Assessment of Skin Autofluorescence and Its Association with Glycated Hemoglobin, Cardiovascular Risk Markers, and Concomitant Chronic Diseases in Children with Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
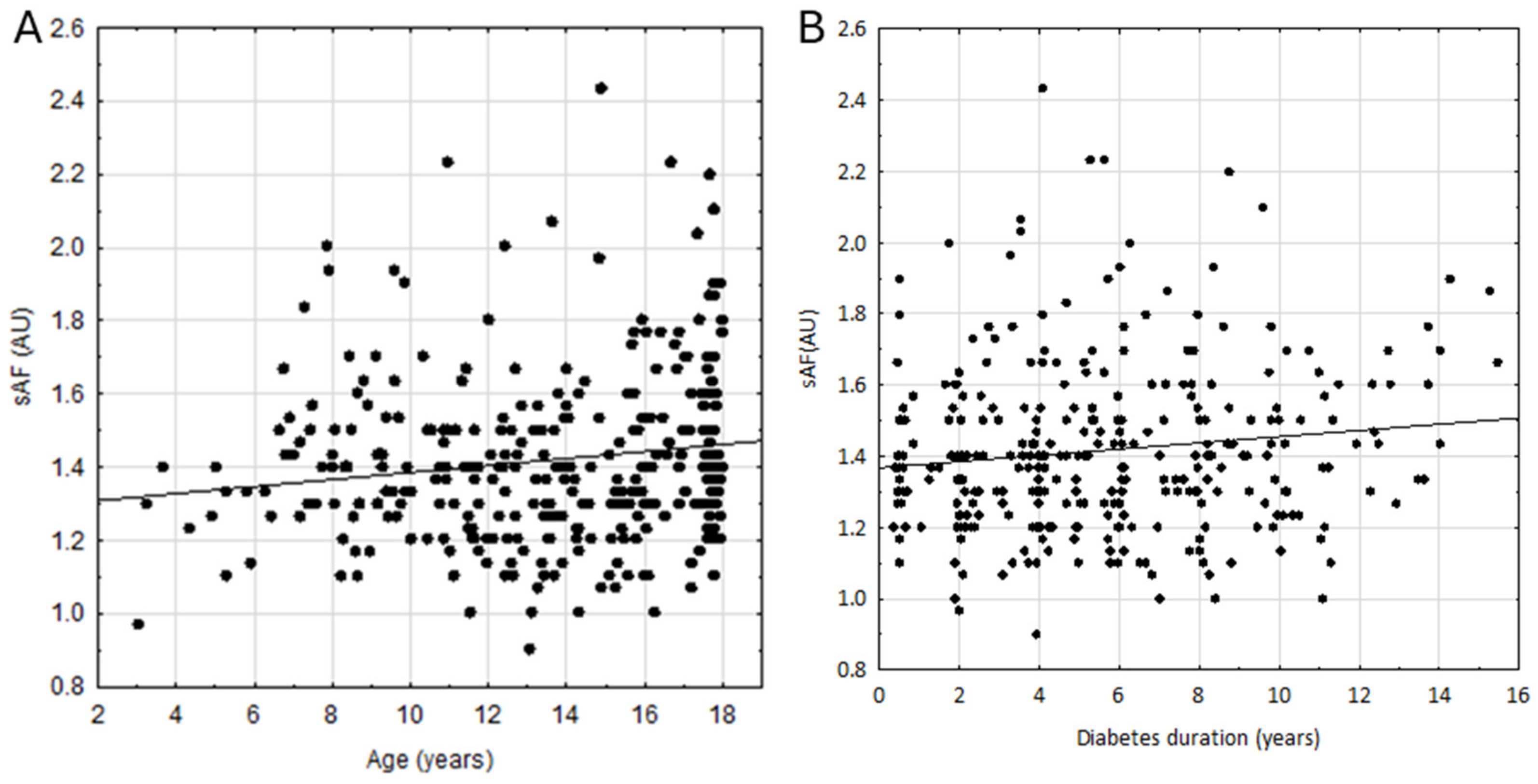
3. Results
4. Discussion
4.1. Metabolic Control
4.2. CV Risk and Microvascular Complications
4.3. Common Comorbidities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Bjornstad, P.; Dart, A.; Donaghue, K.C.; Dost, A.; Feldman, E.L.; Tan, G.S.; Wadwa, R.P.; Zabeen, B.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Microvascular and macrovascular complications in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1432–1450. [Google Scholar] [CrossRef]
- Fröhlich-Reiterer, E.; Elbarbary, N.S.; Simmons, K.; Buckingham, B.; Humayun, K.N.; Johannsen, J.; Holl, R.W.; Betz, S.; Mahmud, F.H. ISPAD Clinical Practice Consensus Guidelines 2022: Other complications and associated conditions in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2022, 23, 1451–1467. [Google Scholar] [CrossRef]
- Nathan, D.M. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef]
- Bry, L.; Chen, P.C.; Sacks, D.B. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin. Chem. 2001, 47, 153–163. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef]
- Sugisawa, E.; Miura, J.; Iwamoto, Y.; Uchigata, Y. Skin autofluorescence reflects integration of past long-term glycemic control in patients with type 1 diabetes. Diabetes Care 2013, 36, 2339–2345. [Google Scholar] [CrossRef][Green Version]
- Conway, B.N.; Aroda, V.R.; Maynard, J.D.; Matter, N.; Fernandez, S.; Ratner, R.E.; Orchard, T.J. Skin intrinsic fluorescence is associated with coronary artery disease in individuals with long duration of type 1 diabetes. Diabetes Care 2012, 35, 2331–2336. [Google Scholar] [CrossRef]
- Monnier, V.M.; Sell, D.R.; Gao, X.; Genuth, S.M.; Lachin, J.M.; Bebu, I. Plasma advanced glycation end products and the subsequent risk of microvascular complications in type 1 diabetes in the DCCT/EDIC. BMJ Open Diabetes Res. Care 2022, 10, e002667. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Naskret, D.; Zozulinska-Ziolkiewicz, D.; Pilacinski, S.; Uruska, A.; Grzelka, A.; Wegner, M.; Wierusz-Wysocka, B. Skin autofluorescence is associated with carotid intima-media thickness, diabetic microangiopathy, and long-lasting metabolic control in type 1 diabetic patients. Results from Poznan Prospective Study. Microvasc. Res. 2015, 98, 62–67. [Google Scholar] [CrossRef]
- Blanc-Bisson, C.; Velayoudom-Cephise, F.L.; Cougnard-Gregoire, A.; Helmer, C.; Rajaobelina, K.; Delcourt, C.; Alexandre, L.; Blanco, L.; Mohammedi, K.; Monlun, M.; et al. Skin autofluorescence predicts major adverse cardiovascular events in patients with type 1 diabetes: A 7-year follow-up study. Cardiovasc. Diabetol. 2018, 17, 82. [Google Scholar] [CrossRef]
- Banser, A.; Naafs, J.C.; Hoorweg-Nijman, J.J.; van de Garde, E.M.; van der Vorst, M.M. Advanced glycation end products, measured in skin, vs. HbA1c in children with type 1 diabetes mellitus. Pediatr. Diabetes 2016, 17, 426–432. [Google Scholar] [CrossRef]
