Exploring the Significance of Gut Microbiota in Diabetes Pathogenesis and Management—A Narrative Review
Abstract
1. Introduction
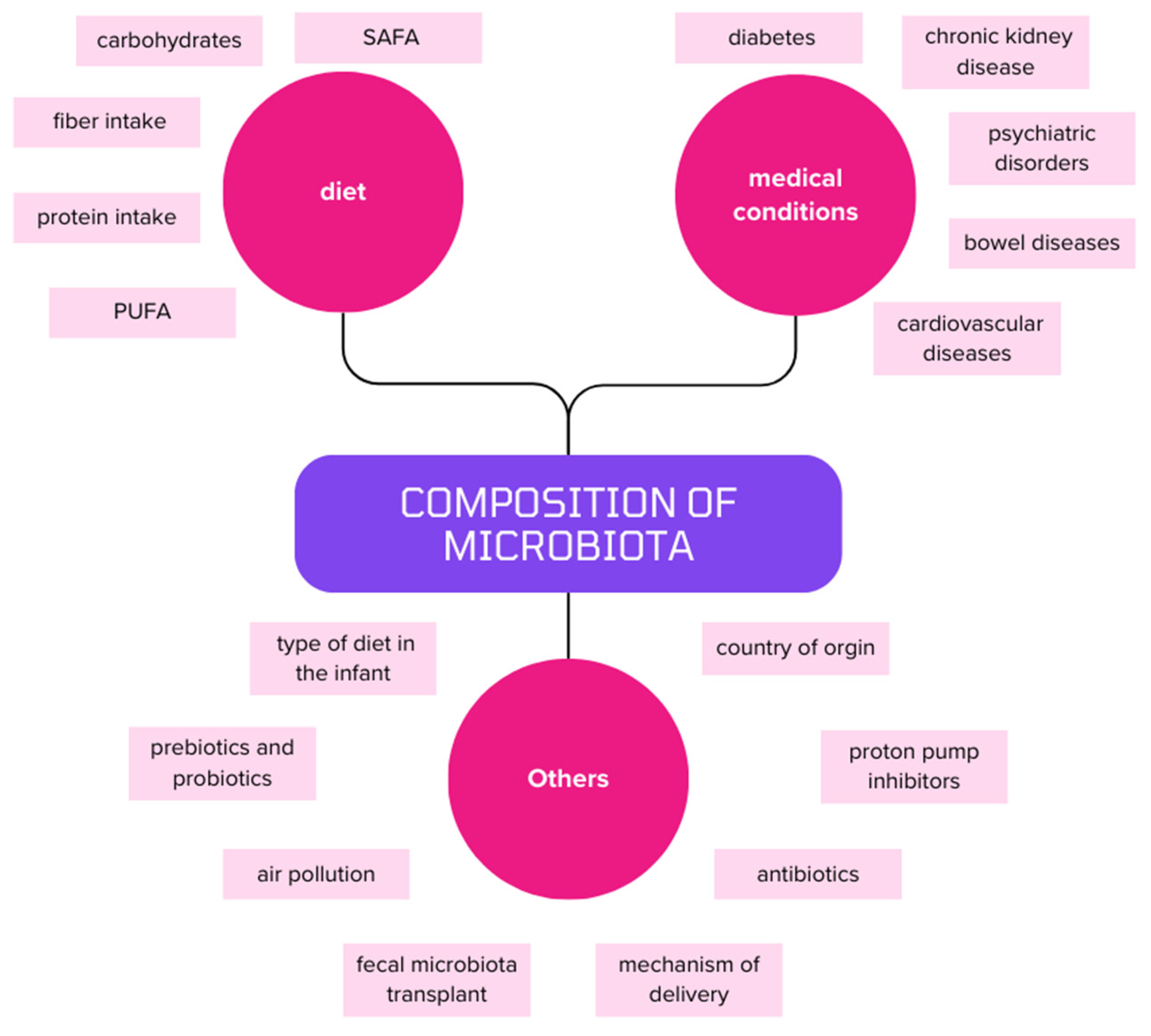
2. The Microbiota and the Consequences of Its Disorders
2.1. Compositional Changes in Microbiota of T2DM Patients
2.2. The Role of the Gut Microbiota in the Pathogenesis of T2DM
2.2.1. SCFAs
2.2.2. BCAAs
2.2.3. Bile Acids
2.2.4. LPS
3. Gut Microbiota—Potential Therapeutic Target for the Management of Diabetes
4. Gut Microbiota—Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beydag-Tasöz, B.S.; Yennek, S.; Grapin-Botton, A. Towards a Better Understanding of Diabetes Mellitus Using Organoid Models. Nat. Rev. Endocrinol. 2023, 19, 232–248. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2011, 34, S62–S69. [Google Scholar] [CrossRef]
- Crasto, W.; Patel, V.; Davies, M.J.; Khunti, K. Prevention of Microvascular Complications of Diabetes. Endocrinol. Metab. Clin. N. Am. 2021, 50, 431–455. [Google Scholar] [CrossRef]
- Fayfman, M.; Pasquel, F.J.; Umpierrez, G.E. Management of Hyperglycemic Crises. Med. Clin. N. Am. 2017, 101, 587–606. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Chong, K.; Chang, J.K.; Chuang, L. Recent Advances in the Treatment of Type 2 Diabetes Mellitus Using New Drug Therapies. Kaohsiung J. Med. Sci. 2024, 40, 212–220. [Google Scholar] [CrossRef]
- Gajewska, A.; Wasiak, J.; Sapeda, N.; Młynarska, E.; Rysz, J.; Franczyk, B. SGLT2 Inhibitors in Kidney Diseases—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4959. [Google Scholar] [CrossRef]
- Tripathi, B.K.; Srivastava, A.K. Diabetes Mellitus: Complications and Therapeutics. Med. Sci. Monit. 2006, 12, RA130–RA147. [Google Scholar]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Zhang, Y.; Li, X. Comprehensive Relationships between Gut Microbiome and Faecal Metabolome in Individuals with Type 2 Diabetes and Its Complications. Endocrine 2019, 66, 526–537. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Pantazi, A.C.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Lupu, V.V.; Kassim, M.A.K.; Mihai, L.; Frecus, C.E.; Chirila, S.I.; Lupu, A.; et al. Development of Gut Microbiota in the First 1000 Days after Birth and Potential Interventions. Nutrients 2023, 15, 3647. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Ke, H. Investigating the Causal Relationship between Gut Microbiota and Crohn’s Disease: A Mendelian Randomization Study. Gastroenterology 2024, 166, 354–355. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; -Or-Rashid, M.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The Gut Microbiota and the Brain–Gut–Kidney Axis in Hypertension and Chronic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Wasiak, J.; Gawlik-Kotelnicka, O. Intestinal Permeability and Its Significance in Psychiatric Disorders—A Narrative Review and Future Perspectives. Behav. Brain Res. 2023, 448, 114459. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Riedl, R.A.; Atkinson, S.N.; Burnett, C.M.L.; Grobe, J.L.; Kirby, J.R. The Gut Microbiome, Energy Homeostasis, and Implications for Hypertension. Curr. Hypertens. Rep. 2017, 19, 27. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- McCallum, G.; Tropini, C. The Gut Microbiota and Its Biogeography. Nat. Rev. Microbiol. 2024, 22, 105–118. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s Role in Health and Diseases. Environ. Sci. Pollut. Res. 2021, 28, 36967–36983. [Google Scholar] [CrossRef]
