A Nutrigenomic View on the Premature-Aging Disease Fanconi Anemia
Abstract
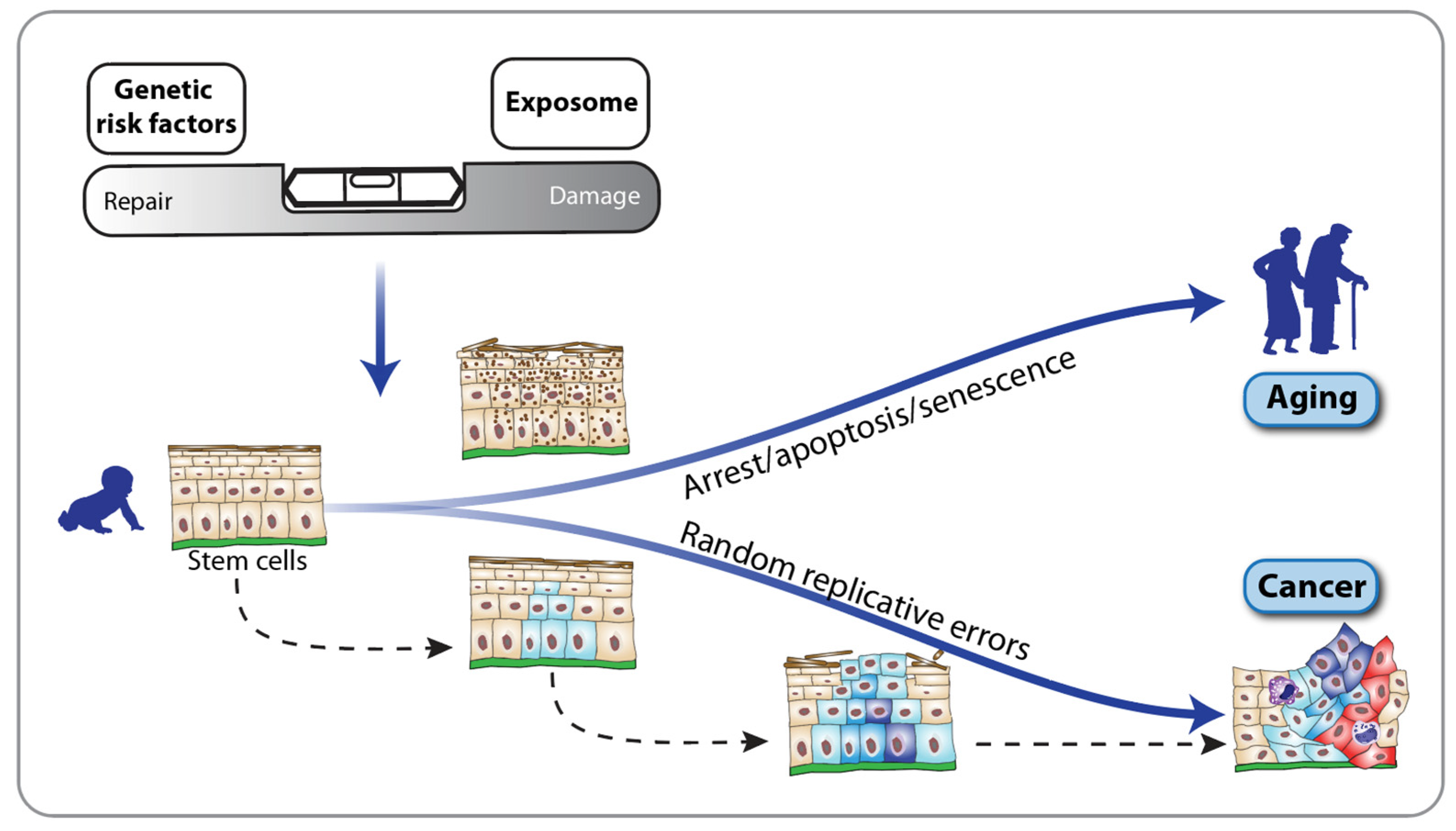
1. Introduction
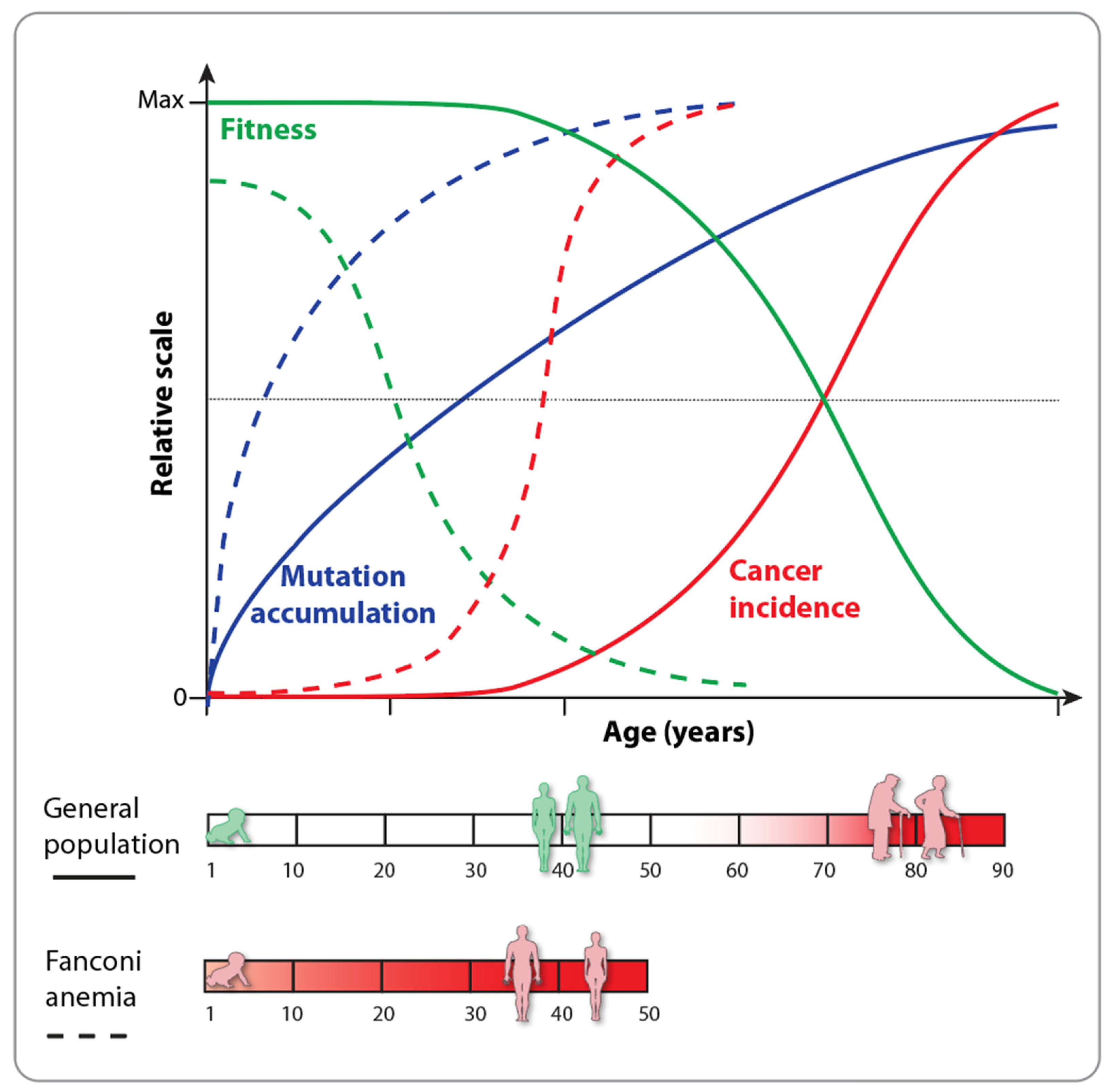
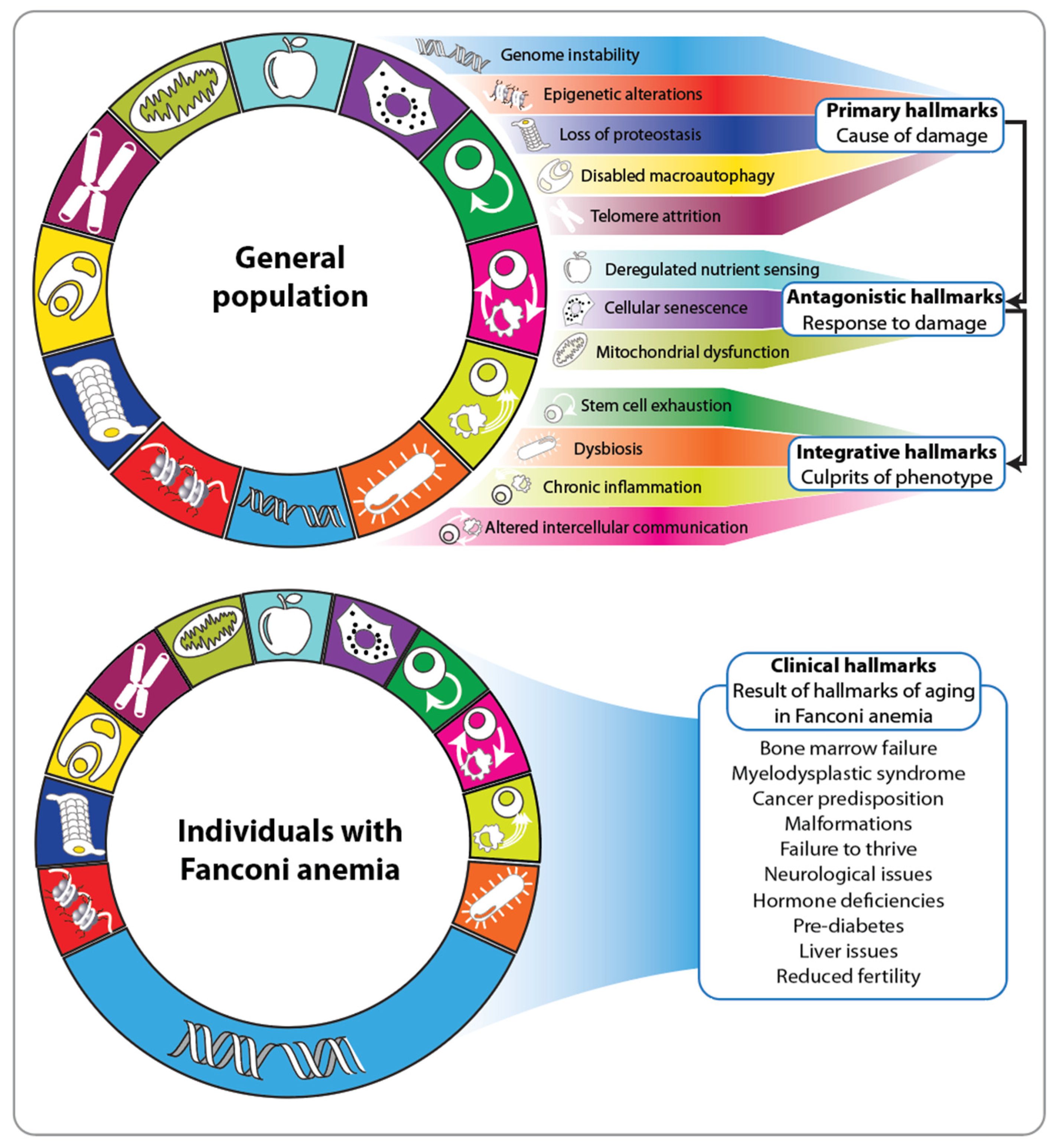
2. Fanconi Anemia and the Hallmarks of Aging
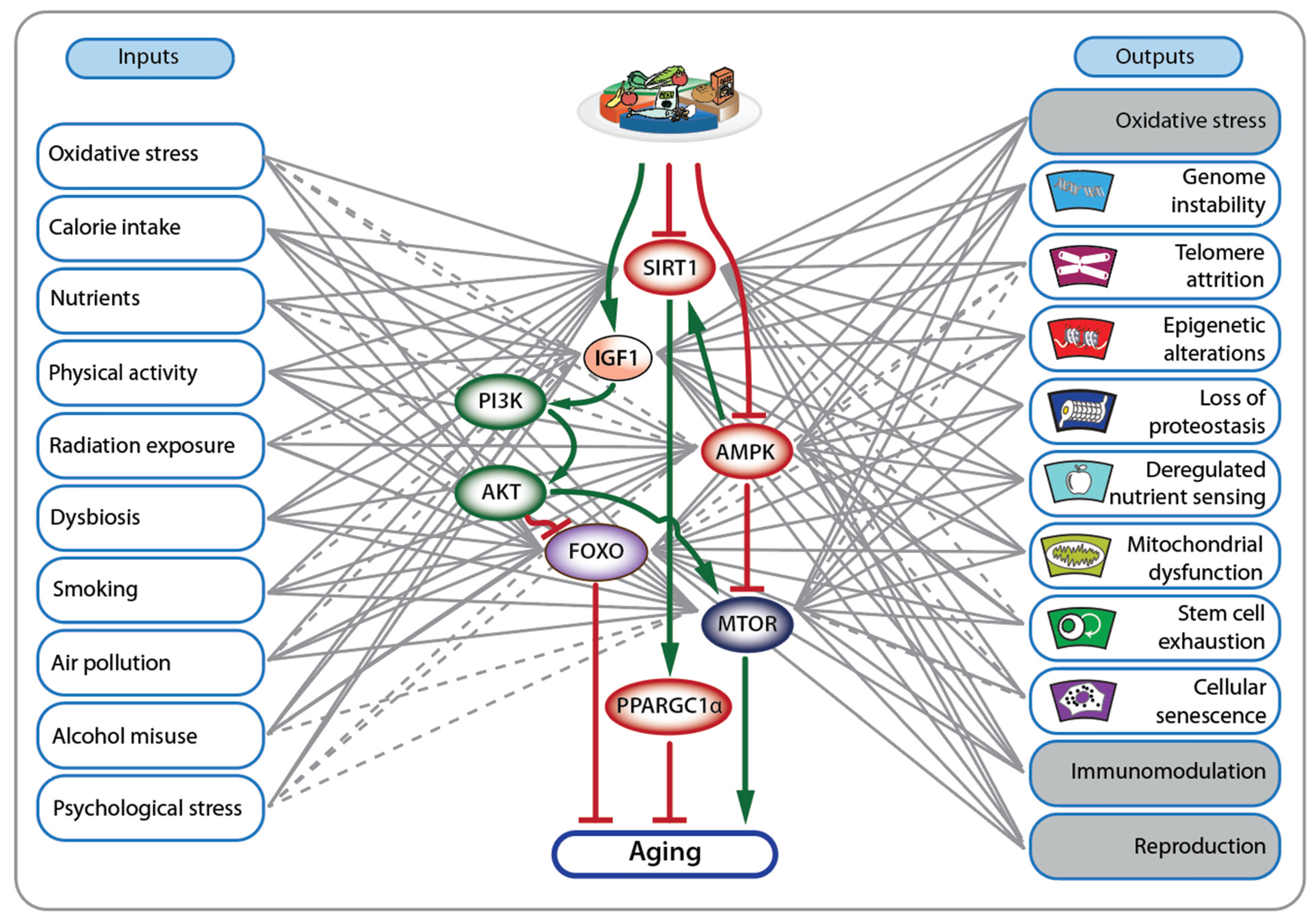
3. Nutrigenomic Principles and Fanconi Anemia
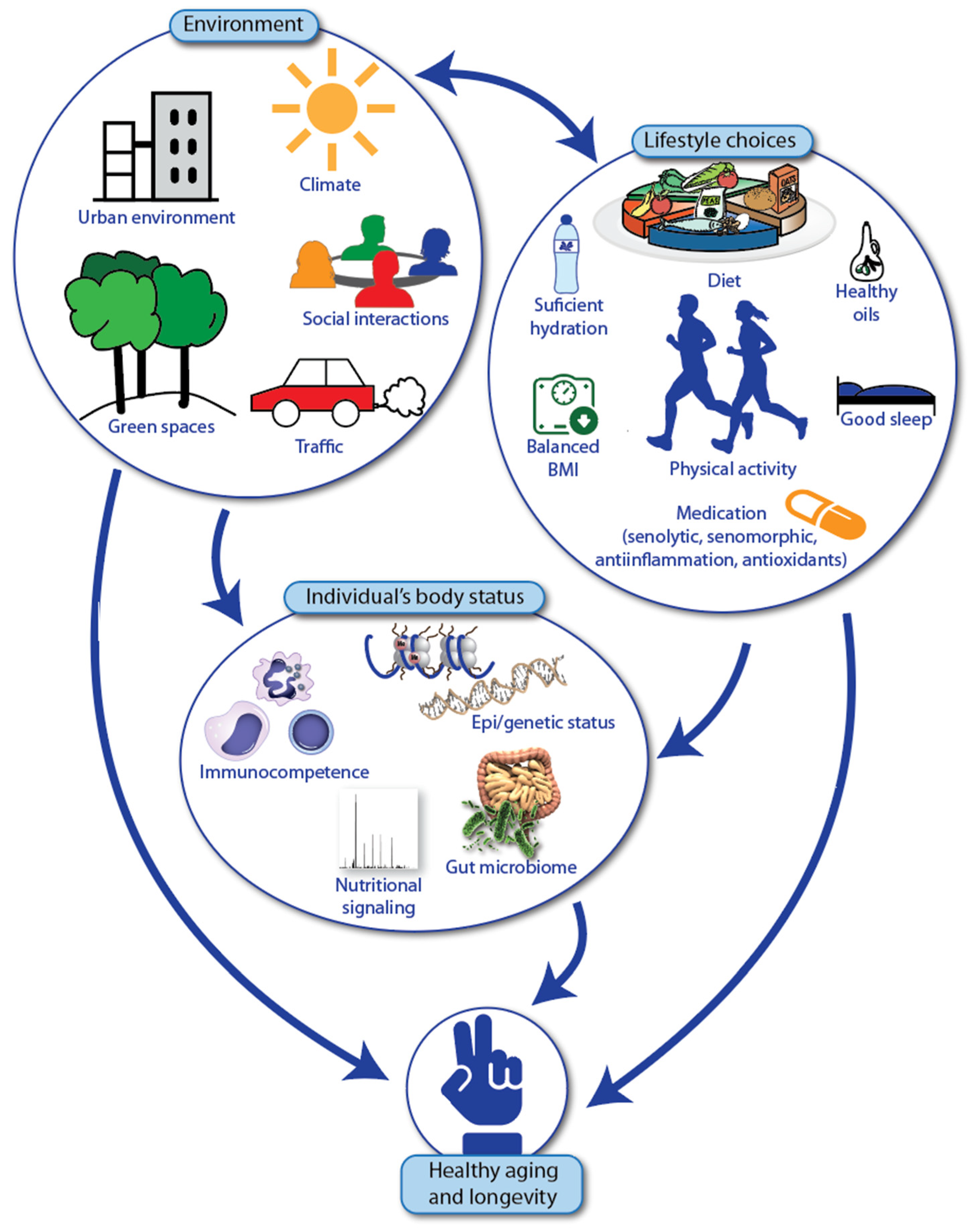
4. Healthy Aging and Longevity of Individuals with Fanconi Anemia
- A plant-based diet composed of fruits, vegetables, leafy greens (foods rich in polyphenols, antioxidants, folate, fibers, potassium, etc.), nuts and seeds, such as walnuts, flaxseed and rapeseed oil (foods rich in the essential ω-3 fatty acid α-linolenic acid);
- Fish and fish oil (containing the marine ω-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid);
- Physical activity, normal-range BMI (body mass index) and low alcohol intake;
- Avoiding saturated fatty acids (animal products, palm oil, coconut oil) and trans-fatty acids (hardened fats).
- Enjoy vegetables, fruits, whole grains, beans, legumes, nuts, plant-based proteins, lean animal proteins, skinless poultry, fish and seafood.
- Limit sweetened drinks, alcohol, sodium, red and processed meats, refined carbohydrates like added sugars and processed grain foods, full-fat dairy products, highly processed foods, tropical oils like coconut and palm.
- Avoid trans-fats and partially hydrogenated oils.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 2023, 35, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Milholland, B.; Vijg, J. Why Gilgamesh failed: The mechanistic basis of the limits to human lifespan. Nat. Aging 2022, 2, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Schoenhofen, E.A.; Wyszynski, D.F.; Andersen, S.; Pennington, J.; Young, R.; Terry, D.F.; Perls, T.T. Characteristics of 32 supercentenarians. J. Am. Geriatr. Soc. 2006, 54, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Velleuer, E. Vitamin D and aging: Central role of immunocompetence. Nutrients 2024, 16, 398. [Google Scholar] [CrossRef] [PubMed]
