Salidroside: A Promising Agent in Bone Metabolism Modulation
Abstract
1. Introduction
2. Biochemical Structure of Salidroside
3. Influence of Salidroside on Bone Metabolism
3.1. In Vitro Studies
3.1.1. Proliferation and Viability of Osteoblast Precursors
3.1.2. Activation and Expression of Bone Morphogenetic Proteins
3.1.3. Alkaline Phosphatase Activity and Mineralization
3.1.4. Adenosine Monophosphate-Activated Protein Kinase Activation
3.1.5. Cellular and Molecular Impact on Endothelial Cells
Improvement of Proliferation and Viability of Endothelial Cells
Stimulation of Migration and Capillary Tube Formation
Activation of the HIF-1α/VEGF Signaling Pathway
3.1.6. Effects of Salidroside on Glucocorticoid-Induced Osteoporosis
3.1.7. Salidroside’s Role in Mitigating Osteoporosis through Antioxidant Activity
3.2. In Vivo Studies
3.2.1. Protective Effects against Oxidative Stress
3.2.2. Effects on Knee Osteoarthritis in Mice
3.2.3. Angiogenesis in Mouse Embryonic Metatarsals
3.2.4. Osteogenesis and Bone Healing
3.2.5. Effects on Osteoporosis Model in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Panossian, A.; Wikman, G. Effects of Adaptogens on the Central Nervous System and the Molecular Mechanisms Associated with Their Stress-Protective Activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef]
- Darbinyan, V.; Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Malmström, C.; Panossian, A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord. J. Psychiatry 2000, 61, 343–348. [Google Scholar] [CrossRef]
- Olsson, E.M.; von Schéele, B.; Panossian, A.G. A randomised double-blind placebo-controlled parallel-group study of the standardised extract SHR-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Medica 2009, 75, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. Rhodiola rosea: A possible plant adaptogen. Altern. Med. Rev. 2001, 6, 293–302. [Google Scholar]
- Brown, R.P.; Gerbarg, P.L.; Ramazanov, Z. Rhodiola rosea: A phytomedicinal overview. HerbalGram 2002, 56, 40–52. [Google Scholar]
- Ishaque, S.; Shamseer, L.; Bukutu, C.; Vohra, S. Rhodiola rosea for physical and mental fatigue: A systematic review. BMC Complement. Altern. Med. 2012, 12, 70. [Google Scholar] [CrossRef]
- Mao, Y.; Li, Q.; Yao, L.; Jiang, Z.; Jin, G.; Zhao, X. Salidroside: Advances in pharmacological activities and molecular mechanisms of action. J. Ethnopharmacol. 2020, 261, 113112. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.; Leung, G.P.; Yue, P.Y.; Wong, R.N. A review of the pharmacological effects of Rhodiola rosea L. and its bioactive compounds. Phytother. Res. 2012, 26, 1727–1734. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, Y.; Li, Y.; Lu, D.; Wang, X.; Ji, X. Salidroside regulates cardiac dysfunction in heart failure by enhancing mitochondrial biogenesis and inhibiting oxidative stress. Biomed. Pharmacother. 2018, 105, 119–128. [Google Scholar] [CrossRef]
- Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea: An herb with anti-stress, anti-aging, and anti-cancer properties. Cancer Cell Int. 2019, 19, 202. [Google Scholar]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology, and economic burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; Liu, E.; Vandenput, L.; McCloskey, E.V. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2022, 17, 104. [Google Scholar] [CrossRef]
- Pisani, P.; Renna, M.D.; Conversano, F.; Casciaro, E.; Di Paola, M.; Quarta, E.; Muratore, M.; Casciaro, S. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J. Orthop. 2016, 7, 171–181. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Xu, H. Protective effects of Rhodiola rosea on oxidative stress and liver injury in rats. Mol. Cell. Biochem. 2009, 331, 31–41. [Google Scholar]
- Fan, X.J.; Wang, Y.; Wang, L.; Zhu, M. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol. Rep. 2016, 6, 3559–3567. [Google Scholar] [CrossRef]
- György, Z.; Hohtola, A. Production of cinnamyl glycosides in compact callus aggregate cultures of Rhodiola rosea through biotransformation of cinnamyl alcohol. In Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants; Jain, S.M., Saxena, P.K., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 305–312. [Google Scholar]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology, and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Guo, H.; Li, W.; Yan, S.; Zhao, S. Phytochemical analysis of traditional Chinese medicine using liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2015, 1428, 155–165. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sowa-Kasprzak, K. Salidroside as an adaptogen compound. Nat. Prod. Commun. 2013, 8, 1435–1440. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2009, 118, 183–212. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhang, N.F.; Mao, G.X.; He, X.B.; Zhan, Y.C.; Deng, H.B.; Wang, Z. Salidroside stimulates osteoblast differentiation through BMP signaling pathway. Food Chem. Toxicol. 2013, 62, 499–505. [Google Scholar] [CrossRef]
- Pan, X.; Peng, X.; Jiang, C.; Yu, H.; Yang, Y.; Chen, H. Salidroside promotes the osteogenic differentiation of rat bone marrow mesenchymal stem cells through the BMP/Smad pathway. Drug Des. Dev. Ther. 2019, 13, 2501–2511. [Google Scholar]
- Li, J.; Guo, W. The effects of salidroside on bone marrow mesenchymal stem cells and their application in the treatment of osteoporosis. Life Sci. 2018, 207, 333–340. [Google Scholar] [CrossRef]
- Pan, X.; Liu, H.; Liu, J.; Shu, B.; Sun, L. Salidroside promotes osteogenic differentiation and bone formation in primary rat bone marrow stromal cells. Exp. Ther. Med. 2013, 5, 1265–1273. [Google Scholar] [CrossRef]
- Xie, B.; Zhou, H.; Liu, H.; Liao, S.; Zhou, C.; Xu, D. Salidroside alleviates dexamethasone-induced inhibition of bone formation via transforming growth factor-beta/Smad2/3 signaling pathway. Phytother. Res. 2023, 37, 1938–1950. [Google Scholar] [CrossRef]
