A Comparative Study Evaluating the Effectiveness of Folate-Based B Vitamin Intervention on Cognitive Function of Older Adults under Mandatory Folic Acid Fortification Policy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Selection Process
2.6. Data Collection Process
2.7. Study Risk of Bias Assessment
2.8. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Characteristics of Individual Trials
3.3. Effect of Folate-Based B Vitamin Supplementation on Cognitive Performance of Older Adults Based on Mandatory FA Fortification
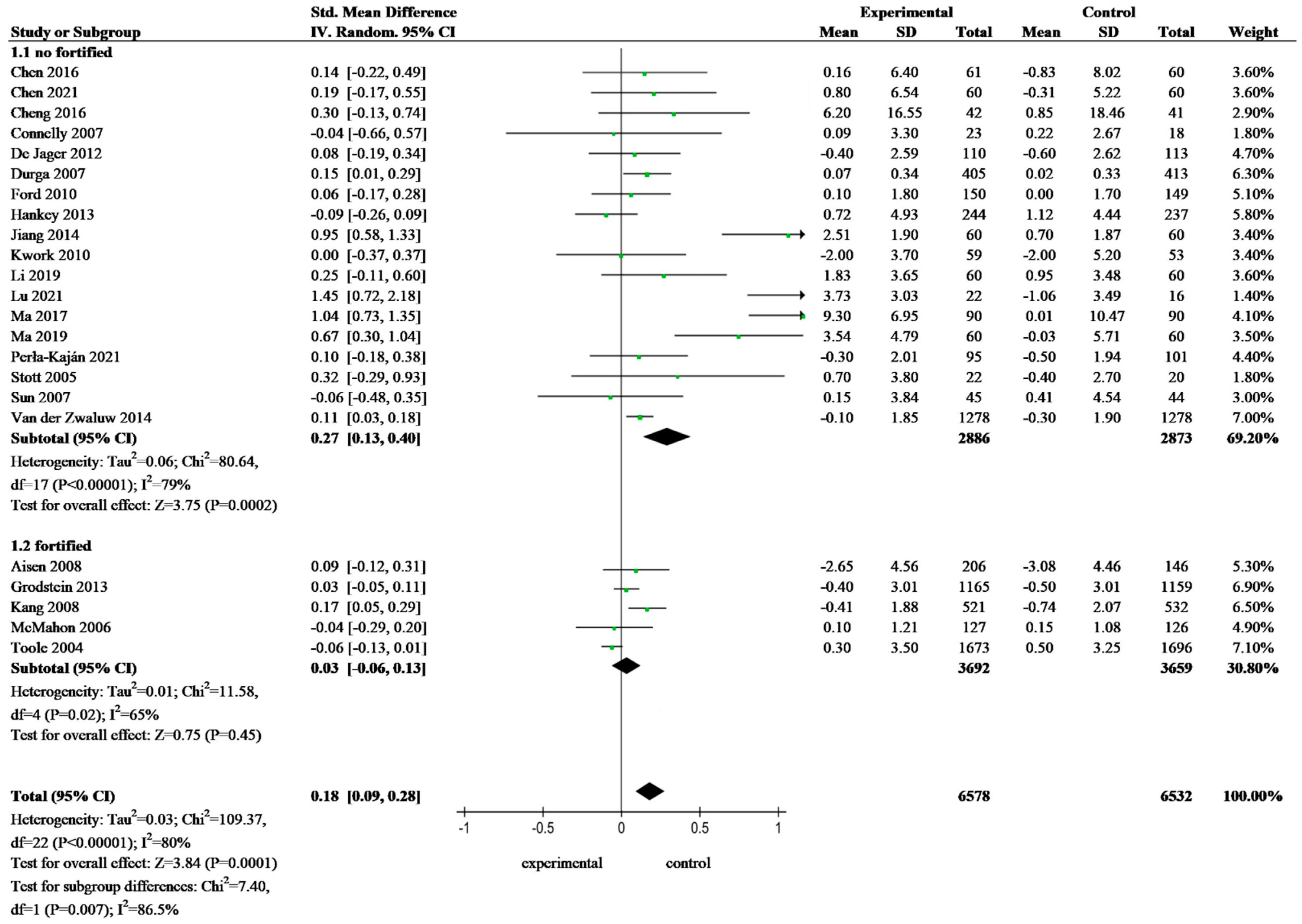
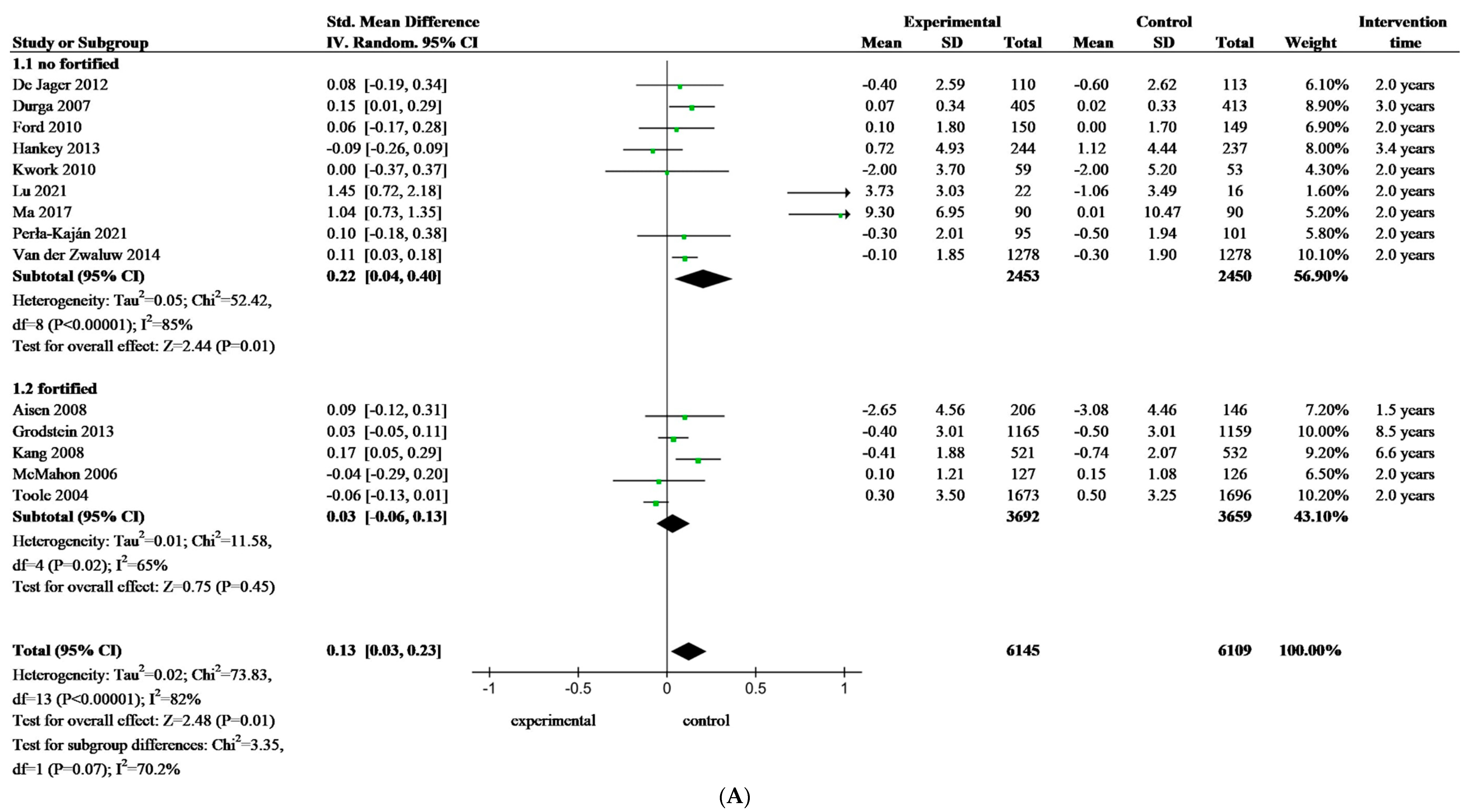
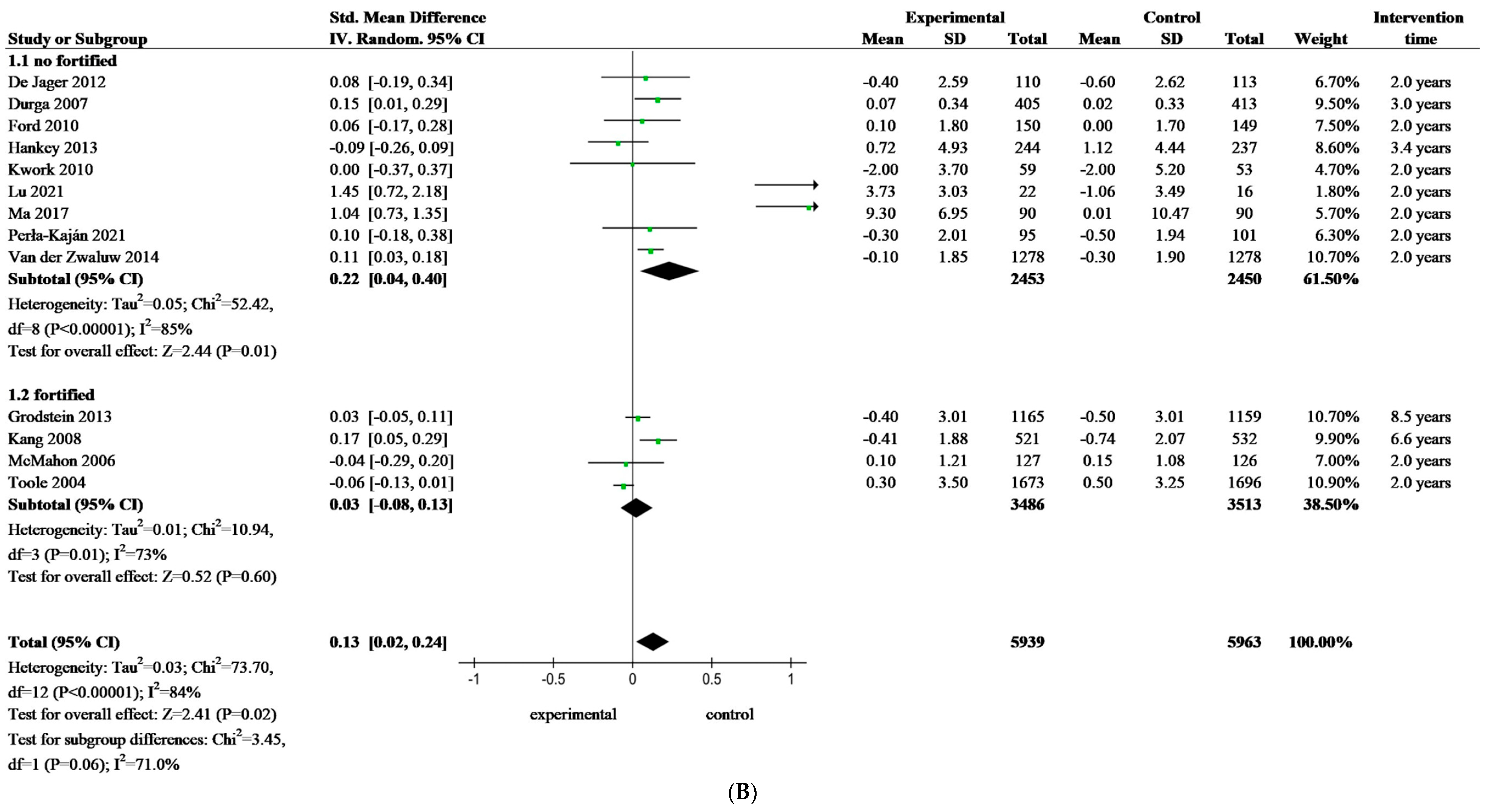
3.4. Meta-Analysis of Folate-Based B Vitamin on Cognitive Performance of Older Adults
3.5. Risk of Bias within Studies
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Li, S.; Guo, Y.; Men, J.; Fu, H.; Xu, T. The preventive efficacy of vitamin B supplements on the cognitive decline of elderly adults: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 367. [Google Scholar] [CrossRef]
- Chang, J.; Liu, M.; Liu, C.; Zhou, S.; Jiao, Y.; Sun, H.; Ji, Y. Effects of vitamins and polyunsaturated fatty acids on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Eur. J. Nutr. 2024, 63, 1003–1022. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Ward, M.; Hoey, L.; Hughes, C.F.; Pentieva, K. Addressing optimal folate and related B-vitamin status through the lifecycle: Health impacts and challenges. Proc. Nutr. Soc. 2019, 78, 449–462. [Google Scholar] [CrossRef]
- Zanin Palchetti, C.; Gomes Gonçalves, N.; Vidal Ferreira, N.; Santos, I.S.; Andrade Lotufo, P.; Bensenor, I.M.; Suemoto, C.K.; Marchioni, D.M.L. Dietary folate intake and its association with longitudinal changes in cognition function. Clin. Nutr. ESPEN 2023, 55, 332–339. [Google Scholar] [CrossRef]
- Ling, Y.; Yuan, S.; Huang, X.; Tan, S.; Cheng, H.; Xu, A.; Lyu, J. Associations of Folate/Folic Acid Supplementation Alone and in Combination with Other B Vitamins on Dementia Risk and Brain Structure: Evidence from 466,224 UK Biobank Participants. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Xing, Y.; Jia, J.; Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 931–949. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Denton, D.A.; Di Nisio, M.; Chong, L.Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, M.A.; Vernooij, R.W.; Martínez, G.; et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018, 12, CD011906. [Google Scholar] [CrossRef]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- Steluti, J.; Selhub, J.; Paul, L.; Reginaldo, C.; Fisberg, R.M.; Marchioni, D.M.L. An overview of folate status in a population-based study from São Paulo, Brazil and the potential impact of 10 years of national folic acid fortification policy. Eur. J. Clin. Nutr. 2017, 71, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Macpherson, H.; Pipingas, A. Improved blood biomarkers but no cognitive effects from 16 weeks of multivitamin supplementation in healthy older adults. Nutrients 2015, 7, 3796–3812. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, H.; Rowsell, R.; Cox, K.H.; Scholey, A.; Pipingas, A. Acute mood but not cognitive improvements following administration of a single multivitamin and mineral supplement in healthy women aged 50 and above: A randomised controlled trial. Age 2015, 37, 9782. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Page, R.; Morrell, C.; Shea, T.B. Maintenance of Cognitive Performance and Mood for Individuals with Alzheimer’s Disease Following Consumption of a Nutraceutical Formulation: A One-Year, Open-Label Study. J. Alzheimer’s Dis. 2016, 51, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Chan, A.; Paskavitz, J.; Shea, T.B. Efficacy of a vitamin/nutriceutical formulation for moderate-stage to later-stage Alzheimer’s disease: A placebo-controlled pilot study. Am. J. Alzheimer’s Dis. Demen. 2009, 24, 27–33. [Google Scholar] [CrossRef] [PubMed]