- Vacante, M.; D’Agata, V.; Motta, M.; Malaguarnera, G.; Biondi, A.; Basile, F.; Malaguarnera, M.; Gagliano, C.; Drago, F.; Salamone, S. Centenarians and supercentenarians: A black swan. Emerging social, medical and surgical problems. BMC Surg. 2012, 12 (Suppl. S1), S36. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, aging and successful aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef]
- Carlberg, C.; Ulven, S.M.; Velleuer, E. Aging: How Science Works; Springer Textbook; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Gregory, T.R. Understanding natural selection: Essential concepts and common misconceptions. Evol. Educ. Outreach 2009, 2, 156–175. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie restriction and aging in humans. Annu. Rev. Nutr. 2020, 40, 105–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, O.; Stewart, C.L. Accelerated aging syndromes, are they relevant to normal human aging? Aging 2011, 3, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gil, L.; Pascual-Ahuir, A.; Proft, M. Genomic instability and epigenetic changes during aging. Int. J. Mol. Sci. 2023, 24, 14279. [Google Scholar] [CrossRef] [PubMed]
- Coppede, F. Mutations involved in pemature-ageing syndromes. Appl. Clin. Genet. 2021, 14, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, F.; Kornak, U.; Wollnik, B. Premature aging disorders: A clinical and genetic compendium. Clin. Genet. 2021, 99, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, S.; Kreienkamp, R. DNA repair defects and genome instability in Hutchinson-Gilford Progeria Syndrome. Curr. Opin. Cell Biol. 2015, 34, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vijg, J. From DNA damage to mutations: All roads lead to aging. Ageing Res. Rev. 2021, 68, 101316. [Google Scholar] [CrossRef] [PubMed]
- Lobitz, S.; Velleuer, E. Guido Fanconi (1892–1979): A jack of all trades. Nat. Rev. Cancer 2006, 6, 893–898. [Google Scholar] [CrossRef]
