Causal Relationships between Polyunsaturated Fatty Acids and Colon Polyps: A Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
2. Methods
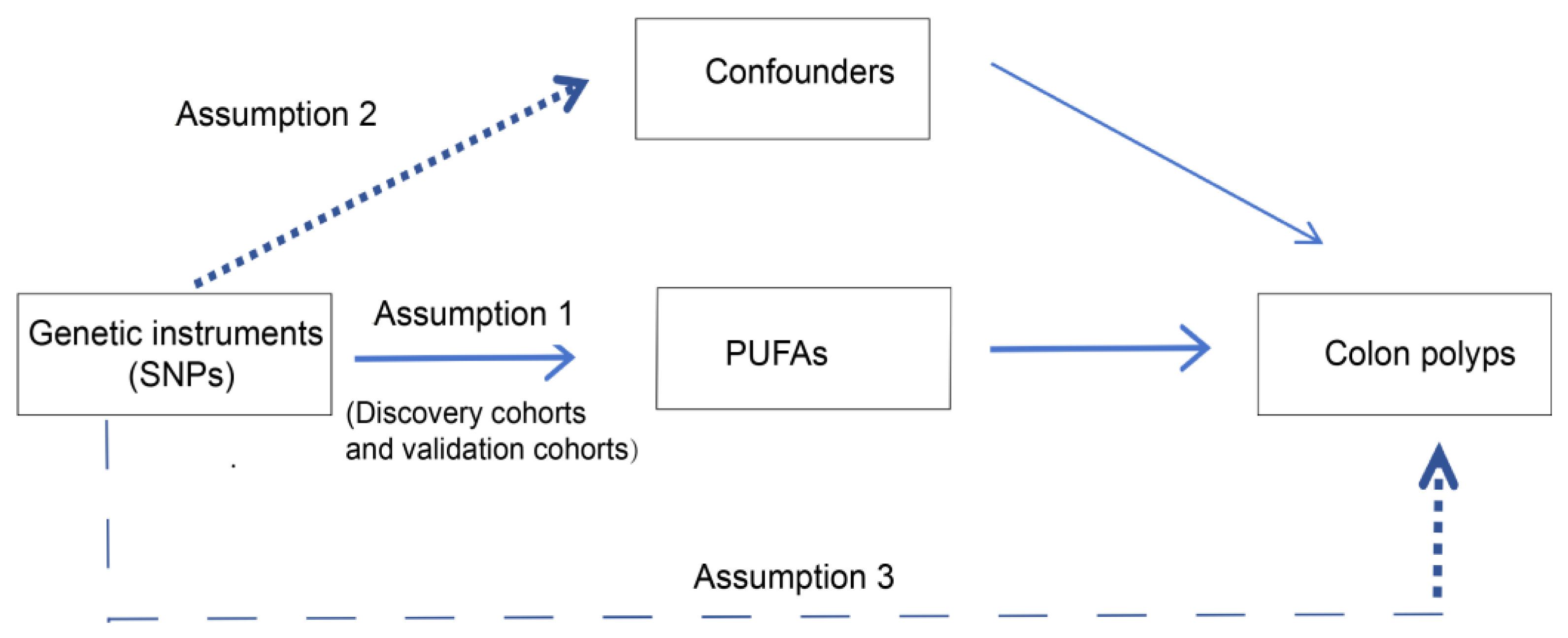
2.1. Study Design
2.2. Data Sources and Participants
2.3. Selection of SNPs
2.4. MR Analysis
2.5. Statistical Analysis
3. Result
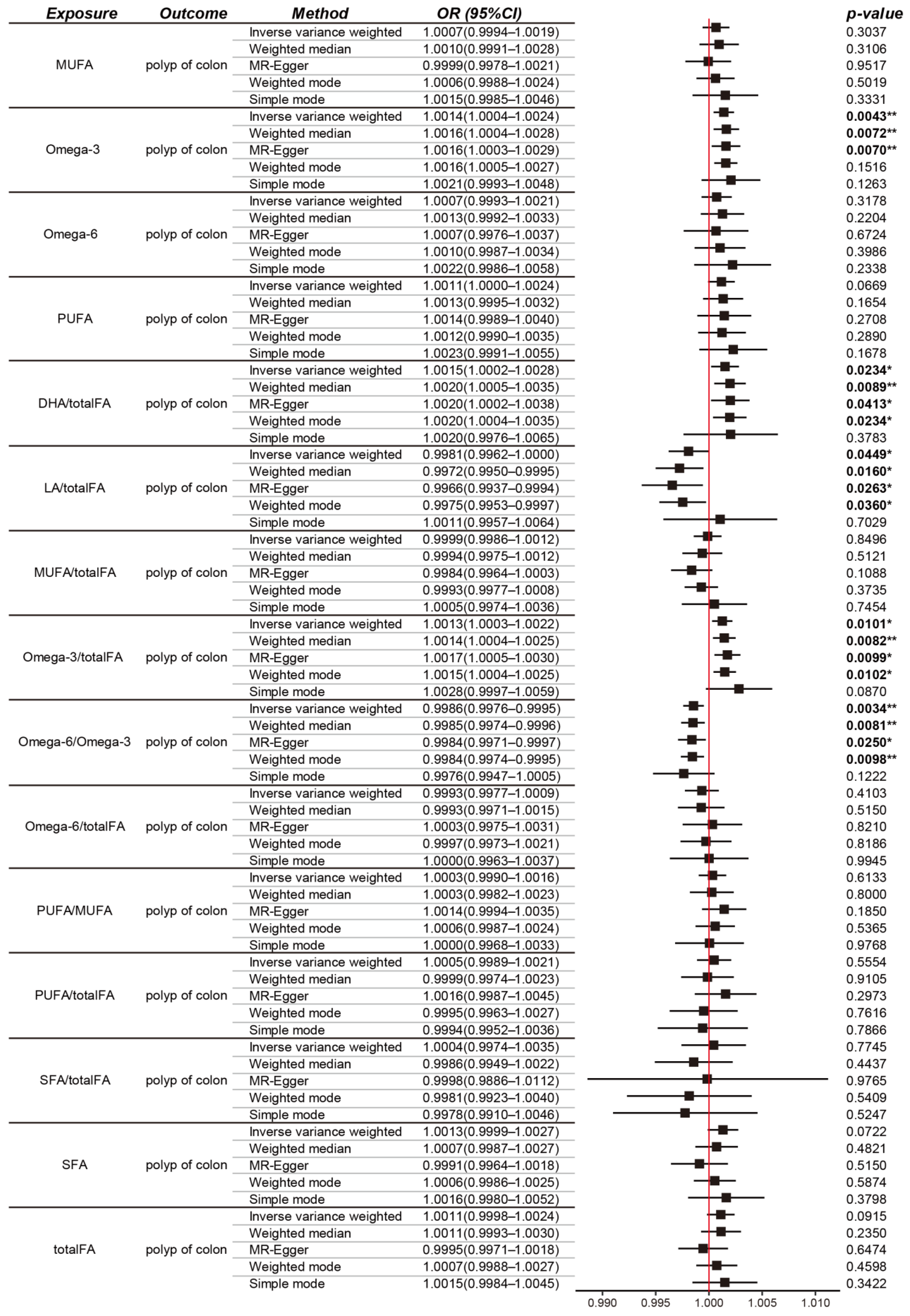
3.1. Causal Effects of Fatty Acids on Colon Polyps
3.2. Characteristics of Selected SNPs
3.3. Sensitivity and Heterogeneity Analysis
3.4. MR Study on Omega-3 Fatty Acids and Polyps in Other Body Systems
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corley, D.A.; Levin, T.R.; Doubeni, C.A. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 2014, 370, 2541. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar]
- Atkin, W.S.; Valori, R.; Kuipers, E.J.; Hoff, G.; Senore, C.; Segnan, N.; Jover, R.; Schmiegel, W.; Lambert, R.; Pox, C. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition—Colonoscopic surveillance following adenoma removal. Endoscopy 2012, 44 (Suppl. S3), SE151–SE163. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Mangu, P.B.; Gruber, S.B.; Hamilton, S.R.; Kalady, M.F.; Lau, M.W.Y.; Lu, K.H.; Roach, N.; Limburg, P.J. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J. Clin. Oncol. 2015, 33, 209–217. [Google Scholar] [CrossRef]
- Song, M.; Nishihara, R.; Cao, Y.; Chun, E.; Qian, Z.R.; Mima, K.; Inamura, K.; Masuki, Y.; Nowak, J.A.; Nosho, K.; et al. Marine omega-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer Characterized by Tumor-Infiltrating T Cells. JAMA Oncol. 2016, 2, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Pot, G.K.; Geelen, A.; van Heijningen, E.M.; Siezen, C.L.E.; van Kranen, H.J.; Kampman, E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: An endoscopy-based case-control study. Int. J. Cancer 2008, 123, 1974–1977. [Google Scholar] [CrossRef]