- van Uffelen, J.G.; Chin APaw, M.J.; Hopman-Rock, M.; van Mechelen, W. The effect of walking and vitamin B supplementation on quality of life in community-dwelling adults with mild cognitive impairment: A randomized, controlled trial. Qual Life Res. 2007, 16, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Fan, J.; Li, M.; Dong, C.; Gao, Y.; Fu, M.; Huang, G.; Liu, H. Effects of Folic Acid Combined with DHA Supplementation on Cognitive Function and Amyloid-β-Related Biomarkers in Older Adults with Mild Cognitive Impairment by a Randomized, Double Blind, Placebo-Controlled Trial. J. Alzheimer’s Dis. 2021, 81, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Oulhaj, A.; Jernerén, F.; Refsum, H.; Smith, A.D.; de Jager, C.A. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 50, 547–557. [Google Scholar] [CrossRef] [PubMed]
- van Soest, A.P.M.; van de Rest, O.; Witkamp, R.F.; de Groot, L.C.P.G.M. Positive effects of folic acid supplementation on cognitive aging are dependent on ω-3 fatty acid status: A post hoc analysis of the FACIT trial. Am. J. Clin. Nutr. 2021, 113, 801–809. [Google Scholar] [CrossRef]
- Douaud, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef]
- Jernerén, F.; Elshorbagy, A.K.; Oulhaj, A.; Smith, S.M.; Refsum, H.; Smith, A.D. Brain atrophy in cognitively impaired elderly: The importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Schneider, L.S.; Sano, M.; Diaz-Arrastia, R.; van Dyck, C.H.; Weiner, M.F.; Bottiglieri, T.; Jin, S.; Stokes, K.T.; Thomas, R.G.; et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: A randomized controlled trial. JAMA 2008, 300, 1774–1783. [Google Scholar] [CrossRef]
- Brady, C.B.; Gaziano, J.M.; Cxypoliski, R.A.; Guarino, P.D.; Kaufman, J.S.; Warren, S.R.; Hartigan, P.; Goldfarb, D.S.; Jamison, R.L. Homocysteine lowering and cognition in CKD: The Veterans Affairs homocysteine study. Am. J. Kidney Dis. 2009, 54, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Remington, R.; Kotyla, E.; Lepore, A.; Zemianek, J.; Shea, T.B. A vitamin/nutriceutical formulation improves memory and cognitive performance in community-dwelling adults without dementia. J. Nutr. Health Aging 2010, 14, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; O’Brien, J.; Kang, J.H.; Dushkes, R.; Cook, N.R.; Okereke, O.; Manson, J.E.; Glynn, R.J.; Buring, J.E.; Gaziano, M.; et al. Long-term multivitamin supplementation and cognitive function in men: A randomized trial. Ann. Intern. Med. 2013, 159, 806–814. [Google Scholar] [CrossRef]
- Harris, E.; Macpherson, H.; Vitetta, L.; Kirk, J.; Sali, A.; Pipingas, A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: A randomised, placebo-controlled trial. Hum. Psychopharmacol. 2012, 27, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Cook, N.; Manson, J.; Buring, J.E.; Albert, C.M.; Grodstein, F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2008, 88, 1602–1610. [Google Scholar] [CrossRef]
- McMahon, J.A.; Green, T.J.; Skeaff, C.M.; Knight, R.G.; Mann, J.I.; Williams, S.M. A controlled trial of homocysteine lowering and cognitive performance. N. Engl. J. Med. 2006, 354, 2764–2772. [Google Scholar] [CrossRef]
- Macpherson, H.; Ellis, K.A.; Sali, A.; Pipingas, A. Memory improvements in elderly women following 16 weeks treatment with a combined multivitamin, mineral and herbal supplement: A randomized controlled trial. Psychopharmacology 2012, 220, 351–365. [Google Scholar] [CrossRef]
- Rommer, P.S.; Fuchs, D.; Leblhuber, F.; Schroth, R.; Greilberger, M.; Tafeit, E.; Greilberger, J. Lowered Levels of Carbonyl Proteins after Vitamin B Supplementation in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Neurodegener. Dis. 2016, 16, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Sommer, B.R.; Hoff, A.L.; Costa, M. Folic acid supplementation in dementia: A preliminary report. J. Geriatr. Psychiatry Neurol. 2003, 16, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Toole, J.F.; Malinow, M.R.; Chambless, L.E.; Spence, J.D.; Pettigrew, L.C.; Howard, V.J.; Sides, E.G.; Wang, C.H.; Stampfer, M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004, 291, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.G.; Batterham, P.J.; Mackinnon, A.J.; Jorm, A.F.; Hickie, I.; Fenech, M.; Kljakovic, M.; Crisp, D.; Christensen, H. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms—The Beyond Ageing Project: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, V.A.; Kesse-Guyot, E.; Barberger-Gateau, P.; Fezeu, L.; Hercberg, S.; Galan, P. Cognitive function after supplementation with B vitamins and long-chain omega-3 fatty acids: Ancillary findings from the SU.FOL.OM3 randomized trial. Am. J. Clin. Nutr. 2011, 94, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.; Calvaresi, E.; Hughes, D. Short-term folate, vitamin B-12 or vitamin B-6 supplementation slightly affects memory performance but not mood in women of various ages. J. Nutr. 2002, 132, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Cockle, S.M.; Haller, J.; Kimber, S.; Dawe, R.A.; Hindmarch, I. The influence of multivitamins on cognitive function and mood in the elderly. Aging Ment. Health 2000, 4, 339–353. [Google Scholar] [CrossRef]
- Clarke, R.; Harrison, G.; Richards, S.; Vital Trial Collaborative Group. Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia. J. Intern. Med. 2003, 254, 67–75. [Google Scholar]
- Connelly, P.J.; Prentice, N.P.; Cousland, G.; Bonham, J. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2008, 23, 155–160. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Xu, W.; Huang, G. Folic Acid Supplementation Mitigates Alzheimer’s Disease by Reducing Inflammation: A Randomized Controlled Trial. Mediators Inflamm. 2016, 2016, 5912146. [Google Scholar] [CrossRef]
- Cheng, D.; Kong, H.; Pang, W.; Yang, H.; Lu, H.; Huang, C.; Jiang, Y. B vitamin supplementation improves cognitive function in the middle aged and elderly with hyperhomocysteinemia. Nutr. Neurosci. 2016, 19, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ge, B.; Zhou, D.; Li, M.; Li, W.; Ma, F.; Liu, Z.; Ji, Y.; Huang, G. Effects of Folic Acid and Vitamin B12 Supplementation on Cognitive Impairment and Inflammation in Patients with Alzheimer’s Disease: A Randomized, Single-Blinded, Placebo-Controlled Trial. J. Prev. Alzheimer’s Dis. 2021, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- de Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.J.; de Groot, L.C.; Joosten, L.W.; Bloo, R.J.; Clarke, R.; Ueland, P.M.; Schneede, J.; Blom, H.J.; Hoefnagels, W.H.; van Staveren, W.A. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: A randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 84, 361–370. [Google Scholar] [CrossRef]
- Fioravanti, M.; Ferrario, E.; Massaia, M.; Cappa, G.; Rivolta, G.; Grossi, E.; Buckley, A.E. Low folate levels in the cognitive decline of elderly patients and the efficacy of folate as a treatment for improving memory deficits. Arch. Gerontol. Geriatr. 1998, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H.; Flicker, L.; Alfonso, H.; Thomas, J.; Clarnette, R.; Martins, R.; Almeida, O.P. Vitamins B(12), B(6), and folic acid for cognition in older men. Neurology 2010, 75, 1540–1547. [Google Scholar] [CrossRef]
- Gong, X.; Shi, L.; Wu, Y.; Luo, Y.; Kwok, T. B Vitamin Supplementation Slows Cognitive Decline in Mild Cognitive Impairment Patients with Frontal Lobe Atrophy. J. Alzheimers Dis. 2022, 89, 1453–1461. [Google Scholar] [CrossRef]
- Hama, Y.; Hamano, T.; Shirafuji, N.; Hayashi, K.; Ueno, A.; Enomoto, S.; Nagata, M.; Kimura, H.; Matsunaga, A.; Ikawa, M.; et al. Influences of Folate Supplementation on Homocysteine and Cognition in Patients with Folate Deficiency and Cognitive Impairment. Nutrients 2020, 12, 3138. [Google Scholar] [CrossRef]
- Hankey, G.J.; Ford, A.H.; Yi, Q.; Eikelboom, J.W.; Lees, K.R.; Chen, C.; Xavier, D.; Navarro, J.C.; Ranawaka, U.K.; Uddin, W.; et al. Effect of B vitamins and lowering homocysteine on cognitive impairment in patients with previous stroke or transient ischemic attack: A prespecified secondary analysis of a randomized, placebo-controlled trial and meta-analysis. Stroke 2013, 44, 2232–2239. [Google Scholar] [CrossRef]
- Jiang, B.; Ding, C.Y.; Yao, G.E.; Yao, C.S.; Zhang, Y.Y.; Ge, J.L.; Qiu, E.C. Intervention Effect of Folic Acid and Vitamin B12 on Vascular Cognitive Impairment Complicated with Hyperhomocysteinemia. J. Med. Biochem. 2014, 33, 169–174. [Google Scholar] [CrossRef][Green Version]