- Rageul, J.; Kim, H. Fanconi anemia and the underlying causes of genomic instability. Environ. Mol. Mutagen. 2020, 61, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, A.; Alter, B.P. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010, 24, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Velleuer, E.; Dietrich, R. Fanconi anemia: Young patients at high risk for squamous cell carcinoma. Mol. Cell. Pediatr. 2014, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Garcia-de-Teresa, B.; Rodriguez, A.; Frias, S. Chromosome instability in Fanconi anemia: From breaks to phenotypic consequences. Genes 2020, 11, 1528. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.E.; Eapen, M.; MacMillan, M.L.; Harris, R.E.; Pasquini, R.; Boulad, F.; Zhang, M.J.; Auerbach, A.D. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood 2007, 109, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, E. Improving survival for Fanconi anemia patients. Blood 2015, 125, 3676. [Google Scholar] [CrossRef] [PubMed]
- Paustian, L.; Chao, M.M.; Hanenberg, H.; Schindler, D.; Neitzel, H.; Kratz, C.P.; Ebell, W. Androgen therapy in Fanconi anemia: Aretrospective analysis of 30 years in Germany. Pediatr. Hematol. Oncol. 2016, 33, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Cle, D.V. Treatment of inherited bone marrow failure syndromes beyond transplantation. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.R.; Kim, M.O.; Korbee, L.; Wilson, K.A.; Ris, M.D.; Eyal, O.; Sherafat-Kazemzadeh, R.; Bollepalli, S.; Harris, R.; Jeng, M.R.; et al. Oxandrolone for the treatment of bone marrow failure in Fanconi anemia. Pediatr. Blood Cancer 2014, 61, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Scheckenbach, K.; Morgan, M.; Filger-Brillinger, J.; Sandmann, M.; Strimling, B.; Scheurlen, W.; Schindler, D.; Gobel, U.; Hanenberg, H. Treatment of the bone marrow failure in Fanconi anemia patients with danazol. Blood Cells Mol. Dis. 2012, 48, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Fiesco-Roa, M.O.; Giri, N.; McReynolds, L.J.; Best, A.F.; Alter, B.P. Genotype-phenotype associations in Fanconi anemia: A literature review. Blood Rev. 2019, 37, 100589. [Google Scholar] [CrossRef] [PubMed]
