Association between Dietary Acid Load and Chronic Kidney Disease in the Chinese Population: A Comprehensive Analysis of the China Health and Nutrition Survey (2009)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Participants
2.2. Predictors—Dietary Acid Load Estimations
2.3. Outcomes—Definition of CKD
2.4. Statistical Analysis
3. Results
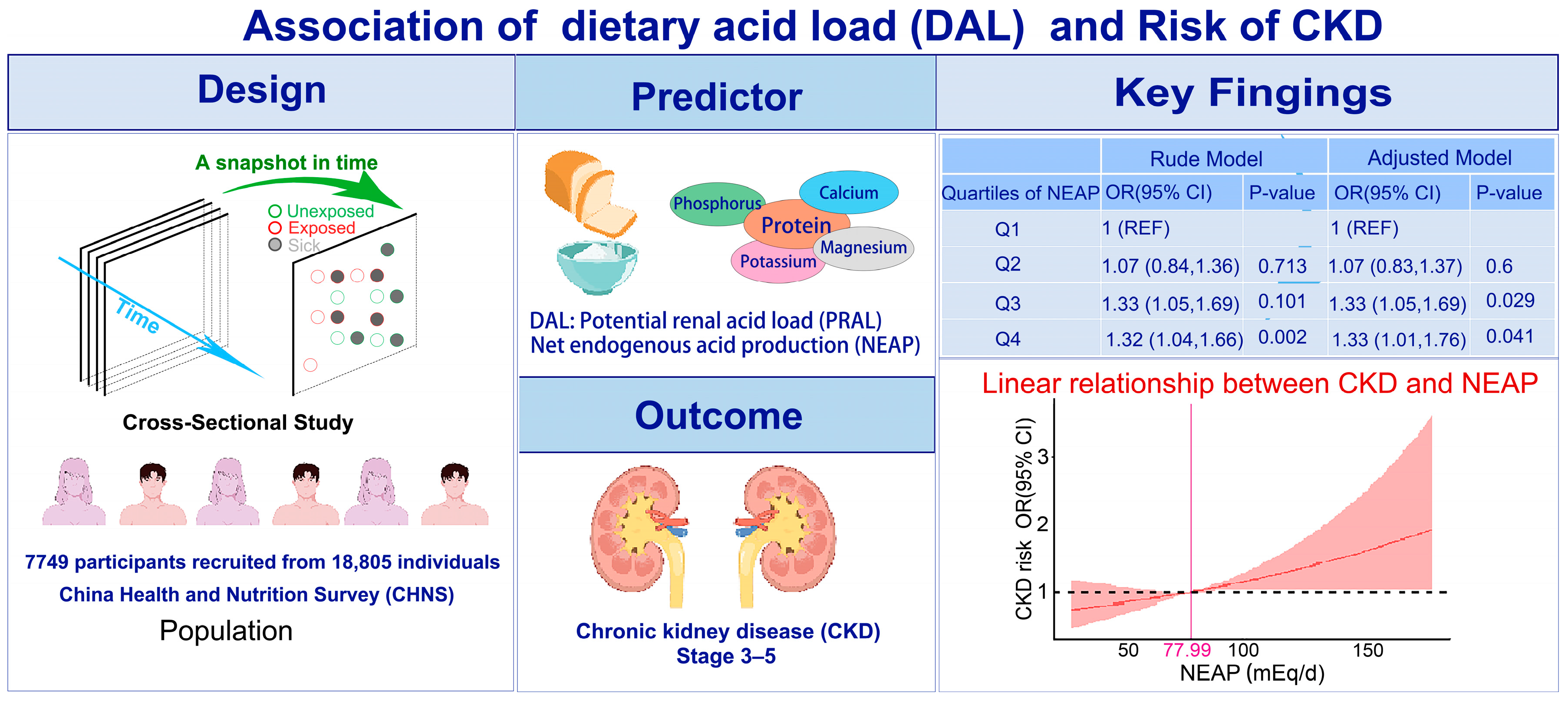
3.1. Baseline Characteristics
3.2. Association between DAL and CKD
3.3. Subgroup Analysis for the Association between DAL and CKD
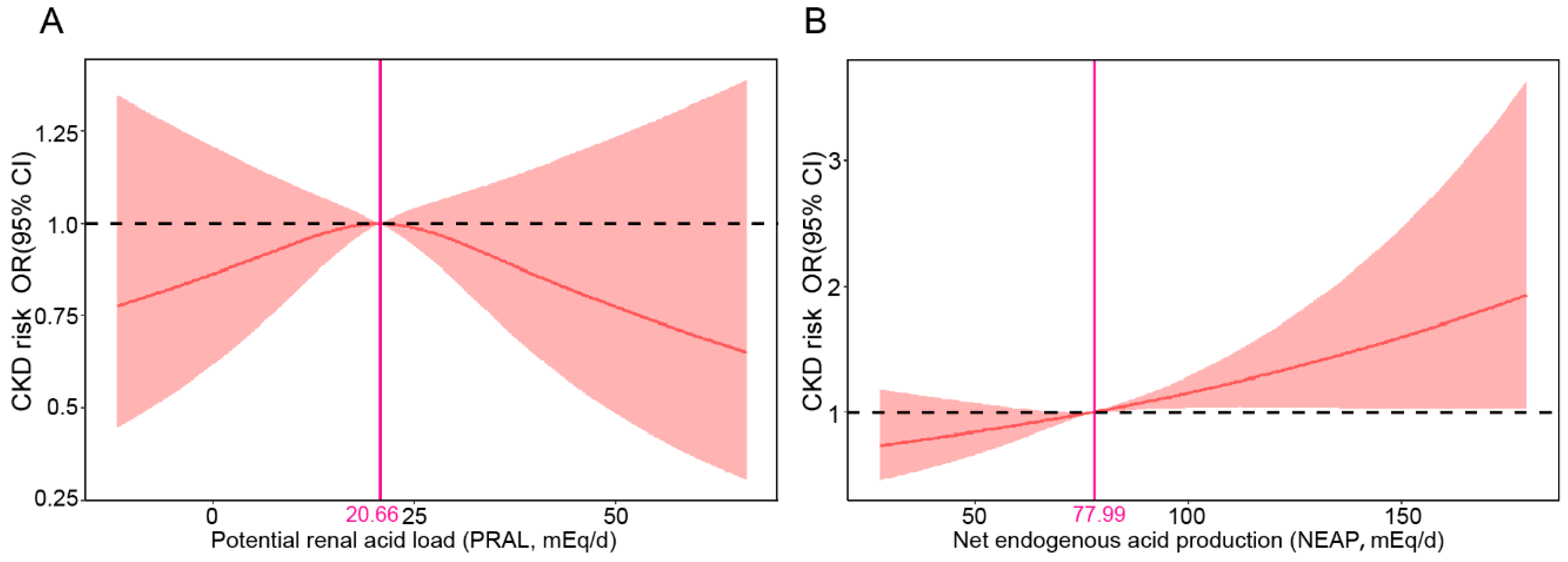
3.4. RCS Analysis of DAL and Risk of CKD
3.5. Dietary Intakes and CKD Risk in an Energy-Adjusted Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Drawz, P.; Rahman, M. Chronic kidney disease. Ann. Intern. Med. 2015, 162, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543s–1548s. [Google Scholar] [CrossRef]
- MacLaughlin, H.L.; Friedman, A.N.; Ikizler, T.A. Nutrition in Kidney Disease: Core Curriculum 2022. Am. J. Kidney Dis. 2022, 79, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, D.; Vellanki, K.; Kramer, H. The Western Diet and Chronic Kidney Disease. Curr. Hypertens. Rep. 2015, 17, 16. [Google Scholar] [CrossRef]
- Harambat, J.; Kunzmann, K.; Azukaitis, K.; Bayazit, A.K.; Canpolat, N.; Doyon, A.; Duzova, A.; Niemirska, A.; Sözeri, B.; Thurn-Valsassina, D.; et al. Metabolic acidosis is common and associates with disease progression in children with chronic kidney disease. Kidney Int. 2017, 92, 1507–1514. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Nitta, K.; Tsuchiya, K. Recent advances in the pathophysiology and management of protein-energy wasting in chronic kidney disease. Ren. Replace. Ther. 2016, 2, 4. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Metabolic Acidosis of CKD: An Update. Am. J. Kidney Dis. 2016, 67, 307–317. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, C.; Wang, R.; Zhang, Z.; Huang, X.; Halimulati, M.; Sun, M.; Ma, Y.; Zhang, Z. Association between Dietary Acid Load and Hyperuricemia in Chinese Adults: Analysis of the China Health and Nutrition Survey (2009). Nutrients 2023, 15, 1806. [Google Scholar] [CrossRef]