- Kwok, T.; Lee, J.; Law, C.B.; Pan, P.C.; Yung, C.Y.; Choi, K.C.; Lam, L.C. A randomized placebo controlled trial of homocysteine lowering to reduce cognitive decline in older demented people. Clin. Nutr. 2011, 30, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.; Wu, Y.; Lee, J.; Lee, R.; Yung, C.Y.; Choi, G.; Lee, V.; Harrison, J.; Lam, L.; Mok, V. A randomized placebo-controlled trial of using B vitamins to prevent cognitive decline in older mild cognitive impairment patients. Clin. Nutr. 2020, 39, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Lewerin, C.; Matousek, M.; Steen, G.; Johansson, B.; Steen, B.; Nilsson-Ehle, H. Significant correlations of plasma homocysteine and serum methylmalonic acid with movement and cognitive performance in elderly subjects but no improvement from short-term vitamin therapy: A placebo-controlled randomized study. Am. J. Clin. Nutr. 2005, 81, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, W.; Gao, Y.; Chen, Y.; Bai, D.; Weng, J.; Du, Y.; Ma, F.; Wang, X.; Liu, H.; et al. Effect of folic acid combined with docosahexaenoic acid intervention on mild cognitive impairment in elderly: A randomized double-blind, placebo-controlled trial. Eur. J. Nutr. 2021, 60, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Fang, Y.; Zhou, Y.; Che, M.; Shen, J.; Liu, Q.; Zhang, H.; Pan, S.; Lin, Y.; Wang, Q.; et al. A pilot study of thiamin and folic acid in hemodialysis patients with cognitive impairment. Ren. Fail. 2021, 43, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wu, T.; Zhao, J.; Han, F.; Marseglia, A.; Liu, H.; Huang, G. Effects of 6-Month Folic Acid Supplementation on Cognitive Function and Blood Biomarkers in Mild Cognitive Impairment: A Randomized Controlled Trial in China. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wu, T.; Zhao, J.; Song, A.; Liu, H.; Xu, W.; Huang, G. Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Sci. Rep. 2016, 6, 37486. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Q.; Zhou, X.; Zhao, J.; Song, A.; Li, W.; Liu, H.; Xu, W.; Huang, G. Effects of folic acid supplementation on cognitive function and Aβ-related biomarkers in mild cognitive impairment: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 345–356. [Google Scholar] [CrossRef]
- Ma, F.; Zhou, X.; Li, Q.; Zhao, J.; Song, A.; An, P.; Du, Y.; Xu, W.; Huang, G. Effects of Folic Acid and Vitamin B12, Alone and in Combination on Cognitive Function and Inflammatory Factors in the Elderly with Mild Cognitive Impairment: A Single-blind Experimental Design. Curr. Alzheimer Res. 2019, 16, 622–632. [Google Scholar] [CrossRef]
- Pathansali, R.; Mangoni, A.A.; Creagh-Brown, B.; Lan, Z.C.; Ngow, G.L.; Yuan, X.F.; Ouldred, E.L.; Sherwood, R.A.; Swift, C.G.; Jackson, S.H. Effects of folic acid supplementation on psychomotor performance and hemorheology in healthy elderly subjects. Arch. Gerontol. Geriatr. 2006, 43, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Perła-Kaján, J.; Włoczkowska, O.; Zioła-Frankowska, A.; Frankowski, M.; Smith, A.D.; de Jager, C.A.; Refsum, H.; Jakubowski, H. Paraoxonase 1, B Vitamins Supplementation, and Mild Cognitive Impairment. J. Alzheimer’s Dis. 2021, 81, 1211–1229. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Doshanjh, L.; Fishman, P.; Luo, Y.; Smyers, K.; Page, R.; Morrell, C.; et al. A Phase II Randomized Clinical Trial of a Nutritional Formulation for Cognition and Mood in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Lortie, J.J.; Hoffmann, H.; Page, R.; Morrell, C.; Shea, T.B. A Nutritional Formulation for Cognitive Performance in Mild Cognitive Impairment: A Placebo-Controlled Trial with an Open-Label Extension. J. Alzheimer’s Dis. 2015, 48, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Stott, D.J.; MacIntosh, G.; Lowe, G.D.; Rumley, A.; McMahon, A.D.; Langhorne, P.; Tait, R.C.; O’Reilly, D.S.; Spilg, E.G.; MacDonald, J.B.; et al. Randomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease. Am. J. Clin. Nutr. 2005, 82, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, C.J.; Chien, K.L.; Chen, S.T.; Chen, R.C. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: A 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin. Ther. 2007, 29, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group; Armitage, J.M.; Bowman, L.; Clarke, R.J.; Wallendszus, K.; Bulbulia, R.; Rahimi, K.; Haynes, R.; Parish, S.; Sleight, P.; et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: A randomized trial. JAMA 2010, 303, 2486–2494. [Google Scholar]