- Huntley, R.; Jensen, E.; Gopalakrishnan, R.; Mansky, K.C. Bone morphogenetic proteins: Their role in regulating osteoclast differentiation. Bone Rep. 2019, 10, 100207. [Google Scholar] [CrossRef]
- Simic, P.; Culej, J.B.; Orlic, I.; Grgurevic, L.; Draca, N.; Spaventi, R.; Vukicevic, S. Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J. Biol. Chem. 2006, 281, 25509–25521. [Google Scholar] [CrossRef]
- Fu, S.; Yan, M.; Fan, Q.; Xu, J. Salidroside promotes osteoblast proliferation and differentiation via the activation of AMPK to inhibit bone resorption in knee osteoarthritis mice. Tissue Cell 2022, 79, 101917. [Google Scholar] [CrossRef]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Dirckx, N.; Van Hul, M.; Maes, C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth defects research. Part C Embryo Today Rev. 2013, 99, 170–191. [Google Scholar] [CrossRef]
- Clarkin, C.E.; Gerstenfeld, L.C. VEGF and bone cell signalling: An essential vessel for communication? Cell Biochem. Funct. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, P.; Qin, Q.; Jiang, K.; Luo, Y.; Xiang, C.; He, J.; Chen, L.; Jiang, D.; Cui, W.; et al. 3D-printed bone regeneration scaffolds modulate bone metabolic homeostasis through vascularization for osteoporotic bone defects. Biomaterials 2024, 311, 122699. [Google Scholar] [CrossRef]
- Tong, X.; Chen, X.; Zhang, S.; Huang, M.; Shen, X.; Xu, J.; Zou, J. The Effect of Exercise on the Prevention of Osteoporosis and Bone Angiogenesis. BioMed Res. Int. 2019, 2019, 8171897. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, J.; Chen, Y.; Jin, X.; Li, Z.; Wen, X.; Xia, Q.; Wang, Y. Salidroside improves angiogenesis-osteogenesis coupling by regulating the HIF-1α/VEGF signalling pathway in the bone environment. Eur. J. Pharmacol. 2020, 884, 173394. [Google Scholar] [CrossRef]
- Guo, X.Q.; Qi, L.; Yang, J.; Wang, Y.; Wang, C.; Li, Z.M.; Li, L.; Qu, Y.; Wang, D.; Han, Z.M. Salidroside accelerates fracture healing through cell-autonomous and non-autonomous effects on osteoblasts. Cell Tissue Res. 2017, 367, 197–211. [Google Scholar] [CrossRef]
- Xue, X.; Feng, Z.; Li, Z.; Pan, X. Salidroside inhibits steroid-induced avascular necrosis of the femoral head via the PI3K/Akt signaling pathway: In vitro and in vivo studies. Mol. Med. Rep. 2018, 17, 3751–3757. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chang, Y.Y.; Zhang, X.M.; Gao, M.T.; Zhang, Q.L.; Li, X.; Zhang, L.; Yao, W.F. Salidroside protects against osteoporosis in ovariectomized rats by inhibiting oxidative stress and promoting osteogenesis via Nrf2 activation. Phytomedicine 2022, 99, 154020. [Google Scholar] [CrossRef]
- Zhang, J.K.; Yang, L.; Meng, G.L.; Yuan, Z.; Fan, J.; Li, D.; Liu, J. Protection by salidroside against bone loss via inhibition of oxidative stress and bone-resorbing mediators. PLoS ONE 2013, 8, e57251. [Google Scholar] [CrossRef]