- Mangano, K.M.; Walsh, S.J.; Kenny, A.M.; Insogna, K.L.; Kerstetter, J.E. Dietary acid load is associated with lower bone mineral density in men with low intake of dietary calcium. J. Bone Min. Res. 2014, 29, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Najafabadi, M.M.; Bellissimo, N.; Azadbakht, L. Association of dietary acid load with cardiovascular disease risk factors in patients with diabetic nephropathy. Nutrition 2015, 31, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Kim, G.; Hong, N.; Lee, Y.H.; Kim, D.W.; Shin, H.J.; Lee, B.W.; Kang, E.S.; Lee, I.K.; Cha, B.S. Association between dietary acid load and the risk of cardiovascular disease: Nationwide surveys (KNHANES 2008-2011). Cardiovasc. Diabetol. 2016, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Dimitriou, T.; Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am. J. Clin. Nutr. 2003, 77, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Moço, S.A.; Antunes, M.L.; Ferreira, A.S.; Moreira, A.C. Dietary Acid Load and Relationship with Albuminuria and Glomerular Filtration Rate in Individuals with Chronic Kidney Disease at Predialysis State. Nutrients 2021, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Tilea, A.M.; Saran, R.; Rios-Burrows, N.; Williams, D.E.; Powe, N.R. High Dietary Acid Load Predicts ESRD among Adults with CKD. J. Am. Soc. Nephrol. 2015, 26, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Crews, D.C.; Banerjee, T.; Wesson, D.E.; Morgenstern, H.; Saran, R.; Burrows, N.R.; Williams, D.E.; Powe, N.R. Race/Ethnicity, Dietary Acid Load, and Risk of End-Stage Renal Disease among US Adults with Chronic Kidney Disease. Am. J. Nephrol. 2018, 47, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, H.; Du, S. China Health and Nutrition Survey, 1989–2019. In Encyclopedia of Gerontology and Population Aging; Gu, D., Dupre, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–6. [Google Scholar]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C., Jr.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef]