- Tan, H.K.; Narasimhalu, K.; Ting, S.K.S.; Hameed, S.; Chang, H.M.; De Silva, D.A.; Chen, C.L.H.; Tan, E.K. B-vitamin supplementation on mitigating post-stroke cognition and neuropsychiatric sequelae: A randomized controlled trial. Int. J. Stroke 2023, 18, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.K.S.; Earnest, A.; Li, H.; Hameed, S.; Chang, H.M.; Chen, C.L.H.; Tan, E.K. B vitamins and cognition in subjects with small vessel disease: A Substudy of VITATOPS, a randomized, placebo-controlled trial. J. Neurol. Sci. 2017, 379, 124–126. [Google Scholar] [CrossRef]
- van Uffelen, J.G.; Chinapaw, M.J.; van Mechelen, W.; Hopman-Rock, M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br. J. Sports Med. 2008, 42, 344–351. [Google Scholar] [CrossRef]
- van der Zwaluw, N.L.; Dhonukshe-Rutten, R.A.; van Wijngaarden, J.P.; Brouwer-Brolsma, E.M.; van de Rest, O.; In ‘t Veld, P.H.; Enneman, A.W.; van Dijk, S.C.; Ham, A.C.; Swart, K.M.; et al. Results of 2-year vitamin B treatment on cognitive performance: Secondary data from an RCT. Neurology 2014, 83, 2158–2166. [Google Scholar] [CrossRef]
- van Soest, A.P.M.; van de Rest, O.; Witkamp, R.F.; Cederholm, T.; de Groot, L.C.P.G.M. DHA status influences effects of B-vitamin supplementation on cognitive ageing: A post-hoc analysis of the B-proof trial. Eur. J. Nutr. 2022, 61, 3731–3739. [Google Scholar] [CrossRef]
- Wolters, M.; Hickstein, M.; Flintermann, A.; Tewes, U.; Hahn, A. Cognitive performance in relation to vitamin status in healthy elderly German women-the effect of 6-month multivitamin supplementation. Prev. Med. 2005, 41, 253–259. [Google Scholar] [CrossRef]
- Holmes, M.V.; Newcombe, P.; Hubacek, J.A.; Sofat, R.; Ricketts, S.L.; Cooper, J.; Breteler, M.M.; Bautista, L.E.; Sharma, P.; Whittaker, J.C.; et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: A meta-analysis of genetic studies and randomised trials. Lancet 2011, 378, 584–594. [Google Scholar] [CrossRef]
- Centeno Tablante, E.; Pachón, H.; Guetterman, H.; Finkelstein, J.L. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database Syst. Rev. 2019, 7, CD012150. [Google Scholar] [CrossRef]
- Jacques, P.F.; Selhub, J.; Bostom, A.G.; Wilson, P.W.; Rosenberg, I.H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 1999, 340, 1449–1454. [Google Scholar] [CrossRef]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 2007, 85, 193–200. [Google Scholar] [CrossRef]
- Santhosh-Kumar, C.R.; Bisping, J.S.; Kick, S.D.; Deutsch, J.C.; Kolhouse, J.F. Folate sufficient subjects do not accumulate additional folates during supplementation. Am. J. Hematol. 2000, 64, 71–72. [Google Scholar] [CrossRef]
- Gregory, J.F., 3rd; Williamson, J.; Bailey, L.B.; Toth, J.P. Urinary excretion of [2H4] folate by nonpregnant women following a single oral dose of [2H4] folic acid is a functional index of folate nutritional status. J. Nutr. 1998, 128, 1907–1912. [Google Scholar] [CrossRef]
- Patanwala, I.; King, M.J.; Barrett, D.A.; Rose, J.; Jackson, R.; Hudson, M.; Philo, M.; Dainty, J.R.; Wright, A.J.; Finglas, P.M.; et al. Folic acid handling by the human gut: Implications for food fortification and supplementation. Am. J. Clin. Nutr. 2014, 100, 593–599. [Google Scholar] [CrossRef]
- Bailey, R.L.; Mills, J.L.; Yetley, E.A.; Gahche, J.J.; Pfeiffer, C.M.; Dwyer, J.T.; Dodd, K.W.; Sempos, C.T.; Betz, J.M.; Picciano, M.F. Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged > or =60 y in the United States. Am. J. Clin. Nutr. 2010, 92, 383–389. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Chen, Y.; Ma, F.; Huang, G.; Li, W. Baseline folic acid status affects the effectiveness of folic acid supplements in cognitively relevant outcomes in older adults: A systematic review. Aging Ment. Health 2022, 26, 457–463. [Google Scholar] [CrossRef]