- van der Heyden, J.C.; Birnie, E.; Mul, D.; Bovenberg, S.; Veeze, H.J.; Aanstoot, H.-J. Increased skin autofluorescence of children and adolescents with type 1 diabetes despite a well-controlled HbA1c: Results from a cohort study. BMC Endocr. Disord. 2016, 16, 49. [Google Scholar] [CrossRef]
- Cho, Y.H.; Craig, M.E.; Januszewski, A.S.; Benitez-Aguirre, P.; Hing, S.; Jenkins, A.J.; Donaghue, K.C. Higher skin autofluorescence in young people with Type 1 diabetes and microvascular complications. Diabet. Med. 2017, 34, 543–550. [Google Scholar] [CrossRef]
- Koetsier, M.; Lutgers, H.; de Jonge, C.; Links, T.; Smit, A.; Graaff, R. Reference values of skin autofluorescence. Diabetes Technol. Ther. 2010, 12, 399–403. [Google Scholar] [CrossRef]
- Klenovics, K.S.; Kollárová, R.; Hodosy, J.; Celec, P.; Šebeková, K. Reference values of skin autofluorescence as an estimation of tissue accumulation of advanced glycation end products in a general Slovak population. Diabet. Med. 2014, 31, 581–585. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Kimhofer, T.; Ahmad, S.; AlAma, M.N.; Mosli, H.H.; Hindawi, S.I.; Mook-Kanamori, D.O.; Šebeková, K.; Damanhouri, Z.A.; Holmes, E. Ethnicity and skin autofluorescence-based risk-engines for cardiovascular disease and diabetes mellitus. PLoS ONE 2017, 12, e0185175. [Google Scholar] [CrossRef]
- Jankowska, M.; Bobeff, K.; Baranowska-Jaźwiecka, A.; Mianowska, M.; Lubnauer, A.; Michalak, A.; Młynarski, W.; Szadkowska, A.; Mianowska, B. Evaluation of skin autofluorescence as a surrogate of advanced glycation end products accumulation in children and adolescents with normal haemoglobin A1c values. Pediatr. Endocrinol. Diabetes Metab. 2020, 26, 1–9. [Google Scholar] [CrossRef]
- Mulder, D.J.; Van De Water, T.; Lutgers, H.L.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: An overview of current clinical studies, evidence, and limitations. Diabetes Technol. Ther. 2006, 8, 523–535. [Google Scholar] [CrossRef]
- Kułaga, Z.; Litwin, M.; Tkaczyk, M.; Palczewska, I.; Zajączkowska, M.; Zwolińska, D.; Krynicki, T.; Wasilewska, A.; Moczulska, A.; Morawiec-Knysak, A.; et al. Polish 2010 growth references for school-aged children and adolescents. Eur. J. Pediatr. 2011, 170, 599–609. [Google Scholar] [CrossRef]
- Shah, S.; Baez, E.A.; Felipe, D.L.; Maynard, J.D.; Hempe, J.M.; Chalew, S.A. Advanced glycation endproducts in children with diabetes. J. Pediatr. 2013, 163, 1427–1431. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Razavi, Z.; Ehsani, A.H.; Firooz, A.; Afazeli, S. Clinical Significance of Non-invasive Skin Autofluorescence Measurement in Patients with Diabetes: A Systematic Review and Meta-analysis. EClinicalMedicine 2021, 42, 101194. [Google Scholar] [CrossRef]
- Felipe, D.L.; Hempe, J.M.; Liu, S.; Matter, N.; Maynard, J.; Linares, C.; Chalew, S.A. Skin Intrinsic Fluorescence Is Associated with Hemoglobin A 1c and Hemoglobin Glycation Index but Not Mean Blood Glucose in Children with Type 1 Diabetes. Diabetes Care 2011, 34, 1816–1820. [Google Scholar] [CrossRef]