- Scialla, J.J.; Anderson, C.A. Dietary acid load: A novel nutritional target in chronic kidney disease? Adv. Chronic Kidney Dis. 2013, 20, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Frassetto, L.A.; Sellmeyer, D.E.; Merriam, R.L.; Morris Jr, R.C. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am. J. Clin. Nutr. 2002, 76, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.A.; Lanham-New, S.A.; Macdonald, H.M.; Remer, T.; Sebastian, A.; Tucker, K.L.; Tylavsky, F.A. Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J. Nutr. 2007, 137, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am. J. Clin. Nutr. 1994, 59, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Coresh, J.; Grams, M.E.; Steffen, L.M.; Anderson, C.A.; Appel, L.J.; Crews, D.C. Dietary Acid Load and Incident Chronic Kidney Disease: Results from the ARIC Study. Am. J. Nephrol. 2015, 42, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Appel, L.J.; Astor, B.C.; Miller, E.R., 3rd; Beddhu, S.; Woodward, M.; Parekh, R.S.; Anderson, C.A. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012, 82, 106–112. [Google Scholar] [CrossRef]
- Kabasawa, K.; Hosojima, M.; Takachi, R.; Nakamura, K.; Ito, Y.; Saito, A.; Sawada, N.; Tsugane, S.; Tanaka, J.; Narita, I. Association of estimated dietary acid load with albuminuria in Japanese adults: A cross-sectional study. BMC Nephrol. 2019, 20, 194. [Google Scholar] [CrossRef]
- Pike, M.; Stewart, T.G.; Morse, J.; Ormsby, P.; Siew, E.D.; Hung, A.; Abdel-Kader, K.; Ikizler, T.A.; Lipworth, L.; Robinson-Cohen, C. APOL1, Acid Load, and CKD Progression. Kidney Int. Rep. 2019, 4, 946–954. [Google Scholar] [CrossRef]