- Ford, A.H.; Almeida, O.P. Effect of Vitamin B Supplementation on Cognitive Function in the Elderly: A Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 419–434. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, J.; Yuan, C.; Ding, D. Vitamin B12, B6, or Folate and Cognitive Function in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2020, 77, 781–794. [Google Scholar] [CrossRef]
- Shah, R.C.; Kamphuis, P.J.; Leurgans, S.; Swinkels, S.H.; Sadowsky, C.H.; Bongers, A.; Rappaport, S.A.; Quinn, J.F.; Wieggers, R.L.; Scheltens, P.; et al. The S-Connect study: Results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 59. [Google Scholar] [CrossRef]
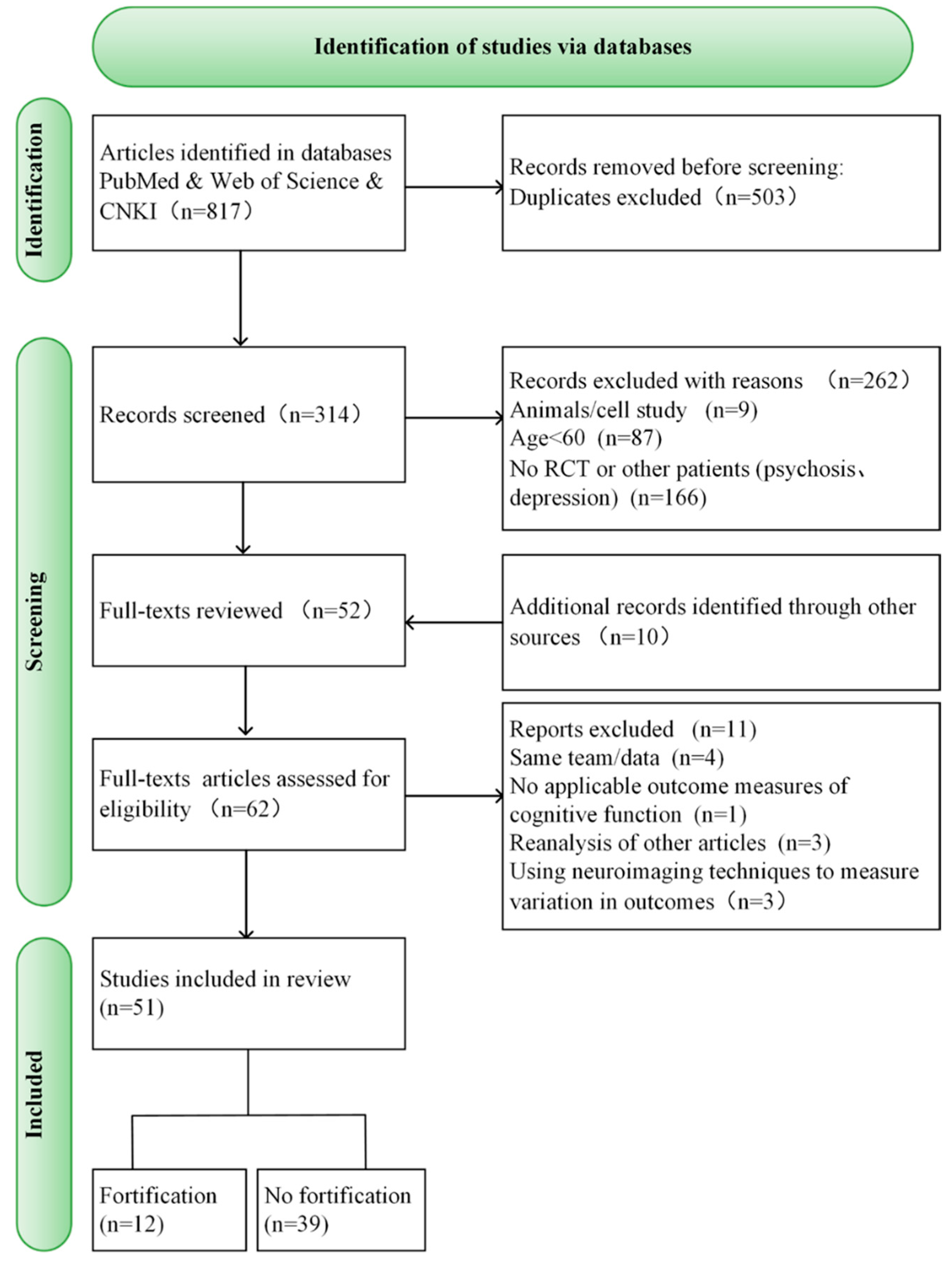
| Included in Review | Excluded from Review | |
|---|---|---|
| Patient population | Senior citizens with an average or median age of over 60 years | Pre-existing mental disorders Children and adolescents Pregnant women |
| Intervention | FA supplementation either alone or in conjunction with B6/B12 or other micro-nutrients | Additional interventions other than stated on the left The use of vitamin B was not specified |
| Comparison | Control group (placebo) | Any other |
| Outcomes | cognitive outcome measures | Non-cognitive outcome measures Studies lacking data on change of cognitive measures |
| Study design | RCTs (randomized controlled trials) | Not RCT |
| Fortification | Study and Year | Country | Participants | Age (y) e | Female (%) | Folate Concentration at Baseline | Folate Status | Supplement | Duration of Treatment | Efficiency | Fortification Food | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Folic Acid | Vitamin B12 | Vitamin B6 | |||||||||||
| Yes | Aisen et al., 2008 [23] | USA | 409 individuals with mild to moderate AD | I: 75.70 ± 8.00 C: 77.30 ± 7.90 | 55.99 | I: 25.74 ± 17.65 nmol/L C: 23.59 ± 14.35 nmol/L | Fully a | Yes | Yes | Yes | 1.5 years | Partly effective d | Maize flour; rice; wheat flour |
| Brady et al., 2009 [24] | USA | 659 elders | I: 63.20 ± 12.20 C: 64.20 ± 11.20 | 1.67 | I: 15.9 ng/mL C:14.8 ng/mL | Fully a | Yes | Yes | Yes | 5 years | Ineffective | ||
| Chan et al., 2010 [25] | USA | 584 elders | No details | No Data | No details | No details | Yes | Yes | No | either 2 weeks or 3 months | Effective | ||
| Grodstein et al., 2013 [26] | USA | 5947 elders | I: 71.60 ± 6.00 C: 71.60 ± 5.90 | 0 | No details | No details | Yes | Yes | Yes | 8.5 years | Ineffective | ||
| Harris et al., 2012 [27] | Australia | 51 elders | I: 62.00 ± 3.90 C: 62.40 ± 6.60 | 0 | I: 575.0 ± 228.1 ng/mL C: 533.0± 209.8 ng/mL | Fully a | Yes | Yes | Yes | 2 months | Effective | Wheat flour | |
| Kang et al., 2008 [28] | USA | 5442 elders | I: 71.30 ± 4.20 C: 71.30 ± 4.20 | 100 | No details | No details | Yes | Yes | Yes | 6.6 years | Ineffective | ||
| McMahon et al., 2006 [29] | New Zealand | 276 elders | I: 73.60 ± 5.80 C: 73.40 ± 5.70 | 40.58 | I: 10.0 ± 5.0 ng/mL C: 10.0 ± 5.0 ng/mL | Fully a | Yes | Yes | Yes | 2 years | Partly effective d | Wheat flour | |
| Macpherson et al., 2012 [30] | Australia | 56 elders | I: 71.90 ± 4.80 C: 70.30 ± 4.30 | 100 | No details | No details | Yes | Yes | Yes | 4 months | Partly effective d | ||
| Rommer et al., 2015 [31] | Australia | 48 patients with MCI/AD | I: 76.40 ± 6.70 C: 63.30 ± 13.70 | 39.58 | No details | No details | Yes | Yes | Yes | 3 months | Ineffective | ||
| Sommer et al., 2003 [32] | USA | 11 patients with AD | T: 76.70 ± 4.10 | 42.86 | No details | No details | Yes | No | No | 10 weeks | Ineffective | ||
| Toole et al., 2004 [33] | USA, Canada and Scotland | 3680 elders | I: 66.40 ± 10.80 C: 66.20 ± 10.80 | 37.40 | No details | No details | Yes | Yes | Yes | 2 years | Ineffective | ||
| Walker et al., 2012 [34] | Australian | 900 elders | I: 65.92 ± 4.30 C: 65.97 ± 4.18 | 60.22 | I: 572.54 ± 266.32 nmol/L C: 557.09 ± 277.50 nmol/L | Fully a | Yes | Yes | No | 2 years | Effective | ||
| No | Andreeva et al., 2011 [35] | France | 871 elders | I: 61.40 ± 8.70 C: 60.90 ± 8.90 | 21.70 | I: 6.90 ± 3.50 ng/mL C: 7.00± 3.70 ng/mL | Insufficiency a | Yes | Yes | Yes | 4 years | Ineffective | |
| Bryan et al., 2002 [36] | Australia | 75 elders | T:74.08 ± 5.75 | 100 | No details | No details | Yes | Yes | Yes | 5 weeks | Effective | ||
| Cockle et al., 2000 [37] | UK | 139 healthy elders | I: 70.70 ± 5.60 C: 70.20 ± 5.40 | 63.31 | No details | No details | Yes | Yes | Yes | 6 months | Ineffective | ||