- Czarnik, W.; Fularski, P.; Gajewska, A.; Jakubowska, P.; Uszok, Z.; Młynarska, E.; Rysz, J.; Franczyk, B. The Role of Intestinal Microbiota and Diet as Modulating Factors in the Course of Alzheimer’s and Parkinson’s Diseases. Nutrients 2024, 16, 308. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Naik, S.S.; Ramphall, S.; Rijal, S.; Prakash, V.; Ekladios, H.; Mulayamkuzhiyil Saju, J.; Mandal, N.; Kham, N.I.; Shahid, R.; Venugopal, S. Association of Gut Microbial Dysbiosis and Hypertension: A Systematic Review. Cureus 2022, 14, e29927. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Barrientos-Durán, A.; Fuentes-López, A.; de Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Impact of Gut Microbiota on Brain and Behaviour. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 552–558. [Google Scholar] [CrossRef]
- McNeil, N.I. The Contribution of the Large Intestine to Energy Supplies in Man. Am. J. Clin. Nutr. 1984, 39, 338–342. [Google Scholar] [CrossRef]
- Furness, J.B. The Enteric Nervous System and Neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Cavin, J.-B.; Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A. Acute Regulation of Intestinal Ion Transport and Permeability in Response to Luminal Nutrients: The Role of the Enteric Nervous System. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G254–G264. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Rudzki, L.; Maes, M. The Microbiota-Gut-Immune-Glia (MGIG) Axis in Major Depression. Mol. Neurobiol. 2020, 57, 4269–4295. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A Comparative Review of Toll-Like Receptor 4 Expression and Functionality in Different Animal Species. Front. Immunol. 2014, 5, 96623. [Google Scholar] [CrossRef]
- Berkes, J. Intestinal Epithelial Responses to Enteric Pathogens: Effects on the Tight Junction Barrier, Ion Transport, and Inflammation. Gut 2003, 52, 439–451. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut as a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 269575. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA Axis Dysregulation in Severe Mental Illness: Can We Shift the Blame to Gut Microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G. Chronic Stress and Diabetes Mellitus: Interwoven Pathologies. Curr. Diabetes Rev. 2020, 16, 546–556. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Kealy, J.; Greene, C.; Campbell, M. Blood-Brain Barrier Regulation in Psychiatric Disorders. Neurosci. Lett. 2020, 726, 133664. [Google Scholar] [CrossRef]
- Sandiego, C.M.; Gallezot, J.-D.; Pittman, B.; Nabulsi, N.; Lim, K.; Lin, S.-F.; Matuskey, D.; Lee, J.-Y.; O’Connor, K.C.; Huang, Y.; et al. Imaging Robust Microglial Activation after Lipopolysaccharide Administration in Humans with PET. Proc. Natl. Acad. Sci. USA 2015, 112, 12468–12473. [Google Scholar] [CrossRef]
