9 | 52.3 | 3.8 | <0.001 | |
| Transferrin (mg/dL) | 200–400 | 173 (155–199) | 166 (149–189) | 195 (170–221) | - | <0.001 |
| Total cholesterol (mg/dL) | 115–190 | 176 (148–219) | 160 (127–196) | 209.5 (172.5–256.5) | 186.5 (162–207) | <0.001 |
| HDL cholesterol (mg/dL) | >45 for F, >40 for M | 44 (36–56) | 40 (35–51) | 41.5 (36–53) | 52 (44–59.5) | <0.001 |
| Total number of lymphocytes (/1 mm3) | 1–3 | 1.5 (1.1–2.1) | 1.3 (1–1.7) | 1.3 (1.1–1.7) | 2.3 (1.8–2.8) | <0.001 |
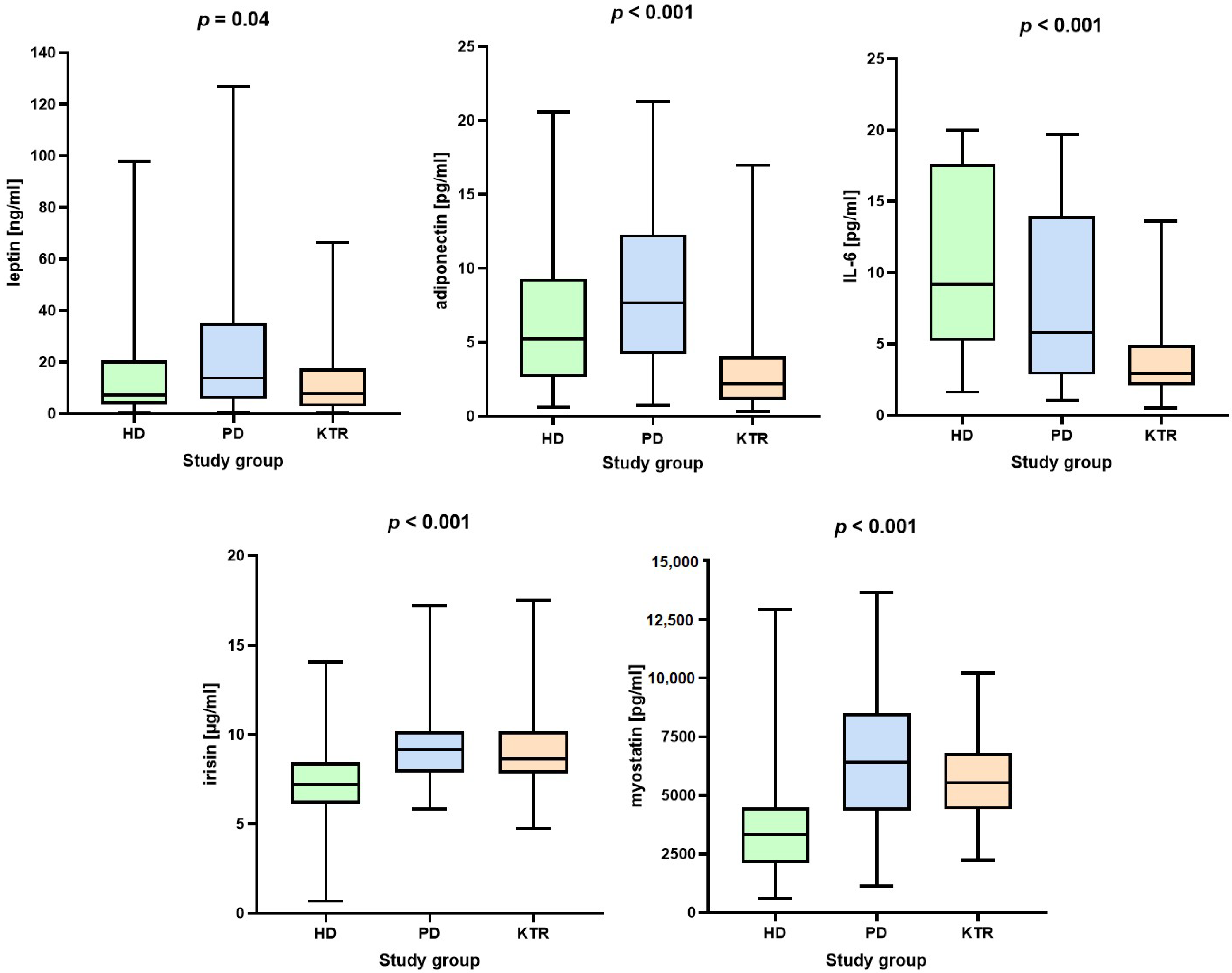
| Adipokines and myokines | ||||||
| All | HD patients | PD patients | KTR | p-values | ||
| Leptin (ng/mL) | 10.1 (3.7–22.6) | 7.2 (3.6–19.9) | 13.8 (6.2–34.4) | 7.8 (2.8–17.1) | 0.040 | |
| Adiponectin (μg/mL) | 4.3 (2–9.4) | 5.3 (2.7–9.3) | 7.7 (4.3–12.3) | 2.2 (1.1–4) | <0.001 | |
| IL-6 (pg/mL) | 5.9 (2.8–13.3) | 9.2 (5.3–17.6) | 5.8 (2.9–13.9) | 2.9 (2.1–4.9) | <0.001 | |
| Irisin (μg/mL) | 8.1 (7.1–9.5) | 7.2 (6.2–8.4) | 9.1 (7.9–10.2) | 8.6 (7.8–10.2) | <0.001 | |
| Myostatin (pg/mL) | 4448 (3047.4–6438) | 3334 (2149–4460) | 6418 (4366–8396) | 5536 (4406–6730) | <0.001 | |
| 95% CI | ||||
|---|---|---|---|---|
| Estimates | Lower | Higher | p-Values | |
| Initial model | ||||
| Age (in years) | 0.019 | −0.012 | 0.051 | 0.231 |
| Sex (men) | −0.137 | −1.311 | 1.037 | 0.819 |
| Albumin (g/dL) | −0.065 | −0.176 | 0.045 | 0.247 |
| Leptin (ng/mL) | 0.005 | −0.017 | 0.026 | 0.667 |
| Adiponectin (µg/mL) | −0.022 | −0.133 | 0.089 | 0.155 |
| Irisin (μg/mL) | −0.072 | −0.296 | 0.151 | 0.526 |
| Myostatin (pg/mL) | −0.005 | −0.001 | 0.001 | 0.002 |
| IL-6 (pg/mL) | 0.004 | −0.077 | 0.085 | 0.917 |
| Final model | ||||
| Age (in years) | 0.0019 | −0.009 | 0.048 | 0.19 |
| Albumin (g/dL) | −0.0725 | −0.177 | 0.032 | 0.17 |