| Clarke et al., 2003 [38] | UK | 149 patients with MCI/AD | T: 75.00 | No Data | T: 7.1 nmol/L | Insufficiency a | Yes | Yes | No | 3 months | Partly effective d | ||
| Connelly et al., 2007 [39] | UK | 57 patients with AD | I: 75.65 ± 5.94 C: 77.60 ± 6.89 | 50.88 | I: 9.77 ± 5.66 mg/L C: 8.71 ± 4.54 mg/L | Fully a | Yes | No | No | 6 months | Ineffective | ||
| Chen et al., 2016 [40] | China | 121 patients with AD | I: 68.10 ± 8.50 C: 67.63 ± 7.92 | 50.41 | I: 11.98 (8.04–17.68) nmol/L C: 10.76(7.53–16.37) nmol/L | Insufficiency b | Yes | No | No | 6 months | Effective | ||
| Cheng et al., 2016 [41] | China | 104 healthy elders | I: 74.30 ± 9.60 C: 72.50 ± 7.00 | 51.92 | I: 9.48 ± 5.2 ng/mL C: 9.90 ± 4.0 ng/mL | Fully a | Yes | Yes | Yes | 14 weeks | Effective | ||
| Chen et al., 2021 [42] | China | 120 patients with AD | I: 68.58 ± 7.29 C: 68.02 ± 8.34 | 46.67 | I: 14.37 (9.52–19.32) nmol/L C: 16.08(11.35–24.58) nmol/L | Insufficiency a | Yes | Yes | No | 6 months | Effective | ||
| De Jager et al., 2012 [43] | UK | 266 patients with MCI | I: 76.80 ± 5.10 C: 76.70 ± 4.80 | 64.13 | I: 22.6 (20.0–25.5) nmol/L C: 23.0 (20.4–26.0) nmol/L | Fully c | Yes | Yes | Yes | 2 years | Effective | ||
| Durga et al., 2007 [44] | The Netherlands | 818 elders | I: 60.0 ± 5.0 C: 60.0 ± 6.0 | 28.36 | I: 12 (9–15) nmol/L C: 12 (10–15) nmol/L | Insufficiency a | Yes | No | No | 3 years | Effective | ||
| Eussen et al., 2006 [45] | The Netherlands | 195 elders | I: 83 ± 6 C: 82 ± 5 | 76.41 | I: 591 ± 203 nmol/L C: 680 ± 280 nmol/L | Fully a | Yes | Yes | No | 6 months | Ineffective | ||
| Fioravanti et al., 1997 [46] | Italy | 30 healthy elders | I: 80.25 ± 5.78 C: 80.21 ± 5.45 | 83.33 | I: 2.34 ± 0.51 ng/mL C: 2.21 ± 0.68 ng/mL | Insufficiency a | Yes | No | No | 2 months | Effective | ||
| Ford et al., 2010 [47] | Australia | 299 healthy elders | I: 79.30 ± 2.80 C: 78.70 ± 2.70 | 0 | I: 24.00 ± 0.61 nmol/L C: 24.40 ± 0.61 nmol/L | Fully c | Yes | Yes | Yes | 2 years | Ineffective | ||
| Gong et al., 2022 [48] | Hongkong | 279 patients with MCI | I: 76.90 ± 5.40 C: 76.72 ± 5.22 | 41.86 | I: 28.26 ± 8.06 nmol/L C: 28.82 ± 8.65 nmol/L | Fully a | Yes | Yes | No | 2 years | Effective | ||
| Hama et al., 2020 [49] | Japan | 45 elders | T: 79.7 ± 7.9 | 62.2 | No details | Insufficiency b | Yes | No | No | 28–63 days | Effective | ||
| Hankey et al., 2013 [50] | Australia | 2214 elders | T: 63.60 ± 11.80 | 32.7 | No details | No details | Yes | Yes | Yes | 3.4 years | Partly effective d | ||
| Jiang et al., 2014 [51] | China | 120 healthy elders | T: 63.00 ± 1.90 | 35 | I: 2.74 ± 0.65 ng/mL C: 2.83 ± 0.80 ng/mL | Insufficiency a | Yes | Yes | No | 6 months | Effective | ||
| Kwok et al., 2010 [52] | Hongkong | 140 patients with AD | I: 79.10 ± 6.70 C: 77.20 ± 7.90 | 63.57 | I: 21.70 ± 9.10 nmol/L C: 20.0 ± 7.0 nmol/L | Fully a | Yes | Yes | No | 2 years | Ineffective | ||
| Kwok et al., 2019 [53] | Hongkong | 279 patients with MCI | I: 77.80 ± 5.55 C: 78.00 ± 5.30 | 40.5 | I: 27.80 ± 8.00 nmol/L C: 29.40 ± 8.60 nmol/L | Fully a | Yes | Yes | No | 2 years | Partly effective d | ||
| Lewerin et al., 2005 [54] | Sweden | 209 healthy elders | I: 75.70 ± 4.70 C: 75.60 ± 4.00 | 55.90 | I: 15.7 ± 6.1 nmol/L C: 16.4 ± 5.1 nmol/L | Insufficiency a | Yes | Yes | Yes | 4 months | Ineffective | ||
| Li et al., 2019 [55] | China | 240 patients with MCI | I: 70.20 ± 6.13 C: 70.38 ± 6.73 | 58.75 | I: 8.41 ± 4.15 ng/mL C: 7.99 ± 4.84 ng/mL | Insufficiency a | Yes | No | No | 6 months | Effective | ||
| Lu et al., 2021 [56] | China | 50 healthy elders | I: 66.16 ± 7.61 C: 69.00 ± 10.80 | 74 | I: 7.96 ± 2.10 ng/mL C: 8.77 ± 4.05 ng/mL | Fully a | Yes | Yes | No | 2 years | Effective | ||
| Ma et al., 2015 [57] | China | 180 patients with MCI | I: 74.82 ± 2.75 C: 74.63 ± 3.21 | 57.22 | I: 7.01 ± 3.64 ng/mL C: 5.79 ± 2.67 ng/mL | Insufficiency b | Yes | No | No | 6 months | Effective | ||
| Ma et al., 2016 [58] | China | 168 patients with MCI | I: 73.71 ± 2.57 C: 73.52 ± 3.03 | 68.45 | I: 7.01 ± 1.01 ng/mL C: 6.33 ± 0.97 ng/mL | Fully a | Yes | No | No | 1 years | Effective | ||