- Scialla, J.J.; Asplin, J.; Dobre, M.; Chang, A.R.; Lash, J.; Hsu, C.Y.; Kallem, R.R.; Hamm, L.L.; Feldman, H.I.; Chen, J.; et al. Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int. 2017, 91, 204–215. [Google Scholar] [CrossRef]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Morris, R.C., Jr.; Sellmeyer, D.E.; Todd, K.; Sebastian, A. Diet, evolution and aging—The pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur. J. Nutr. 2001, 40, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.; Paciência, I.; Rufo, J.C.; Mendes, F.C.; Farraia, M.; Barros, R.; Silva, D.; Delgado, L.; Padrão, P.; Moreira, A.; et al. Dietary Acid Load: A Novel Nutritional Target in Overweight/Obese Children with Asthma? Nutrients 2019, 11, 2255. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.; Kozan, P.; Samocha-Bonet, D. The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie 2016, 124, 171–177. [Google Scholar] [CrossRef]
- Ko, B.J.; Chang, Y.; Ryu, S.; Kim, E.M.; Lee, M.Y.; Hyun, Y.Y.; Lee, K.B. Dietary acid load and chronic kidney disease in elderly adults: Protein and potassium intake. PLoS ONE 2017, 12, e0185069. [Google Scholar] [CrossRef] [PubMed]
- Truche, A.S.; Bailly, S.; Fabre, O.; Legrand, R.; Zaoui, P. A Specific High-Protein Weight Loss Program Does Not Impair Renal Function in Patients Who Are Overweight/Obese. Nutrients 2022, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Borrelli, S.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017, 91, 1224–1235. [Google Scholar] [CrossRef]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef]
- Kamper, A.L.; Strandgaard, S. Long-Term Effects of High-Protein Diets on Renal Function. Annu. Rev. Nutr. 2017, 37, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kramer, H.M.; Fouque, D. High-protein diet is bad for kidney health: Unleashing the taboo. Nephrol. Dial. Transpl. 2020, 35, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Crews, D.C.; Grams, M.E.; Steffen, L.M.; Levey, A.S.; Miller, E.R., 3rd; Appel, L.J.; Coresh, J. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am. J. Kidney Dis. 2016, 68, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Jones, G.W.; Di Lauro, M.; Zaitseva, A.P.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 2534. [Google Scholar] [CrossRef]