- Januszewski, A.S.; Xu, D.; Cho, Y.H.; Benitez-Aguirre, P.Z.; O’neal, D.N.; Craig, M.E.; Donaghue, K.C.; Jenkins, A.J. Skin autofluorescence in people with type 1 diabetes and people without diabetes: An eight-decade cross-sectional study with evidence of accelerated aging and associations with complications. Diabet. Med. 2021, 38, e14432. [Google Scholar] [CrossRef]
- Samuelsson, U.; Anderzén, J.; Gudbjörnsdottir, S.; Steineck, I.; Åkesson, K.; Hanberger, L. Teenage girls with type 1 diabetes have poorer metabolic control than boys and face more complications in early adulthood. J. Diabetes Complicat. 2016, 30, 917–922. [Google Scholar] [CrossRef]
- Jarosz-Chobot, P.; Polańska, J.; Myśliwiec, M.; Szadkowska, A.; Fendler, W.; Kamińska, H.; Chumiecki, M.; Mianowska, B.; Techmańska, I.; Sztangierska, B.; et al. PolPeDiab study group. Multicenter cross-sectional analysis of values of glycated haemoglobin (HbA1c) in Polish children and adolescents with long-term type 1 diabetes in Poland: PolPeDiab study group. Pediatr. Endocrinol. Diabetes Metab. 2012, 18, 125. [Google Scholar]
- Báez, E.A.; Shah, S.; Felipe, D.; Maynard, J.; Lefevre, S.; Chalew, S.A. Skin advanced glycation endproducts are elevated at onset of type 1 diabetes in youth. J. Pediatr. Endocrinol. Metab. 2015, 28, 133–137. [Google Scholar] [CrossRef]
- Barat, P.; Cammas, B.; Lacoste, A.; Harambat, J.; Vautier, V.; Nacka, F.; Corcuff, J.-B. Advanced glycation end products in children with type 1 diabetes: Family matters? Diabetes Care 2012, 35, 6479. [Google Scholar] [CrossRef]
- Reurean-Pintilei, D.; Stoian, A.P.; Potcovaru, C.-G.; Salmen, T.; Cinteză, D.; Stoica, R.-A.; Lazăr, S.; Timar, B. Skin Autofluorescence as a Potential Adjunctive Marker for Cardiovascular Risk Assessment in Type 2 Diabetes: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3889. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Lutgers, H.L.; Links, T.P.; Graaff, R.; Baynes, J.W.; Gans, R.O.; Smit, A.J. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care 2007, 30, 107–112. [Google Scholar] [CrossRef]
- Mulder, D.J.; van Haelst, P.L.; Gross, S.; de Leeuw, K.; Bijzet, J.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin autofluorescence is elevated in patients with stable coronary artery disease and is associated with serum levels of neopterin and the soluble receptor for advanced glycation end products. Atherosclerosis 2008, 197, 217–223. [Google Scholar] [CrossRef]
- Mulder, D.J.; van Haelst, P.L.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin autofluorescence is elevated in acute myocardial infarction and is associated with the one-year incidence of major adverse cardiac events. Neth. Heart J. 2009, 17, 162–168. [Google Scholar] [CrossRef][Green Version]