| Ma et al., 2017 [59] | China | 180 patients with MCI | I: 74.82 ± 2.75 C: 74.63 ± 3.21 | 57.22 | I: 7.01 ± 0.64 ng/mL C: 5.79 ± 0.67 ng/mL | Insufficiency b | Yes | No | No | 2 years | Effective | ||
| Ma et al., 2019 [60] | China | 240 patients with MCI | I: 68.42 ± 3.62 C: 68.54 ± 3.90 | 64.17 | I: 7.71 ± 1.17 ng/mL C: 7.61 ± 0.60 ng/mL | Insufficiency b | Yes | Yes | No | 6 months | Effective | ||
| Pathansali et al., 2005 [61] | UK | 24 healthy elders | I: 72.30 ± 6.00 C: 73.80 ± 5.30 | 87.50 | I: 6.0 ± 2.3 μg/L C: 6.6 ± 2.8 μg/L | Insufficiency a | Yes | No | No | 1 months | Ineffective | ||
| Perła-Kaján et al., 2021 [62] | UK | 196 patients with MCI | T: 77.60 ± 4.80 | 60 | No details | No details | Yes | Yes | Yes | 2 years | Effective | ||
| Remington et al., 2014 [63] | New England | 106 patients with AD | T: 77.80± 9.30 | No Data | No details | No details | Yes | Yes | No | 9 months | Effective | ||
| Remington et al., 2015 [64] | New England | 34 patients with MCI | T: 65.90 ± 11.30 | No Data | No details | No details | Yes | Yes | No | 1 years | Effective | ||
| Stott, 2005 [65] | UK | 185 elders | I: 72.60 ± 6.40 C: 72.80 ± 5.40 | 44.68 | I: 320 ± 122 ng/mL C: 269 ± 87 ng/mL | Fully a | Yes | Yes | Yes | 3 months | Partly effective d | ||
| Sun et al., 2007 [66] | Taiwan | 89 patients with AD | I: 74.90 ± 7.10 C: 74.60 ± 7.50 | 49.44 | I: 9.0 ± 4.50 ng/mL C: 8.40 ± 6.60 ng/mL | Insufficiency a | Yes | Yes | Yes | 26 week | Partly effective d | ||
| Search et al., 2010 [67] | UK | 12064 elders | T: 64.20± 8.90 | 17.01 | No details | No details | Yes | Yes | No | 6.7 years | Partly effective d | ||
| Tan et al., 2022 [68] | Singapore | 707 elders | I: 61.51 ± 11.28 C: 60.22 ± 11.47 | 31.82 | I: 17.10 ± 8.32 μmol/L C: 16.62 ± 7.77 μmol/L | Fully a | Yes | Yes | Yes | 5 years | Ineffective | ||
| Ting et al., 2017 [69] | Singapore | 230 elders | I: 68.00 C: 66.00 | 39.57 | No details | No details | Yes | Yes | Yes | 5 years | Partly effective d | ||
| van Uffelen et al., 2008 [70] | The Netherlands | 152 patients with MCI | I: 75.40 ± 2.80 C: 74.90 ± 3.00 | 44.08 | No details | No details | Yes | Yes | Yes | 1 years | Ineffective | ||
| van der Zwaluw et al., 2014 [71] | The Netherlands | 2919 elders | T: 74.1 ± 6.5 | 50.00 | I: 19.2 (14.0–22.6) nmol/L C: 18.9 (14.2–22.7) nmol/L | Fully a | Yes | Yes | No | 2 years | Partly effective d | ||
| van Soest et al., 2022 [72] | The Netherlands | 191 elders | I: 70.30 ± 5.10 C: 72.70 ± 6.30 | 44 | I: 16.9 (13.9–22.4) nmol/L C: 17.7 (14.3–24.7) nmol/L | Fully a | Yes | Yes | No | 2 years | Effective | ||
| Wolters et al., 2005 [73] | Germany | 220 elders | I: 63.00 C: 64.00 | 100 | No details | No details | Yes | Yes | Yes | 6 months | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chen, X.; Chen, Y.; Yan, J.; Huang, G.; Li, W. A Comparative Study Evaluating the Effectiveness of Folate-Based B Vitamin Intervention on Cognitive Function of Older Adults under Mandatory Folic Acid Fortification Policy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2024, 16, 2199. https://doi.org/10.3390/nu16142199
Zhang L, Chen X, Chen Y, Yan J, Huang G, Li W. A Comparative Study Evaluating the Effectiveness of Folate-Based B Vitamin Intervention on Cognitive Function of Older Adults under Mandatory Folic Acid Fortification Policy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2024; 16(14):2199. https://doi.org/10.3390/nu16142199
Chicago/Turabian StyleZhang, Liyang, Xukun Chen, Yongjie Chen, Jing Yan, Guowei Huang, and Wen Li. 2024. "A Comparative Study Evaluating the Effectiveness of Folate-Based B Vitamin Intervention on Cognitive Function of Older Adults under Mandatory Folic Acid Fortification Policy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 16, no. 14: 2199. https://doi.org/10.3390/nu16142199
APA StyleZhang, L., Chen, X., Chen, Y., Yan, J., Huang, G., & Li, W. (2024). A Comparative Study Evaluating the Effectiveness of Folate-Based B Vitamin Intervention on Cognitive Function of Older Adults under Mandatory Folic Acid Fortification Policy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 16(14), 2199. https://doi.org/10.3390/nu16142199