- Steinberg-Shemer, O.; Goldberg, T.A.; Yacobovich, J.; Levin, C.; Koren, A.; Revel-Vilk, S.; Ben-Ami, T.; Kuperman, A.A.; Zemer, V.S.; Toren, A.; et al. Characterization and genotype-phenotype correlation of patients with Fanconi anemia in a multi-ethnic population. Haematologica 2020, 105, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Fiesco-Roa, M.O.; Garcia-de Teresa, B.; Leal-Anaya, P.; van ‘t Hek, R.; Wegman-Ostrosky, T.; Frias, S.; Rodriguez, A. Fanconi anemia and dyskeratosis congenita/telomere biology disorders: Two inherited bone marrow failure syndromes with genomic instability. Front. Oncol. 2022, 12, 949435. [Google Scholar] [CrossRef] [PubMed]
- Bottega, R.; Nicchia, E.; Cappelli, E.; Ravera, S.; De Rocco, D.; Faleschini, M.; Corsolini, F.; Pierri, F.; Calvillo, M.; Russo, G.; et al. Hypomorphic FANCA mutations correlate with mild mitochondrial and clinical phenotype in Fanconi anemia. Haematologica 2018, 103, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Tischkowitz, M.D.; Hodgson, S.V. Fanconi anaemia. J. Med. Genet. 2003, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C. How I manage patients with Fanconi anaemia. Br. J. Haematol. 2017, 178, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Tartiere, A.G.; Freije, J.M.P.; Lopez-Otin, C. The hallmarks of aging as a conceptual framework for health and longevity research. Front. Aging 2024, 5, 1334261. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Mogilenko, D.A.; Shchukina, I.; Artyomov, M.N. Immune ageing at single-cell resolution. Nat. Rev. Immunol. 2022, 22, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Giri, N.; Savage, S.A.; Rosenberg, P.S. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica 2018, 103, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Velleuer, E.; Dietrich, R.; Pomjanski, N.; de Santana Almeida Araujo, I.K.; Silva de Araujo, B.E.; Sroka, I.; Biesterfeld, S.; Bocking, A.; Schramm, M. Diagnostic accuracy of brush biopsy-based cytology for the early detection of oral cancer and precursors in Fanconi anemia. Cancer Cytopathol. 2020, 128, 403–413. [Google Scholar] [CrossRef]