- Cavero-Redondo, I.; Soriano-Cano, A.; Álvarez-Bueno, C.; Cunha, P.G.; Martínez-Hortelano, J.A.; Garrido-Miguel, M.; Berlanga-Macías, C.; Martínez-Vizcaíno, V. Skin autofluorescence–indicated advanced glycation end products as predictors of cardiovascular and all-cause mortality in high-risk subjects: A systematic review and meta-analysis. J. Am. Heart Assoc. 2018, 7, e009833. [Google Scholar] [CrossRef]
- van Waateringe, R.P.; Fokkens, B.T.; Slagter, S.N.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Smit, A.J.; Lutgers, H.L.; Wolffenbuttel, B.H.R. Skin autofluorescence predicts incident type 2 diabetes, cardiovascular disease and mortality in the general population. Diabetologia 2019, 62, 269–280. [Google Scholar] [CrossRef]
- Lentferink, Y.E.; Van Teeseling, L.; Knibbe, C.A.J.; Van Der Vorst, M.M.J. Skin autofluorescence in children with and without obesity. J. Pediatr. Endocrinol. Metab. 2019, 32, 41–47. [Google Scholar] [CrossRef]
- Zawada, A.; Naskret, D.; Niedźwiecki, P.; Grzymisławski, M.; Zozulińska-Ziółkiewicz, D.; Dobrowolska, A. Excess body fat increases the accumulation of advanced glycation end products in the skin of patients with type 1 diabetes. Adv. Clin. Exp. Med. 2020, 29, 1193–1199. [Google Scholar] [CrossRef]
- Speakman, J.R.; Selman, C. Physical activity and resting metabolic rate. Proc. Nutr. Soc. 2003, 62, 621–634. [Google Scholar] [CrossRef]
- Köchli, S.; Endes, K.; Trinkler, M.; Mondoux, M.; Zahner, L.; Hanssen, H. Association of physical fitness with skin autofluorescence-derived advanced glycation end products in children. Pediatr. Res. 2020, 87, 1106–1111. [Google Scholar] [CrossRef]
- Hjerrild, J.N.; Wobbe, A.; Stausholm, M.B.; Larsen, A.E.; Josefsen, C.O.; Malmgaard-Clausen, N.M.; Dela, F.; Kjaer, M.; Magnusson, S.P.; Hansen, M.; et al. Effects of long-term physical activity and diet on skin glycation and Achilles tendon structure. Nutrients 2019, 11, 1409. [Google Scholar] [CrossRef]
- Hirai, T.; Fujiyoshi, K.; Yamada, S.; Matsumoto, T.; Kikuchi, J.; Ishida, K.; Ishida, M.; Yamaoka-Tojo, M.; Inomata, T.; Shigeta, K.; et al. Advanced Glycation End Products Are Associated with Diabetes Status and Physical Functions in Patients with Cardiovascular Disease. Nutrients 2022, 14, 3032. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. C-Reactive Protein, Fibrinogen, and Cardiovascular Disease Prediction. N. Engl. J. Med. 2012, 367, 1310–1320. [Google Scholar] [CrossRef]
- Badimon, L.; Peña, E.; Arderiu, G.; Padró, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-reactive protein in atherothrombosis and angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef]
- Warncke, K.; Liptay, S.; Fröhlich-Reiterer, E.; Scheuing, N.; Schebek, M.; Wolf, J.; Rohrer, T.R.; Meissner, T.; Holl, R.W. Vascular risk factors in children, adolescents, and young adults with type 1 diabetes complicated by celiac disease: Results from the DPV initiative. Pediatr. Diabetes 2016, 17, 191–198. [Google Scholar] [CrossRef]
- Conroy, M.; Allen, N.; Lacey, B.; Soilleux, E.; Littlejohns, T. Association between coeliac disease and cardiovascular disease: Prospective analysis of UK Biobank data. BMJ Med. 2023, 2, e000371. [Google Scholar] [CrossRef]