- He, X.; Wu, K.; Ogino, S.; Giovannucci, E.L.; Chan, A.T.; Song, M. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology 2018, 155, 355–373.e318. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Willett, W.C.; Fuchs, C.S.; Giovannucci, E. Dietary marine n-3 fatty acids in relation to risk of distal colorectal adenoma in women. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Pickens, C.A.; Pereira, M.F.A.; Fenton, J.I. Long-chain omega-6 plasma phospholipid polyunsaturated fatty acids and association with colon adenomas in adult men: A cross-sectional study. Eur. J. Cancer Prev. 2017, 26, 497–505. [Google Scholar] [CrossRef]
- Butler, L.M.; Yuan, J.-M.; Huang, J.Y.; Su, J.; Wang, R.; Koh, W.-P.; Ong, C.-N. Plasma fatty acids and risk of colon and rectal cancers in the Singapore Chinese Health Study. NPJ Precis. Oncol. 2017, 1, 38. [Google Scholar] [CrossRef]
- Khankari, N.K.; Banbury, B.L.; Borges, M.C.; Haycock, P.; Albanes, D.; Arndt, V.; Berndt, S.I.; Bézieau, S.; Brenner, H.; Campbell, P.T.; et al. Mendelian Randomization of Circulating Polyunsaturated Fatty Acids and Colorectal Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2020, 29, 860–870. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Smith, G.D. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Q.; Xue, R.; Liu, X.; Yu, H. Examining the Causal Inference of Leptin and Soluble Plasma Leptin Receptor Levels on Schizophrenia: A Mendelian Randomization Study. Front. Psychiatry 2021, 12, 753224. [Google Scholar] [CrossRef] [PubMed]
- Holme, Ø.; Bretthauer, M.; Eide, T.J.; Løberg, E.M.; Grzyb, K.; Løberg, M.; Kalager, M.; Adami, H.-O.; Kjellevold, Ø.; Hoff, G. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut 2015, 64, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Malau-Aduli, A.E.O.; Nichols, P.D. Enhancing Omega-3 Long-Chain Polyunsaturated Fatty Acid Content of Dairy-Derived Foods for Human Consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chen, C.-Y.; Nie, Y.-H.; Kaliannan, K.; Kang, J.X. Differential Interventional Effects of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on High Fat Diet-Induced Obesity and Hepatic Pathology. Int. J. Mol. Sci. 2023, 24, 17261. [Google Scholar] [CrossRef] [PubMed]