- Li, L.; Qu, Y.; Jin, X.; Guo, X.Q.; Wang, Y.; Qi, L.; Yang, J.; Zhang, P.; Li, L.Z. Protective effect of salidroside against bone loss via hypoxia-inducible factor-1α pathway-induced angiogenesis. Sci. Rep. 2016, 6, 32131. [Google Scholar] [CrossRef]
- Zheng, H.; Qi, S.; Chen, C. Salidroside Improves Bone Histomorphology and Prevents Bone Loss in Ovariectomized Diabetic Rats by Upregulating the OPG/RANKL Ratio. Molecules 2018, 23, 2398. [Google Scholar] [CrossRef]
- Amirtharaj Mosas, K.K.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent Advancements in Materials and Coatings for Biomedical Implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef]
- Yokota, S.; Ishizu, H.; Miyazaki, T.; Takahashi, D.; Iwasaki, N.; Shimizu, T. Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights. Biomedicines 2024, 12, 843. [Google Scholar] [CrossRef]
- Babhulkar, S. Newer trends in complex trauma and fracture nonunion. Injury 2017, 48 (Suppl. 2), S1. [Google Scholar] [CrossRef]
- Ozen, G.; Pedro, S.; Wolfe, F.; Michaud, K. Medications associated with fracture risk in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 1041–1047. [Google Scholar] [CrossRef]
- Qin, Y.; Su, J. Salidroside suppresses cell growth and inflammatory response of fibroblast-like synoviocytes via inhibition of phosphoinositol-3 kinase/threonine kinase signaling in rheumatoid arthritis. Unterdrückung von Zellwachstum und entzündlicher Reaktion fibroblastenartiger Synoviozyten durch Salidrosid via Inhibition des Phosphatidylinositol-3-Kinase-/Proteinkinase-Signalwegs bei rheumatoider Arthritis. Z. Fur Rheumatol. 2024, 83 (Suppl. 1), 78–87. [Google Scholar] [CrossRef]
- Gerbarg, P.L.; Brown, R.P. Pause menopause with Rhodiola rosea, a natural selective estrogen receptor modulator. Phytomedicine Int. J. Phytother. Phytopharm. 2016, 23, 763–769. [Google Scholar] [CrossRef]
- Schinzel, E.; Kast, S.; Kohl, M.; von Stengel, S.; Jakob, F.; Kerschan-Schindl, K.; Kladny, B.; Lange, U.; Peters, S.; Thomasius, F.; et al. The effect of aquatic exercise on bone mineral density in older adults. A systematic review and meta-analysis. Front. Physiol. 2023, 14, 1135663. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef]
- Shams, R.; Drasites, K.P.; Zaman, V.; Matzelle, D.; Shields, D.C.; Garner, D.P.; Sole, C.J.; Haque, A.; Banik, N.L. The Pathophysiology of Osteoporosis after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 3057. [Google Scholar] [CrossRef]
- Turczyn, P.; Wojdasiewicz, P.; Poniatowski, Ł.A.; Purrahman, D.; Maślińska, M.; Żurek, G.; Romanowska-Próchnicka, K.; Żuk, B.; Kwiatkowska, B.; Piechowski-Jóźwiak, B.; et al. Omega-3 fatty acids in the treatment of spinal cord injury: Untapped potential for therapeutic intervention? Mol. Biol. Rep. 2022, 49, 10797–10809. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Y.; Zheng, L.; He, P.; Tan, J.; Cai, J.; Wu, M.; Ye, X. Rosavin: Research Advances in Extraction and Synthesis, Pharmacological Activities and Therapeutic Effects on Diseases of the Characteristic Active Ingredients of Rhodiola rosea L. Molecules 2023, 28, 7412. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Turczyn, P.; Lach-Gruba, A.; Poniatowski, Ł.A.; Purrahman, D.; Mahmoudian-Sani, M.-R.; Szukiewicz, D. The Role of Rosavin in the Pathophysiology of Bone Metabolism. Int. J. Mol. Sci. 2024, 25, 2117. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Arafat, B.; Gultekin, H.E. 3D-printed dosage forms for oral administration: A review. Drug Deliv. Transl. Res. 2023, 13, 210–223. [Google Scholar]
- Pan, X.; Liu, H.; Liu, J.; Shu, B.; Sun, L. Advances in Oral Drug Delivery Systems: Challenges and Opportunities. Pharmaceutics 2023, 15, 484. [Google Scholar] [CrossRef] [PubMed]


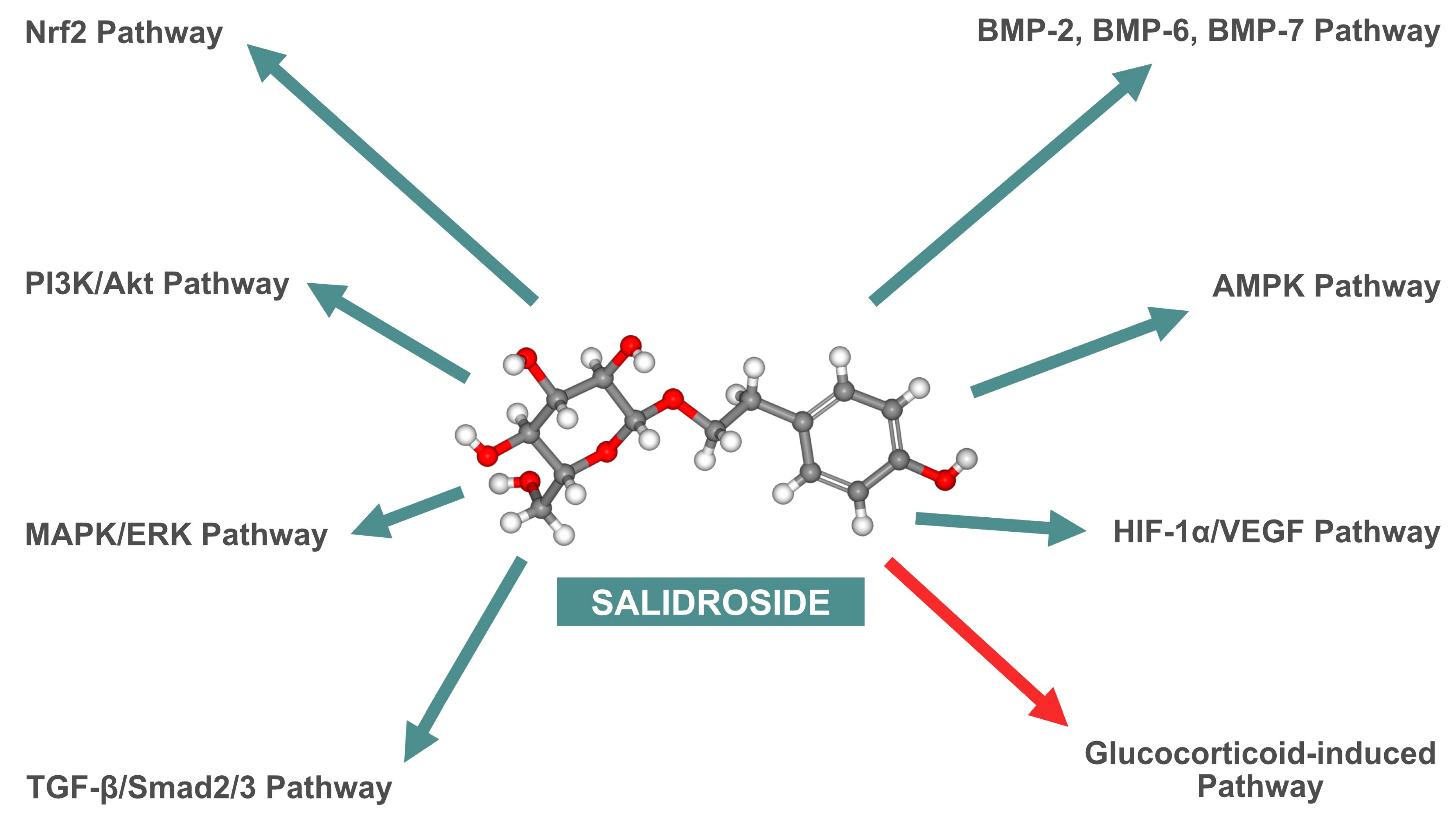
| First Author, Year (Reference) | Cell Lines | Biological Manifestation |
|---|---|---|
| Pan et al., 2013 [24] | rBMSCs | Promotion of osteoblast differentiation and bone formation, upregulation of osteogenic markers ALP and COL1A1. |
| Guo et al., 2017 [35] | MG-63, ROBs | Enhanced proliferation and differentiation of osteoblasts, activation of MAPK/ERK and PI3K/Akt pathways, increased Runx2 and OSX expression. |
| Li et al., 2018 [23] | rBMSCs | Increased osteogenic differentiation, enhanced ALP activity, and mineralization, promotion of bone formation, increased expression of Runx2 and ALP. |
| Xue et al., 2018 [36] | ROBs | Protection against apoptosis, activation of PI3K/Akt pathway, reduction in apoptotic markers such as BAX, CASP3, and CASP9, increased expression of Runx2 and OSX. |
| Chen et al., 2019 [21] | C3H10T1/2, MC3T3-E1, rBMSCs | Increased proliferation and differentiation of osteoblasts, enhanced ALP activity, and mineralization via BMP/Smad pathway activation, increased expression of Runx2 and OSX. |
| Guo et al., 2020 [34] | EA.hy926, HUVECs, MG-63, ROBs | Increased proliferation, migration, capillary formation, and mineralization through HIF-1α/VEGF pathway activation, increased expression of ALP, Runx2, OSX, and VEGF. |
| Fu et al., 2022 [28] | MC3T3-E1 | Enhanced proliferation and differentiation of osteoblasts, AMPK activation, inhibition of bone resorption, increased expression of ALP, COL1A1, OCN, and Runx2. |
| Wang et al., 2022 [37] | ROBs | Protection against oxidative stress, increased Nrf2 activation, decreased Keap1 expression, promotion of osteogenesis, increased expression of ALP and Runx2. |
| Xie et al., 2023 [25] | MC3T3-E1 | Protection against dexamethasone-induced inhibition, increased ALP activity, activation of TGF-β/Smad2/3 pathway, increased expression of OSX and ALP. |
| First Author, Year (Reference) | Animal Model | Biological Effect |
|---|---|---|
| Pan et al., 2013 [24] | Sprague Dawley rats (OVX-induced bone loss model) | Reduced oxidative stress, increased bone mass, reduced RANKL, increased OPG. |