- Kontessis, P.; Jones, S.; Dodds, R.; Trevisan, R.; Nosadini, R.; Fioretto, P.; Borsato, M.; Sacerdoti, D.; Viberti, G. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990, 38, 136–144. [Google Scholar] [CrossRef]
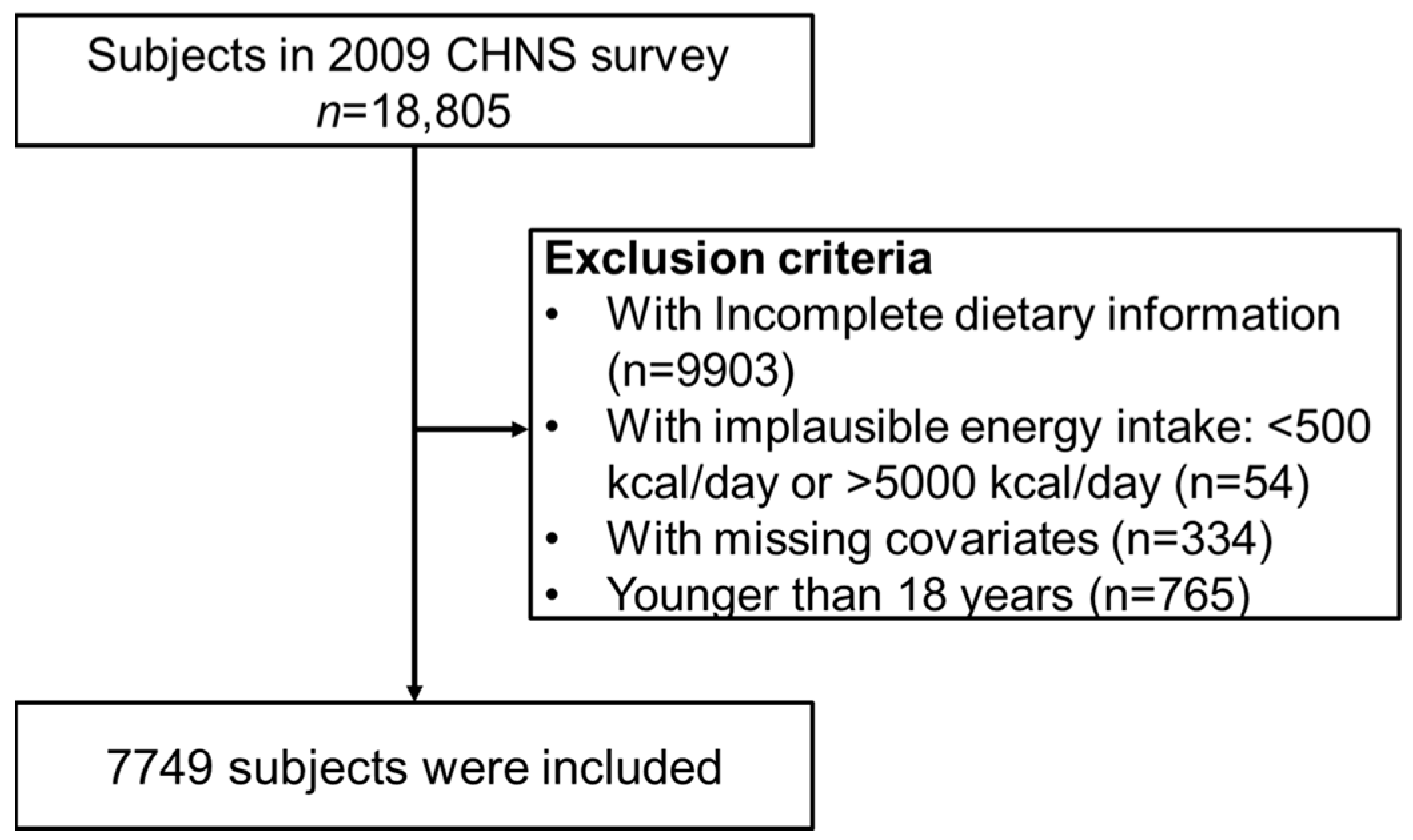

| Variable | Overall (n = 7749) | Non-CKD (n = 6854) | CKD (n = 895) | p | |
|---|---|---|---|---|---|
| Age (y) | 50.2 ± 15.0 | 47.9 ± 13.9 | 67.9 ± 10.8 | <0.001 | |
| Gender | Male | 3639 (46.96%) | 3281 (47.87%) | 358 (40%) | <0.001 |
| Education | Primary | 3417 (44.1%) | 2785 (40.63%) | 632 (70.61%) | <0.001 |
| Middle | 2573 (33.2%) | 2436 (35.54%) | 137 (15.31%) | ||
| High | 886 (11.43%) | 834 (12.17%) | 52 (5.81%) | ||
| Vocational | 520 (6.71%) | 468 (6.83%) | 52 (5.81%) | ||
| University | 353 (4.56%) | 331 (4.83%) | 22 (2.46%) | ||
| Marry | Unmarried | 1163 (15.01%) | 914 (13.34%) | 249 (27.82%) | <0.001 |
| Married | 6586 (84.99%) | 5940 (86.66%) | 646 (72.18%) | ||
| Location | Rural | 4758 (61.04%) | 4316 (62.97%) | 442 (49.39%) | <0.001 |
| Urban | 2991 (38.6%) | 2538 (37.03%) | 453 (50.61%) | ||
| Alcohol | Yes | 2532 (32.68%) | 2359 (34.42%) | 173 (19.33%) | <0.001 |
| Smoking | Yes | 2407 (31.06%) | 2176 (31.75%) | 231 (25.81%) | <0.001 |
| Hip (cm) | 94.4 ± 7.8 | 94.4 ± 7.8 | 94.0 ± 8.5 | 0.056 | |
| Waist (cm) | 82.6 ± 10.3 | 82.4 ± 10.2 | 83.8 ± 10.7 | <0.001 | |
| BMI (kg/m2) | 23.3 ± 3.4 | 23.3 ± 3.4 | 23.3 ± 3.8 | 0.410 | |
| BMI (kg/m2) | <18.5 | 490 (6.32%) | 411 (6.0%) | 79 (8.83%) | 0.005 |
| (18.5, 23.9) | 4186 (54.02%) | 3717 (54.23%) | 469 (52.4%) | ||
| (24, 27.9) | 715 (9.23%) | 622 (9.07%) | 93 (10.39%) | ||
| ≥28 | 2358 (30.43%) | 2104 (30.7%) | 254 (28.38%) | ||
| Hypertension | Yes | 1012 (13.06%) | 732 (10.68%) | 280 (31.28%) | <0.001 |