- Amminger, G.P.; Schafer, M.R.; Papageorgiou, K.; Klier, C.M.; Cotton, S.M.; Harrigan, S.M.; Mackinnon, A.; McGorry, P.D.; Berger, G.E. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: A randomized, placebo-controlled trial. Arch. Gen. Psychiatry 2010, 67, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C.; Wilson, P.W.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory from the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Baracchi, A.; Piani, F.; Esposti, D.D.; Agnoletti, D.; Borghi, C.; D’Addato, S.; Bologna HDP Study Group. When pregnancy-associated hypertriglyceridemia goes above and beyond the risk of pancreatitis. Intern. Emerg. Med. 2024, 19, 477–481. [Google Scholar] [CrossRef]
- da Silva Batista, E.; Nakandakari, S.; da Silva, A.S.A.; Pauli, J.R.; de Moura, L.P.; Ropelle, E.R.; Camargo, E.A.; Cintra, D.E. Omega-3 pleiad: The multipoint anti-inflammatory strategy. Crit. Rev. Food Sci. Nutr. 2024, 64, 4817–4832. [Google Scholar] [CrossRef]
- Bie, N.; Han, L.; Meng, M.; Yan, Z.; Wang, C. The immunomodulatory effect of docosahexaenoic acid (DHA) on the RAW264.7 cells by modification of the membrane structure and function. Food Funct. 2020, 11, 2603–2616. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.Y.; Sokolowska, M.; Alsaaty, S.; Martinez-Anton, A.; Logun, C.; Qi, H.-Y.; Shelhamer, J.H. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A(2) via GPR120 receptor to produce prostaglandin E(2) and plays an anti-inflammatory role in macrophages. Immunology 2014, 143, 81–95. [Google Scholar] [CrossRef]
- Campanari, D.D.; Cipriano, U.G.; Fraga-Silva, T.F.d.C.; Ramalho, L.N.Z.; Ovidio, P.P.; Júnior, A.A.J.; Bonato, V.L.D.; Ferriolli, E. Effect of Dietary Supplementation with Omega-3 Fatty Acid on the Generation of Regulatory T Lymphocytes and on Antioxidant Parameters and Markers of Oxidative Stress in the Liver Tissue of IL-10 Knockout Mice. Nutrients 2024, 16, 634. [Google Scholar] [CrossRef]
- Corteselli, E.M.; Gold, A.; Surratt, J.; Cui, T.; Bromberg, P.; Dailey, L.; Samet, J.M. Supplementation with omega-3 fatty acids potentiates oxidative stress in human airway epithelial cells exposed to ozone. Environ. Res. 2020, 187, 109627. [Google Scholar] [CrossRef]
- Zajdel, A.; Wilczok, A.; Tarkowski, M. Toxic effects of n-3 polyunsaturated fatty acids in human lung A549 cells. Toxicol. In Vitro 2015, 30, 486–491. [Google Scholar] [CrossRef]