- Rummel, N.G.; Butterfield, D.A. Altered Metabolism in Alzheimer Disease Brain: Role of Oxidative Stress. Antioxid. Redox Signal 2022, 36, 1289–1305. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.-C. The Gut-Brain Barrier in Major Depression: Intestinal Mucosal Dysfunction with an Increased Translocation of LPS from Gram Negative Enterobacteria (Leaky Gut) Plays a Role in the Inflammatory Pathophysiology of Depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Jin, R.; Xu, X.; Wei, J.; Huang, H.; Tang, Y.; Zou, C.; Chen, T. Engineered Probiotics Clostridium Butyricum-PMTL007-GLP-1 Improves Blood Pressure via Producing GLP-1 and Modulating Gut Microbiota in Spontaneous Hypertension Rat Models. Microb. Biotechnol. 2023, 16, 799–812. [Google Scholar] [CrossRef]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 433299. [Google Scholar] [CrossRef]
- Principi, N.; Cozzali, R.; Farinelli, E.; Brusaferro, A.; Esposito, S. Gut Dysbiosis and Irritable Bowel Syndrome: The Potential Role of Probiotics. J. Infect. 2018, 76, 111–120. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Sinagra, E.; Barchi, A.; Verga, M.C.; Brinch, D.; Raimondo, D.; Danese, S. Gastroparesis: The Complex Interplay with Microbiota and the Role of Exogenous Infections in the Pathogenesis of the Disease. Microorganisms 2023, 11, 1122. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Chen, F.; Xia, R.; Zhu, D.; Chen, B.; Lin, A.; Zheng, C.; Hou, D.; Li, X.; et al. Fecal Microbiota Transplantation Reverses Insulin Resistance in Type 2 Diabetes: A Randomized, Controlled, Prospective Study. Front. Cell Infect. Microbiol. 2023, 12, 1089991. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Wang, J.; Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut Metagenome in European Women with Normal, Impaired and Diabetic Glucose Control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H.; et al. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H. Bin Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal Relationships among the Gut Microbiome, Short-Chain Fatty Acids and Metabolic Diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, J.; Hao, W.; Zhang, J.; Li, H.; Yang, C.; Yang, J.; Chen, X.; Wang, H. Gut Microbiota and Type 2 Diabetes Mellitus: Association, Mechanism, and Translational Applications. Mediat. Inflamm. 2021, 2021, 5110276. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; Macdougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2018, 26, 43. [Google Scholar]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Qi, Q.; Hruby, A.; Manson, J.A.E.; Willett, W.C.; Wolpin, B.M.; Hu, F.B.; Qi, L. Cumulative Consumption of Branched-Chain Amino Acids and Incidence of Type 2 Diabetes. Int. J. Epidemiol. 2016, 45, 1482–1492. [Google Scholar] [CrossRef]
- Asghari, G.; Farhadnejad, H.; Teymoori, F.; Mirmiran, P.; Tohidi, M.; Azizi, F. High Dietary Intake of Branched-Chain Amino Acids Is Associated with an Increased Risk of Insulin Resistance in Adults. J. Diabetes 2018, 10, 357–364. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature That Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, F.; Sun, D.; Wang, X.; Zhang, X.; Zhang, J.; Yan, F.; Huang, C.; Xie, H.; Lin, C.; et al. Branched-Chain Amino Acids Exacerbate Obesity-Related Hepatic Glucose and Lipid Metabolic Disorders via Attenuating Akt2 Signaling. Diabetes 2020, 69, 1164–1177. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-Chain Amino Acids as Pharmacological Nutrients in Chronic Liver Disease. Hepatology 2011, 54, 1063–1070. [Google Scholar] [CrossRef]