| Adiponectin (µg/mL) | −0.0304 | −0.137 | 0.0758 | 0.57 |
| Myostatin (pg/mL) | −0.0005 | −0.0008 | −0.0002 | 0.0004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaja-Stolc, S.; Chatrenet, A.; Potrykus, M.; Ruszkowski, J.; Torreggiani, M.; Lichodziejewska-Niemierko, M.; Dębska-Ślizień, A.; Piccoli, G.B.; Małgorzewicz, S. Adipokines and Myokines as Markers of Malnutrition and Sarcopenia in Patients Receiving Kidney Replacement Therapy: An Observational, Cross-Sectional Study. Nutrients 2024, 16, 2480. https://doi.org/10.3390/nu16152480
Czaja-Stolc S, Chatrenet A, Potrykus M, Ruszkowski J, Torreggiani M, Lichodziejewska-Niemierko M, Dębska-Ślizień A, Piccoli GB, Małgorzewicz S. Adipokines and Myokines as Markers of Malnutrition and Sarcopenia in Patients Receiving Kidney Replacement Therapy: An Observational, Cross-Sectional Study. Nutrients. 2024; 16(15):2480. https://doi.org/10.3390/nu16152480
Chicago/Turabian StyleCzaja-Stolc, Sylwia, Antoine Chatrenet, Marta Potrykus, Jakub Ruszkowski, Massimo Torreggiani, Monika Lichodziejewska-Niemierko, Alicja Dębska-Ślizień, Giorgina Barbara Piccoli, and Sylwia Małgorzewicz. 2024. "Adipokines and Myokines as Markers of Malnutrition and Sarcopenia in Patients Receiving Kidney Replacement Therapy: An Observational, Cross-Sectional Study" Nutrients 16, no. 15: 2480. https://doi.org/10.3390/nu16152480
APA StyleCzaja-Stolc, S., Chatrenet, A., Potrykus, M., Ruszkowski, J., Torreggiani, M., Lichodziejewska-Niemierko, M., Dębska-Ślizień, A., Piccoli, G. B., & Małgorzewicz, S. (2024). Adipokines and Myokines as Markers of Malnutrition and Sarcopenia in Patients Receiving Kidney Replacement Therapy: An Observational, Cross-Sectional Study. Nutrients, 16(15), 2480. https://doi.org/10.3390/nu16152480