- Park, Y.; Nam, S.; Yi, H.-J.; Hong, H.-J.; Lee, M. Dietary n-3 polyunsaturated fatty acids increase oxidative stress in rats with intracerebral hemorrhagic stroke. Nutr. Res. 2009, 29, 812–818. [Google Scholar] [CrossRef]
- Wenhao, W.; Ajay, G. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar]
- Briata, I.M.; Paleari, L.; Rutigliani, M.; Petrera, M.; Caviglia, S.; Romagnoli, P.; Libera, M.D.; Oppezzi, M.; Puntoni, M.; Siri, G.; et al. A Presurgical Study of Curcumin Combined with Anthocyanin Supplements in Patients with Colorectal Adenomatous Polyps. Int. J. Mol. Sci. 2021, 22, 11024. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Chatterjee, N. A comprehensive evaluation of methods for Mendelian randomization using realistic simulations and an analysis of 38 biomarkers for risk of type 2 diabetes. Int. J. Epidemiol. 2021, 50, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Bevan, R.; Essam, M.; Nickerson, C.; Hungin, P.; Bramble, M.; Rutter, M.D. Adenoma characteristics in the English Bowel Cancer Screening Programme. Colorectal Dis. 2024, 26, 643–649. [Google Scholar] [CrossRef]
- Waldmann, E.; Jiricka, L.; Zessner-Spitzenberg, J.; Majcher, B.; Rockenbauer, L.-M.; Penz, D.; Hinterberger, A.; Trauner, M.; Ferlitsch, M. Differences between men and women with respect to colorectal cancer mortality despite screening colonoscopy. Gastrointest. Endosc. 2024, 99, 998–1005. [Google Scholar] [CrossRef]


| Exposure | GWAS ID | Year | Consortium | Sample Size | Number of SNPs |
|---|---|---|---|---|---|
| MUFA | ebi-a-GCST90092928 | 2022 | NA | 115,006 | 11,590,399 |
| Omega-3 | ebi-a-GCST90092931 | 2022 | NA | 115,006 | 11,590,399 |
| Omega-6 | ebi-a-GCST90092933 | 2022 | NA | 115,006 | 11,590,399 |
| PUFA | ebi-a-GCST90092939 | 2022 | NA | 115,006 | 11,590,399 |
| DHA/totalFA | ebi-a-GCST90092817 | 2022 | NA | 115,006 | 11,590,399 |
| LA/totalFA | ebi-a-GCST90092881 | 2022 | NA | 115,006 | 11,590,399 |
| MUFA/totalFA | ebi-a-GCST90092929 | 2022 | NA | 115,006 | 11,590,399 |
| Omega-3/totalFA | ebi-a-GCST90092932 | 2022 | NA | 115,006 | 11,590,399 |
| Omega-6/Omega-3 | ebi-a-GCST90092934 | 2022 | NA | 115,006 | 11,590,399 |