- Yesair, D.W.; Himmelfarb, P. Hydrolysis of Conjugated Bile Acids by Cell-Free Extracts from Aerobic Bacteria. Appl. Microbiol. 1970, 19, 295–300. [Google Scholar] [CrossRef]
- Devlin, A.S.; Fischbach, M.A. A Biosynthetic Pathway for a Prominent Class of Microbiota-Derived Bile Acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef]
- Shaham, O.; Wei, R.; Wang, T.J.; Ricciardi, C.; Lewis, G.D.; Vasan, R.S.; Carr, S.A.; Thadhani, R.; Gerszten, R.E.; Mootha, V.K. Metabolic Profiling of the Human Response to a Glucose Challenge Reveals Distinct Axes of Insulin Sensitivity. Mol. Syst. Biol. 2008, 4, 214. [Google Scholar] [CrossRef]
- Patti, M.E.; Houten, S.M.; Bianco, A.C.; Bernier, R.; Larsen, P.R.; Holst, J.J.; Badman, M.K.; Maratos-Flier, E.; Mun, E.C.; Pihlajamaki, J.; et al. Serum Bile Acids Are Higher in Humans with Prior Gastric Bypass: Potential Contribution to Improved Glucose and Lipid Metabolism. Obesity 2009, 17, 1671–1677. [Google Scholar] [CrossRef]
- Katsuma, S.; Hirasawa, A.; Tsujimoto, G. Bile Acids Promote Glucagon-like Peptide-1 Secretion through TGR5 in a Murine Enteroendocrine Cell Line STC-1. Biochem. Biophys. Res. Commun. 2005, 329, 386–390. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Chaudhari, S.N.; Harris, D.A.; Aliakbarian, H.; Luo, J.N.; Henke, M.T.; Subramaniam, R.; Vernon, A.H.; Tavakkoli, A.; Sheu, E.G.; Devlin, A.S. Bariatric Surgery Reveals a Gut-Restricted TGR5 Agonist with Anti-Diabetic Effects. Nat. Chem. Biol. 2020, 17, 20–29. [Google Scholar] [CrossRef]
- Thomas, C.; Auwerx, J.; Schoonjans, K. Bile Acids and the Membrane Bile Acid Receptor TGR5—Connecting Nutrition and Metabolism. Thyroid 2008, 18, 167–174. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Ren, S.; Gil, G.; Dent, P. Bile Acids as Regulatory Molecules. J. Lipid Res. 2009, 50, 1509–1520. [Google Scholar] [CrossRef]
- Yamagata, K.; Daitoku, H.; Shimamoto, Y.; Matsuzaki, H.; Hirota, K.; Ishida, J.; Fukamizu, A. Bile Acids Regulate Gluconeogenic Gene Expression via Small Heterodimer Partner-Mediated Repression of Hepatocyte Nuclear Factor 4 and Foxo1. J. Biol. Chem. 2004, 279, 23158–23165. [Google Scholar] [CrossRef]
- Katafuchi, T.; Makishima, M. Molecular Basis of Bile Acid-FXR-FGF15/19 Signaling Axis. Int. J. Mol. Sci. 2022, 23, 6046. [Google Scholar] [CrossRef]
- Kyrou, I.; Weickert, M.O.; Gharanei, S.; Randeva, H.S.; Tan, B.K. Fibroblast Growth Factors: New Insights, New Targets in the Management of Diabetes. Minerva Endocrinol. 2017, 42, 248–270. [Google Scholar] [CrossRef]
- Ryan, K.K.; Tremaroli, V.; Clemmensen, C.; Kovatcheva-Datchary, P.; Myronovych, A.; Karns, R.; Wilson-Pérez, H.E.; Sandoval, D.A.; Kohli, R.; Bäckhed, F.; et al. FXR Is a Molecular Target for the Effects of Vertical Sleeve Gastrectomy. Nature 2014, 509, 183. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Allin, K.H.; Nielsen, T.; Pedersen, O. MECHANISMS IN ENDOCRINOLOGY: Gut Microbiota in Patients with Type 2 Diabetes Mellitus. Eur. J. Endocrinol. 2015, 172, R167–R177. [Google Scholar] [CrossRef]