- Bakker, S.; Tushuizen, M.; Gözütok, E.; Çiftci, A.; Gelderman, K.; Mulder, C.; Simsek, S. Advanced glycation end products (AGEs) and the soluble receptor for AGE (sRAGE) in patients with type 1 diabetes and coeliac disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 230–235. [Google Scholar] [CrossRef]


| Characteristics | T1D Group (N = 348) Median (IQR) or Number (%) | Control Group (N = 85) Median (IQR) or Number (%) |
|---|---|---|
| Gender (male/female) | 182 (52.3%)/166 (47.7%) | 45 (53%)/40 (47%) |
| Insulin therapy (insulin pump/MDI) | 244 (70.1%)/104 (29.9%) | NA |
| Age [years] | 14.30 (11.20–17.11) | 11.01 (8.03–14.10) |
| Diabetes duration [years] | 5.60 (3.10–8.80) | NA |
| Body mass index [z-score] | 0.37 (−0.35–1.03) | 0.08 (−0.25–0.74) |
| Skin autofluorescence, sAF [AU] | 1.40 (1.27–1.53) | 1.20 (1.07–1.30) |
| Daily insulin dose [units per kg per day] | 0.82 (0.65–1.00) | NA |
| HbA1c [% and mmol/mol] | 7.30 (6.70–8.05); 56.00 (50.00–64.00) | 5.10 (4.90–5.20); 32.00 (30.00–33.00) |
| HbA1c-historical [% and mmol/mol] | 7.19 (6.64–7.78); 55.00 (49.00–62.00) | NA |
| C-peptide [ng/dL] | 0.01 (0.01–0.11) | NA |
| Total cholesterol [mg/dL] | 167.00 (149.30–186.00) | NA |
| HDL cholesterol [mg/dL] | 62.73 (54.66–72.22) | NA |
| LDL cholesterol [mg/dL] | 93.00 (79.57–109.83) | NA |
| Triglycerides [mg/dL] | 65.73 (51.36–86.85) | NA |
| Creatinine [mg/dL] | 0.60 (0.50–0.72) | NA |
| CRP [mg/L] | 0.40 (0.20–0.82) | NA |
| Total bilirubin [mg/dL] | 0.57 (0.40–0.76) | NA |
| Albuminuria [mg/L] | 7.28 (5.92–10.00) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowska, M.; Szadkowska, A.; Pietrzak, I.; Chrzanowski, J.; Sołek, J.; Fendler, W.; Mianowska, B. Assessment of Skin Autofluorescence and Its Association with Glycated Hemoglobin, Cardiovascular Risk Markers, and Concomitant Chronic Diseases in Children with Type 1 Diabetes. Nutrients 2024, 16, 1940. https://doi.org/10.3390/nu16121940
Jankowska M, Szadkowska A, Pietrzak I, Chrzanowski J, Sołek J, Fendler W, Mianowska B. Assessment of Skin Autofluorescence and Its Association with Glycated Hemoglobin, Cardiovascular Risk Markers, and Concomitant Chronic Diseases in Children with Type 1 Diabetes. Nutrients. 2024; 16(12):1940. https://doi.org/10.3390/nu16121940
Chicago/Turabian StyleJankowska, Marta, Agnieszka Szadkowska, Iwona Pietrzak, Jędrzej Chrzanowski, Julia Sołek, Wojciech Fendler, and Beata Mianowska. 2024. "Assessment of Skin Autofluorescence and Its Association with Glycated Hemoglobin, Cardiovascular Risk Markers, and Concomitant Chronic Diseases in Children with Type 1 Diabetes" Nutrients 16, no. 12: 1940. https://doi.org/10.3390/nu16121940
APA StyleJankowska, M., Szadkowska, A., Pietrzak, I., Chrzanowski, J., Sołek, J., Fendler, W., & Mianowska, B. (2024). Assessment of Skin Autofluorescence and Its Association with Glycated Hemoglobin, Cardiovascular Risk Markers, and Concomitant Chronic Diseases in Children with Type 1 Diabetes. Nutrients, 16(12), 1940. https://doi.org/10.3390/nu16121940