- Chen, S.H.; Hsiao, S.Y.; Chang, K.Y.; Chang, J.Y. New insights into oral squamous cell carcinoma: From clinical apects to molecular tumorigenesis. Int. J. Mol. Sci. 2021, 22, 2252. [Google Scholar] [CrossRef] [PubMed]
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Frois-Martins, R.; Morelli, M.; et al. The impact of the fungus-host-mcrobiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Muzio, L.L.; Ballini, A.; Cantore, S.; Bottalico, L.; Charitos, I.A.; Ambrosino, M.; Nocini, R.; Malcangi, A.; Dioguardi, M.; Cazzolla, A.P.; et al. Overview of Candida albicans and human papillomavirus (HPV) Iinfection agents and their bomolecular mechanisms in promoting oral cancer in pediatric patients. Biomed. Res. Int. 2021, 2021, 7312611. [Google Scholar] [CrossRef] [PubMed]
- Errazquin, R.; Carrasco, E.; Del Marro, S.; Sunol, A.; Peral, J.; Ortiz, J.; Rubio, J.C.; Segrelles, C.; Duenas, M.; Garrido-Aranda, A.; et al. Early diagnosis of oral cancer and lesions in Fanconi anemia patients: A prospective and longitudinal study using saliva and plasma. Cancers 2023, 15, 1871. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Velleuer, E. Cancer Biology: How Science Works; Springer Textbook; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Velleuer, E.; Dominguez-Huttinger, E.; Rodriguez, A.; Harris, L.A.; Carlberg, C. Concepts of multi-level dynamical modelling: Understanding mechanisms of squamous cell carcinoma development in Fanconi anemia. Front. Genet. 2023, 14, 1254966. [Google Scholar] [CrossRef] [PubMed]
- Berben, L.; Floris, G.; Wildiers, H.; Hatse, S. Cancer and aging: Two tightly interconnected biological processes. Cancers 2021, 13, 1400. [Google Scholar] [CrossRef] [PubMed]