- Harte, A.L.; Varma, M.C.; Tripathi, G.; McGee, K.C.; Al-Daghri, N.M.; Al-Attas, O.S.; Sabico, S.; O’Hare, J.P.; Ceriello, A.; Saravanan, P.; et al. High Fat Intake Leads to Acute Postprandial Exposure to Circulating Endotoxin in Type 2 Diabetic Subjects. Diabetes Care 2012, 35, 375–382. [Google Scholar] [CrossRef]
- Woting, A.; Blaut, M. The Intestinal Microbiota in Metabolic Disease. Nutrients 2016, 8, 202. [Google Scholar] [CrossRef]
- Snelson, M.; de Pasquale, C.; Ekinci, E.I.; Coughlan, M.T. Gut Microbiome, Prebiotics, Intestinal Permeability and Diabetes Complications. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101507. [Google Scholar] [CrossRef]
- Muzio, M.; Polentarutti, N.; Bosisio, D.; Kumar, P.P.M.; Mantovani, A. Toll-like Receptor Family and Signalling Pathway. Biochem. Soc. Trans. 2000, 28, 563–566. [Google Scholar] [CrossRef]
- Lin, K.-I.; Johnson, D.R.; Freund, G.G. LPS-Dependent Suppression of Social Exploration Is Augmented in Type 1 Diabetic Mice. Brain Behav. Immun. 2007, 21, 775–782. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-like Peptide-1 Secretion from L Cells and Alpha Cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Fogelstrand, L.; Hulthe, J.; Hulten, L.M.; Wiklund, O.; Fagerberg, B. Monocytic Expression of CD14 and CD18, Circulating Adhesion Molecules and Inflammatory Markers in Women with Diabetes Mellitus and Impaired Glucose Tolerance. Diabetologia 2004, 47, 1948–1952. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Xu, T.-C.; Liu, Y.; Yu, Z.; Xu, B. Gut-Targeted Therapies for Type 2 Diabetes Mellitus: A Review. World J. Clin. Cases 2024, 12, 1–8. [Google Scholar] [CrossRef]
- Ansari, F.; Neshat, M.; Pourjafar, H.; Jafari, S.M.; Samakkhah, S.A.; Mirzakhani, E. The Role of Probiotics and Prebiotics in Modulating of the Gut-Brain Axis. Front. Nutr. 2023, 10, 1173660. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Samuel, S.M.; Büsselberg, D. The Influence of Gut Microbial Species on Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 8118. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Bednarz, K.; Kowalczyk, K.; Cwynar, M.; Czapla, D.; Czarkowski, W.; Kmita, D.; Nowak, A.; Madej, P. The Role of Glp-1 Receptor Agonists in Insulin Resistance with Concomitant Obesity Treatment in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2022, 23, 4334. [Google Scholar] [CrossRef]
- Carlessi, R.; Chen, Y.; Rowlands, J.; Cruzat, V.F.; Keane, K.N.; Egan, L.; Mamotte, C.; Stokes, R.; Gunton, J.E.; De Bittencourt, P.I.H.; et al. GLP-1 Receptor Signalling Promotes β-Cell Glucose Metabolism via MTOR-Dependent HIF-1α Activation. Sci. Rep. 2017, 7, 2661. [Google Scholar] [CrossRef]