| Omega-6/totalFA | ebi-a-GCST90092935 | 2022 | NA | 115,006 | 11,590,399 |
| PUFA/MUFA | ebi-a-GCST90092940 | 2022 | NA | 115006 | 11,590,399 |
| PUFA/totalFA | ebi-a-GCST90092941 | 2022 | NA | 115,006 | 11,590,399 |
| SFA/totalFA | ebi-a-GCST90092981 | 2022 | NA | 115,006 | 11,590,399 |
| SFA | ebi-a-GCST90092980 | 2022 | NA | 115,006 | 11,590,399 |
| totalFA | ebi-a-GCST90092987 | 2022 | NA | 115,006 | 11,590,399 |
| Outcome | GWAS ID | Year | Consortium | Sample Size | Number of SNPs |
| Polyp of colon | ukb-b-1968 | 2018 | MRC-IEU | 463,010 | 9,851,867 |
| Pleiotropy Test | Heterogeneity Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MR-Egger | MR-Egger | IVW | |||||||
| Intercept | SE | p-Value | Q | Q (df) | p-Value | Q | Q (df) | p-Value | |
| Omega-3 | 4.81 × 10−5 | 5.85 × 10−5 | 0.417 | 24.20 | 34 | 0.893 | 24.39 | 35 | 0.910 |
| DHA/totalFA | −5.68 × 10−5 | 7.32 × 10−5 | 0.447 | 13.74 | 21 | 0.880 | 14.34 | 22 | 0.889 |
| LA/totalFA | 1.03 × 10−4 | 7.56 × 10−5 | 0.185 | 33.74 | 28 | 0.210 | 35.96 | 29 | 0.175 |
| Omega-3/totalFA | −7.45 × 10−5 | 6.01 × 10−5 | 0.227 | 20.27 | 26 | 0.778 | 21.81 | 27 | 0.747 |
| Omega-6/Omega-3 | 2.28 × 10−5 | 6.72 × 10−5 | 0.738 | 11.39 | 23 | 0.979 | 11.51 | 24 | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, N.; Ba, Q.; Lu, Y. Causal Relationships between Polyunsaturated Fatty Acids and Colon Polyps: A Two-Sample Mendelian Randomization Study. Nutrients 2024, 16, 2033. https://doi.org/10.3390/nu16132033
Shen N, Ba Q, Lu Y. Causal Relationships between Polyunsaturated Fatty Acids and Colon Polyps: A Two-Sample Mendelian Randomization Study. Nutrients. 2024; 16(13):2033. https://doi.org/10.3390/nu16132033
Chicago/Turabian StyleShen, Na, Qinwen Ba, and Yanjun Lu. 2024. "Causal Relationships between Polyunsaturated Fatty Acids and Colon Polyps: A Two-Sample Mendelian Randomization Study" Nutrients 16, no. 13: 2033. https://doi.org/10.3390/nu16132033
APA StyleShen, N., Ba, Q., & Lu, Y. (2024). Causal Relationships between Polyunsaturated Fatty Acids and Colon Polyps: A Two-Sample Mendelian Randomization Study. Nutrients, 16(13), 2033. https://doi.org/10.3390/nu16132033