- Templeman, N.M.; Murphy, C.T. Regulation of reproduction and longevity by nutrient-sensing pathways. J. Cell Biol. 2018, 217, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Petryk, A.; Kanakatti Shankar, R.; Giri, N.; Hollenberg, A.N.; Rutter, M.M.; Nathan, B.; Lodish, M.; Alter, B.P.; Stratakis, C.A.; Rose, S.R. Endocrine disorders in Fanconi anemia: Recommendations for screening and treatment. J. Clin. Endocrinol. Metab. 2015, 100, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sipple, J.; Maynard, S.; Mehta, P.A.; Rose, S.R.; Davies, S.M.; Pang, Q. Fanconi anemia links reactive oxygen species to insulin resistance and obesity. Antioxid. Redox Signal. 2012, 17, 1083–1098. [Google Scholar] [CrossRef] [PubMed]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2022, 23, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, R.; de Cabo, R.; Anderson, R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017, 8, 14063. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. AMP-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J. Lipid Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Velleuer, E. Nutrition and epigenetic programming. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Ulven, S.M.; Molnár, F. Nutrigenomics: How Science Works; Springer Textbook; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.A.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8, 998. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and amino acid restriction, aging and disease: From yeast to humans. Trends Endocrinol. Metab. 2014, 25, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Paivarinta, E.; Itkonen, S.T.; Pellinen, T.; Lehtovirta, M.; Erkkola, M.; Pajari, A.M. Replacing animal-based proteins with plant-based proteins changes the composition of a whole Nordic diet: A randomised cinical trial in healthy Finnish adults. Nutrients 2020, 12, 943. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Sack, M.N. Protein deacetylation by sirtuins: Delineating a post-translational regulatory program responsive to nutrient and redox stressors. Cell Mol. Life Sci. 2010, 67, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Kennedy, B.K. Sirtuins in aging and age-related disease. Cell 2006, 126, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Fulco, M.; Sartorelli, V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle 2008, 7, 3669–3679. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMP-activated protein kinase: A target for drugs both ancient and modern. Chem. Biol. 2012, 19, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.A.; Furutani, E.; Liu, S.; Esrick, E.; Cohen, L.E.; Bledsoe, J.; Liu, C.W.; Lu, K.; de Haro, M.J.R.; Surralles, J.; et al. Metformin for treatment of cytopenias in children and young adults with Fanconi anemia. Blood Adv. 2022, 6, 3803–3811. [Google Scholar] [CrossRef] [PubMed]
- Crane, P.A.; Wilkinson, G.; Teare, H. Healthspan versus lifespan: New medicines to close the gap. Nat. Aging 2022, 2, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Portincasa, P. The environment as a determinant of successful aging or frailty. Mech. Ageing Dev. 2020, 188, 111244. [Google Scholar] [CrossRef] [PubMed]
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361, bmj k2173. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.A. Molecular targets for bioactive food components. J. Nutr. 2004, 134, 2492S–2498S. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Velleuer, E.; Molnar, F. Molecular Medicine: How Science Works; Springer Textbook; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Carlberg, C.; Molnár, F. Switching genes on and off: The example of nuclear receptors. Mechanisms of Gene Regulation, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 95–108. [Google Scholar]
- Novershtern, N.; Subramanian, A.; Lawton, L.N.; Mak, R.H.; Haining, W.N.; McConkey, M.E.; Habib, N.; Yosef, N.; Chang, C.Y.; Shay, T.; et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 2011, 144, 296–309. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Z.J.; Yu, X.; Wang, P. Dietary regulation in health and disease. Signal Transduct. Target. Ther. 2022, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, nutrients, and dietary patterns: Interconnections and implications for dietary guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.D.; Philippou, E. Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Lacoppidan, S.A.; Kyro, C.; Loft, S.; Helnaes, A.; Christensen, J.; Hansen, C.P.; Dahm, C.C.; Overvad, K.; Tjonneland, A.; Olsen, A. Adherence to a healthy Nordic food index Is associated with a lower risk of type-2 diabetes. the Danish diet, cancer and health cohort study. Nutrients 2015, 7, 8633–8644. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef] [PubMed]
- Buettner, D.; Skemp, S. Blue Zones: Lessons from the world’s longest lived. Am. J. Lifestyle Med. 2016, 10, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Alcorta, A.; Porta, A.; Tarrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for plant-based diets: Challenges and innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic diets and chronic disease: Weighing the benefits against the risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.L.; Romick-Rosendale, L.; Nelson, A.; Abdullah, S.; Luebbering, N.; Bartlett, J.; Brusadelli, M.; Palumbo, J.S.; Lake, K.; Litts, B.; et al. Tryptophan metabolism is dysregulated in individuals with Fanconi anemia. Blood Adv. 2021, 5, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Kabacik, S.; Lowe, D.; Fransen, L.; Leonard, M.; Ang, S.-L.; Whiteman, C.; Corsi, S.; Cohen, H.; Felton, S.; Bali, R.; et al. The relationship between epigenetic age and the hallmarks of aging in human cells. Nat. Aging 2022, 2, 484–493. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velleuer, E.; Carlberg, C. A Nutrigenomic View on the Premature-Aging Disease Fanconi Anemia. Nutrients 2024, 16, 2271. https://doi.org/10.3390/nu16142271
Velleuer E, Carlberg C. A Nutrigenomic View on the Premature-Aging Disease Fanconi Anemia. Nutrients. 2024; 16(14):2271. https://doi.org/10.3390/nu16142271
Chicago/Turabian StyleVelleuer, Eunike, and Carsten Carlberg. 2024. "A Nutrigenomic View on the Premature-Aging Disease Fanconi Anemia" Nutrients 16, no. 14: 2271. https://doi.org/10.3390/nu16142271
APA StyleVelleuer, E., & Carlberg, C. (2024). A Nutrigenomic View on the Premature-Aging Disease Fanconi Anemia. Nutrients, 16(14), 2271. https://doi.org/10.3390/nu16142271