- Den Besten, G.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; Van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect against High-Fat Diet-Induced Obesity via a Pparg-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the Roles of PPARγ in Adipocytes via Dynamic Change of Transcription Complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, Z.; Duan, C.; Wang, C.; Zhao, Y.; Yang, G.; Gao, L.; Niu, C.; Xu, J.; Li, S. Lactobacillus Plantarum C88 Protects against Aflatoxin B1-Induced Liver Injury in Mice via Inhibition of NF-ΚB-Mediated Inflammatory Responses and Excessive Apoptosis. BMC Microbiol. 2019, 19, 170. [Google Scholar] [CrossRef]
- Khalili, L.; Alipour, B.; Jafar-Abadi, M.A.; Faraji, I.; Hassanalilou, T.; Abbasi, M.M.; Vaghef-Mehrabany, E.; Sani, M.A. The Effects of Lactobacillus Casei on Glycemic Response, Serum Sirtuin1 and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Iran. Biomed. J. 2019, 23, 68. [Google Scholar] [CrossRef]
- Stelmaszyk, A.; Mikołajczak, P.; Dworacka, M. Sirtuin 1 as the Mechanism of Action of Agents Used in the Diabetes Mellitus Pharmacotherapy. Eur. J. Pharmacol. 2021, 907, 174289. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function. Front. Endocrinol. 2019, 10, 415266. [Google Scholar] [CrossRef]
- Mihanfar, A.; Akbarzadeh, M.; Ghazizadeh Darband, S.; Sadighparvar, S.; Majidinia, M. SIRT1: A Promising Therapeutic Target in Type 2 Diabetes Mellitus. Arch. Physiol. Biochem. 2024, 130, 13–28. [Google Scholar] [CrossRef]
- Bourebaba, L.; Marycz, K. Pathophysiological Implication of Fetuin-a Glycoprotein in the Development of Metabolic Disorders: A Concise Review. J. Clin. Med. 2019, 8, 2033. [Google Scholar] [CrossRef]
- Zarfeshani, A.; Khaza’ai, H.; Mohd Ali, R.; Hambali, Z.; Wahle, K.W.J.; Mutalib, M.S.A. Effect of Lactobacillus Casei on the Production of Pro-Inflammatory Markers in Streptozotocin-Induced Diabetic Rats. Probiotics Antimicrob. Proteins 2011, 3, 168–174. [Google Scholar] [CrossRef]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; Vaghef-Mehrabany, L.; Asghari-Jafarabadi, M.; Nakhjavani, M.R.; Mohtadi-Nia, J. Effects of Lactobacillus Casei Supplementation on Disease Activity and Inflammatory Cytokines in Rheumatoid Arthritis Patients: A Randomized Double-Blind Clinical Trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar] [CrossRef]
- Vitetta, L.; Gorgani, N.N.; Vitetta, G.; Henson, J.D. Prebiotics Progress Shifts in the Intestinal Microbiome That Benefits Patients with Type 2 Diabetes Mellitus. Biomolecules 2023, 13, 1307. [Google Scholar] [CrossRef]
- Ojo, O.; Wang, X.; Ojo, O.O.; Brooke, J.; Jiang, Y.; Dong, Q.; Thompson, T. The Effect of Prebiotics and Oral Anti-Diabetic Agents on Gut Microbiome in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Nutrients 2022, 14, 5139. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.; Huang, J.; Yang, Y.; Yang, Q.; Hu, H. Efficacy of Inulin Supplementation in Improving Insulin Control, HbA1c and HOMA-IR in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Biochem. Nutr. 2020, 66, 176–183. [Google Scholar] [CrossRef]
- Soleimani, A.; Motamedzadeh, A.; Zarrati Mojarrad, M.; Bahmani, F.; Amirani, E.; Ostadmohammadi, V.; Tajabadi-Ebrahimi, M.; Asemi, Z. The Effects of Synbiotic Supplementation on Metabolic Status in Diabetic Patients Undergoing Hemodialysis: A Randomized, Double-Blinded, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1248–1256. [Google Scholar] [CrossRef]