| Diabetes | Yes | 216 (2.79%) | 152 (2.22%) | 64 (7.15%) | <0.001 |
| Heart Disease | Yes | 73 (0.94%) | 44 (0.64%) | 29 (3.24%) | <0.001 |
| Fraction | Yes | 365 (4.71%) | 310 (4.52%) | 55 (6.15%) | 0.036 |
| Urea (mg/dL) | 32 (26, 38) | 31 (26, 37) | 37 (31, 44) | <0.001 | |
| Ua (mg/dL) | 4.96 (3.98, 6.05) | 4.84 (3.90, 5.92) | 5.85 (4.97, 6.96) | <0.001 | |
| CRP (mg/L) | 1.00 (0.00, 2.00) | 1.00 (0.00, 2.00) | 2.00 (1.00, 4.00) | <0.001 | |
| eGFR (mL/min/1.73 m2) | 76 (67, 85) | 78 (70, 87) | 55 (49, 58) | <0.001 | |
| PRAL (mEq/d) | 21 (13, 28) | 21 (13, 28) | 21 (13, 28) | 0.808 | |
| NEAP (mEq/d) | 78 (65, 92) | 77 (65, 92) | 80 (67, 95) | <0.001 |
| Nutrients | Overall (n = 7749) | Non-CKD (n = 6854) | CKD (n = 895) | p |
|---|---|---|---|---|
| Energy (Kcal) | 1656 (1354, 1985) | 1659 (1355, 1988) | 1634 (1344, 1967) | 0.238 |
| Carbohydrate (g) | 152 (129, 176) | 153 (129, 177) | 150 (127, 172) | 0.006 |
| Protein (g) | 37 (32, 44) | 37 (32, 44) | 37 (33, 45) | 0.736 |
| Fat (g) | 25 (15, 35) | 25 (15, 35) | 26 (17, 36) | 0.004 |
| Cholesterol (mg) | 209 (101, 343) | 210 (102, 343) | 202 (95, 342) | 0.774 |
| Carbohydrate (g) | 152 (129, 176) | 153 (129, 177) | 150 (127, 172) | 0.006 |
| Calcium (mg) | 180 (142, 241) | 179 (141, 240) | 185 (147, 249) | 0.006 |
| Phosphorus (mg) | 536 (478, 602) | 536 (480, 602) | 524 (463, 599) | 0.002 |
| Potassium (mg) | 922 (777, 1107) | 924 (780, 1109) | 906 (744, 1103) | 0.012 |
| Magnesium (mg) | 154 (133, 182) | 155 (134, 183) | 150 (128, 177) | 0.001 |
| Water (g) | 489 (385, 620) | 490 (386, 620) | 484 (381, 618) | 0.349 |
| PRAL | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Q1 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| Q2 | 1.02 (0.84, 1.25) | 0.839 | 1.04 (0.82, 1.32) | 0.723 | 1.03 (0.84, 1.26) | 0.778 | 1.04 (0.82, 1.32) | 0.715 | 1.02 (0.79, 1.31) | 0.887 |
| Q3 | 1.08 (0.89, 1.31) | 0.452 | 1.15 (0.91, 1.45) | 0.244 | 1.08 (0.88, 1.32) | 0.462 | 1.15 (0.91, 1.45) | 0.243 | 1.11 (0.85, 1.45) | 0.441 |