| Guo et al., 2017 [35] | BALB/c mice (tibia fracture model) | Accelerated fracture healing, enhanced osteoblast proliferation and differentiation, increased HIF-1α and VEGF. |
| Li et al., 2018 [23] | Sprague Dawley rats (OVX-induced OP model) | Increased bone mass and mineral apposition rates, improved bone microarchitecture, increased HIF-1α and VEGF. |
| Xue et al., 2018 [36] | Sprague Dawley rats (SANFH model) | Reduced osteoblast apoptosis, increased osteogenic differentiation via PI3K/Akt, increased Bcl-2, decreased BAX and CASP3. |
| Zheng et al., 2018 [40] | Sprague Dawley rats (OVX-induced OP model with diabetes) | Improved bone histomorphology, prevention of bone loss, upregulation of the OPG/RANKL ratio |
| Chen et al., 2019 [21] | C57BL/6 mice (oxidative stress model) | Preserved bone microstructure, reduced MDA and ROS, increased osteoblast function and antioxidant enzymes such as SOD and GSH-Px. |
| Guo et al., 2020 [34] | C57BL/6 mice (angiogenesis model using mouse embryonic metatarsals) | Enhanced endothelial sprouting, increased VEGF, increased CD31-positive endothelial cells. |
| Fu et al., 2022 [28] | C57BL/6 mice (KOA model) | Reduced inflammation, increased osteogenic protein expression, enhanced AMPK activation, reduced TNF-α, IL-1β, and IL-6. |
| Wang et al., 2022 [37] | Sprague Dawley rats (OVX-induced OP model) | Reduced oxidative stress, increased osteogenesis via Nrf2, increased Runx2, ALP, and OCN. |
| Xie et al., 2023 [25] | C57BL/6 mice (dexamethasone-induced OP model) | Mitigated inhibitory effects of dexamethasone on osteogenesis, activated TGF-β/Smad2/3, increased ALP and COL1A1. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojdasiewicz, P.; Brodacki, S.; Cieślicka, E.; Turczyn, P.; Poniatowski, Ł.A.; Ławniczak, W.; Olczak, M.; Stolarczyk, E.U.; Wróbel, E.; Mikulska, A.; et al. Salidroside: A Promising Agent in Bone Metabolism Modulation. Nutrients 2024, 16, 2387. https://doi.org/10.3390/nu16152387
Wojdasiewicz P, Brodacki S, Cieślicka E, Turczyn P, Poniatowski ŁA, Ławniczak W, Olczak M, Stolarczyk EU, Wróbel E, Mikulska A, et al. Salidroside: A Promising Agent in Bone Metabolism Modulation. Nutrients. 2024; 16(15):2387. https://doi.org/10.3390/nu16152387
Chicago/Turabian StyleWojdasiewicz, Piotr, Stanisław Brodacki, Ewa Cieślicka, Paweł Turczyn, Łukasz A. Poniatowski, Weronika Ławniczak, Mieszko Olczak, Elżbieta U. Stolarczyk, Edyta Wróbel, Agnieszka Mikulska, and et al. 2024. "Salidroside: A Promising Agent in Bone Metabolism Modulation" Nutrients 16, no. 15: 2387. https://doi.org/10.3390/nu16152387
APA StyleWojdasiewicz, P., Brodacki, S., Cieślicka, E., Turczyn, P., Poniatowski, Ł. A., Ławniczak, W., Olczak, M., Stolarczyk, E. U., Wróbel, E., Mikulska, A., Lach-Gruba, A., Żuk, B., Romanowska-Próchnicka, K., & Szukiewicz, D. (2024). Salidroside: A Promising Agent in Bone Metabolism Modulation. Nutrients, 16(15), 2387. https://doi.org/10.3390/nu16152387