- Mazhar, M.; Zhu, Y.; Qin, L. The Interplay of Dietary Fibers and Intestinal Microbiota Affects Type 2 Diabetes by Generating Short-Chain Fatty Acids. Foods 2023, 12, 1023. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, X.; Zhang, X. Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat Metabolic Disorders to Maintain Health. Nutrients 2023, 15, 1539. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Xu, D.; Wang, Q. Probiotics Ameliorates Glycemic Control of Patients with Diabetic Nephropathy: A Randomized Clinical Study. J. Clin. Lab. Anal. 2021, 35, e23650. [Google Scholar] [CrossRef]
- Ding, L.N.; Ding, W.Y.; Ning, J.; Wang, Y.; Yan, Y.; Wang, Z. Bin Effects of Probiotic Supplementation on Inflammatory Markers and Glucose Homeostasis in Adults With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 770861. [Google Scholar] [CrossRef]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-abadi, M. Oligofructose-Enriched Inulin Improves Some Inflammatory Markers and Metabolic Endotoxemia in Women with Type 2 Diabetes Mellitus: A Randomized Controlled Clinical Trial. Nutrition 2014, 30, 418–423. [Google Scholar] [CrossRef]
- Javid, A.Z.; Aminzadeh, M.; Haghighi-Zadeh, M.H.; Jamalvandi, M. The Effects of Synbiotic Supplementation on Glycemic Status, Lipid Profile, and Biomarkers of Oxidative Stress in Type 1 Diabetic Patients. A Placebo-Controlled, Double-Blind, Randomized Clinical Trial. Diabetes Metab. Syndr. Obes. 2020, 13, 607–617. [Google Scholar] [CrossRef]
- Jovanovski, E.; Khayyat, R.; Zurbau, A.; Komishon, A.; Mazhar, N.; Sievenpiper, J.L.; Mejia, S.B.; Ho, H.V.T.; Li, D.; Jenkins, A.L.; et al. Erratum: Should Viscous Fiber Supplements Be Considered in Diabetes Control? Results from a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Care 2019, 42, 755–766. [Google Scholar] [CrossRef]
- Pant, A.; Das, B. Microbiome-Based Therapeutics: Opportunity and Challenges. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2022; Volume 191. [Google Scholar]
- Barone, M.; Rampelli, S.; Biagi, E.; Bertozzi, S.M.; Falchi, F.; Cavalli, A.; Armirotti, A.; Brigidi, P.; Turroni, S.; Candela, M. Searching for New Microbiome-Targeted Therapeutics through a Drug Repurposing Approach. J. Med. Chem. 2021, 64, 17277–17286. [Google Scholar] [CrossRef]
- Lemon, K.P.; Armitage, G.C.; Relman, D.A.; Fischbach, M.A. Microbiota-Targeted Therapies: An Ecological Perspective. Sci. Transl. Med. 2012, 4, rv5–rv137. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y. CRISPR-Based Gene Editing Technology and Its Application in Microbial Engineering. Eng. Microbiol. 2023, 3, 100101. [Google Scholar] [CrossRef]
- Larroya, A.; Pantoja, J.; Codoñer-Franch, P.; Cenit, M.C. Towards Tailored Gut Microbiome-Based and Dietary Interventions for Promoting the Development and Maintenance of a Healthy Brain. Front. Pediatr. 2021, 9, 705859. [Google Scholar] [CrossRef]
- Snydman, D.R. The Safety of Probiotics. Clin. Infect. Dis. 2008, 46, S104–S111. [Google Scholar] [CrossRef]
- Doron, S.I.; Hibberd, P.L.; Gorbach, S.L. Probiotics for Prevention of Antibiotic-Associated Diarrhea. J. Clin. Gastroenterol. 2008, 42, S58–S63. [Google Scholar] [CrossRef]