| Q4 | 1.02 (0.84, 1.24) | 0.844 | 1.15 (0.91, 1.46) | 0.247 | 0.98 (0.80, 1.21) | 0.873 | 1.14 (0.90, 1.45) | 0.275 | 1.08 (0.77, 1.52) | 0.665 |
| NEAP | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Q1 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| Q2 | 1.04 (0.85, 1.28) | 0.713 | 1.07 (0.84, 1.36) | 0.584 | 1.07 (0.87, 1.32) | 0.531 | 1.09 (0.86, 1.39) | 0.482 | 1.07 (0.83, 1.37) | 0.6 |
| Q3 | 1.18 (0.97, 1.45) | 0.101 | 1.33 (1.05, 1.69) | 0.017 | 1.19 (0.97, 1.47) | 0.097 | 1.32 (1.04, 1.67) | 0.023 | 1.33 (1.03, 1.72) | 0.029 |
| Q4 | 1.37 (1.13, 1.67) | 0.002 | 1.32 (1.04, 1.66) | 0.02 | 1.34 (1.09, 1.64) | 0.005 | 1.30 (1.03, 1.64) | 0.03 | 1.33 (1.01, 1.76) | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Fan, X.; Zheng, X.; Xia, P.; Zou, H.; Zhang, Z.; Chen, L. Association between Dietary Acid Load and Chronic Kidney Disease in the Chinese Population: A Comprehensive Analysis of the China Health and Nutrition Survey (2009). Nutrients 2024, 16, 2461. https://doi.org/10.3390/nu16152461
Wang S, Fan X, Zheng X, Xia P, Zou H, Zhang Z, Chen L. Association between Dietary Acid Load and Chronic Kidney Disease in the Chinese Population: A Comprehensive Analysis of the China Health and Nutrition Survey (2009). Nutrients. 2024; 16(15):2461. https://doi.org/10.3390/nu16152461
Chicago/Turabian StyleWang, Shurui, Xiaohong Fan, Xixi Zheng, Peng Xia, Haiou Zou, Zhaofeng Zhang, and Limeng Chen. 2024. "Association between Dietary Acid Load and Chronic Kidney Disease in the Chinese Population: A Comprehensive Analysis of the China Health and Nutrition Survey (2009)" Nutrients 16, no. 15: 2461. https://doi.org/10.3390/nu16152461
APA StyleWang, S., Fan, X., Zheng, X., Xia, P., Zou, H., Zhang, Z., & Chen, L. (2024). Association between Dietary Acid Load and Chronic Kidney Disease in the Chinese Population: A Comprehensive Analysis of the China Health and Nutrition Survey (2009). Nutrients, 16(15), 2461. https://doi.org/10.3390/nu16152461