- Lherm, T.; Monet, C.; Nougière, B.; Soulier, M.; Larbi, D.; Le Gall, C.; Caen, D.; Malbrunot, C. Seven Cases of Fungemia with Saccharomyces Boulardii in Critically Ill Patients. Intensive Care Med. 2002, 28, 797–801. [Google Scholar] [CrossRef]
- Zavišić, G.; Popović, M.; Stojkov, S.; Medić, D.; Gusman, V.; Jovanović Lješković, N.; Jovanović Galović, A. Antibiotic Resistance and Probiotics: Knowledge Gaps, Market Overview and Preliminary Screening. Antibiotics 2023, 12, 1281. [Google Scholar] [CrossRef]
- Hradicka, P.; Adamkova, P.; Lenhardt, L.; Gancarcikova, S.; Iannaccone, S.F.; Demeckova, V. Addressing Safety Concerns of Long-Term Probiotic Use: In Vivo Evidence from a Rat Model. J. Funct. Foods 2023, 104, 105521. [Google Scholar] [CrossRef]
- Ritchie, M.L.; Romanuk, T.N. A Meta-Analysis of Probiotic Efficacy for Gastrointestinal Diseases. PLoS ONE 2012, 7, e34938. [Google Scholar] [CrossRef]

| Therapeutic Intervention | Mechanism of Action | Diabetic Patient Outcomes | References |
|---|---|---|---|
| Probiotics | Introduction of beneficial bacteria to the gut. | Improvement in glycemic control; decrease in fasting blood glucose, HbA1c, and HOMA-IR. | [124] |
| Improvement in glycemic control; decrease in fasting blood glucose, HbA1c, HOMA-IR, TNF-alfa, and CRP. | [125] | ||
| Prebiotics | Non-digestible food ingredients that stimulate the growth of beneficial bacteria. | Decrease in the levels of fasting plasma glucose, HbA1c, Il-6, CRP, and TNF-alfa. | [126] |
| Synbiotics | Combination of probiotics and prebiotics. | Decrease in the levels of fasting plasma glucose, HbA1c, and CRP. | [127] |
| Dietary modifications (e.g., high-fiber diets) | Modifications in dietary habits; consuming beneficial food. | Decrease in the levels of fasting plasma glucose, HbA1c, and HOMA-IR. | [128] |
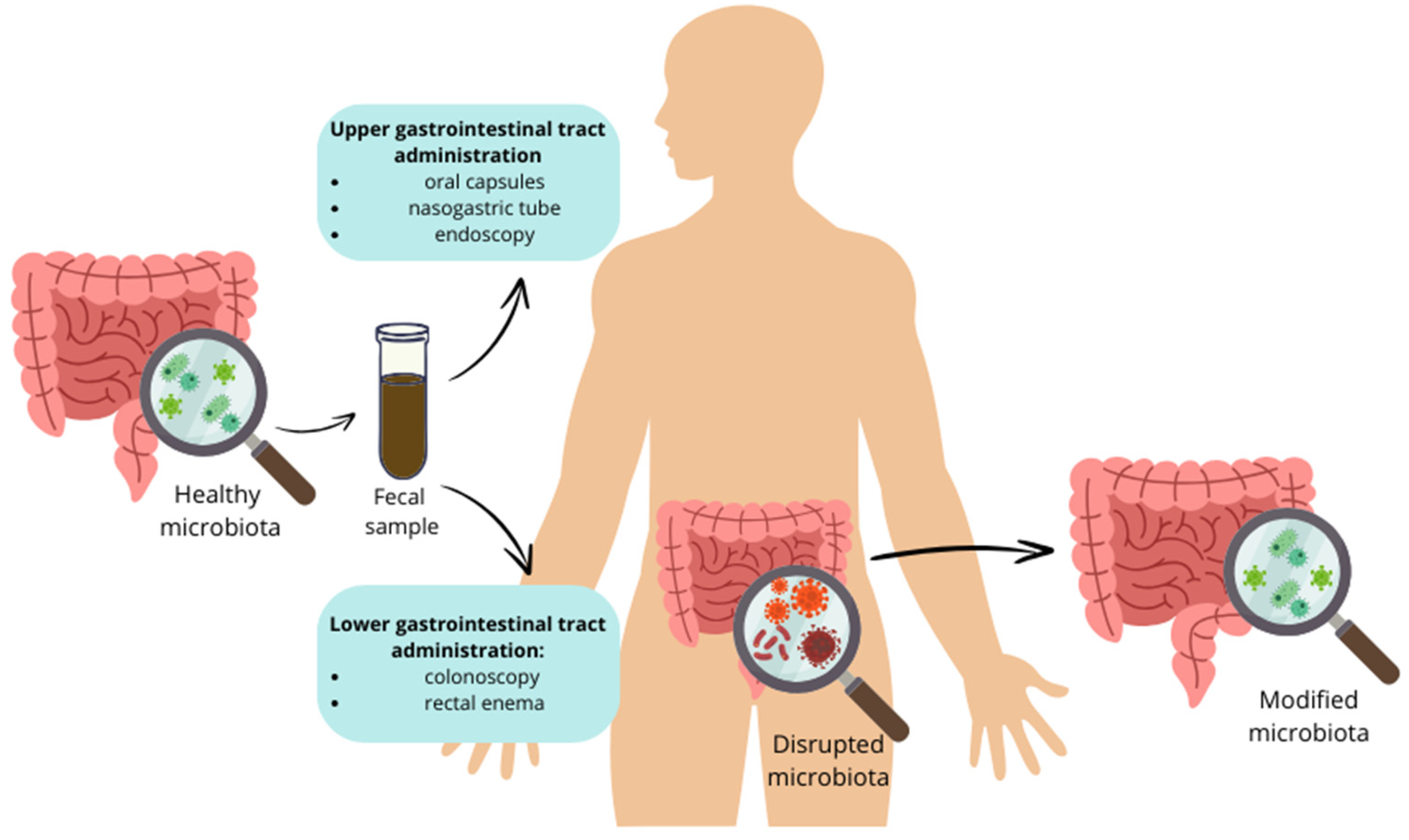
| Fecal microbiota transplantation (FMT) | Transplantation of stool from a healthy donor to the patient’s gut. | Decrease in the levels of fasting and postprandial plasma glucose, HbA1c, HOMA-IR, and BMI. | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarska, E.; Wasiak, J.; Gajewska, A.; Steć, G.; Jasińska, J.; Rysz, J.; Franczyk, B. Exploring the Significance of Gut Microbiota in Diabetes Pathogenesis and Management—A Narrative Review. Nutrients 2024, 16, 1938. https://doi.org/10.3390/nu16121938
Młynarska E, Wasiak J, Gajewska A, Steć G, Jasińska J, Rysz J, Franczyk B. Exploring the Significance of Gut Microbiota in Diabetes Pathogenesis and Management—A Narrative Review. Nutrients. 2024; 16(12):1938. https://doi.org/10.3390/nu16121938
Chicago/Turabian StyleMłynarska, Ewelina, Jakub Wasiak, Agata Gajewska, Greta Steć, Joanna Jasińska, Jacek Rysz, and Beata Franczyk. 2024. "Exploring the Significance of Gut Microbiota in Diabetes Pathogenesis and Management—A Narrative Review" Nutrients 16, no. 12: 1938. https://doi.org/10.3390/nu16121938
APA StyleMłynarska, E., Wasiak, J., Gajewska, A., Steć, G., Jasińska, J., Rysz, J., & Franczyk, B. (2024). Exploring the Significance of Gut Microbiota in Diabetes Pathogenesis and Management—A Narrative Review. Nutrients, 16(12), 1938. https://doi.org/10.3390/nu16121938









