L-Citrulline Supplementation Improves Arterial Blood Flow and Muscle Oxygenation during Handgrip Exercise in Hypertensive Postmenopausal Women
Abstract
1. Introduction
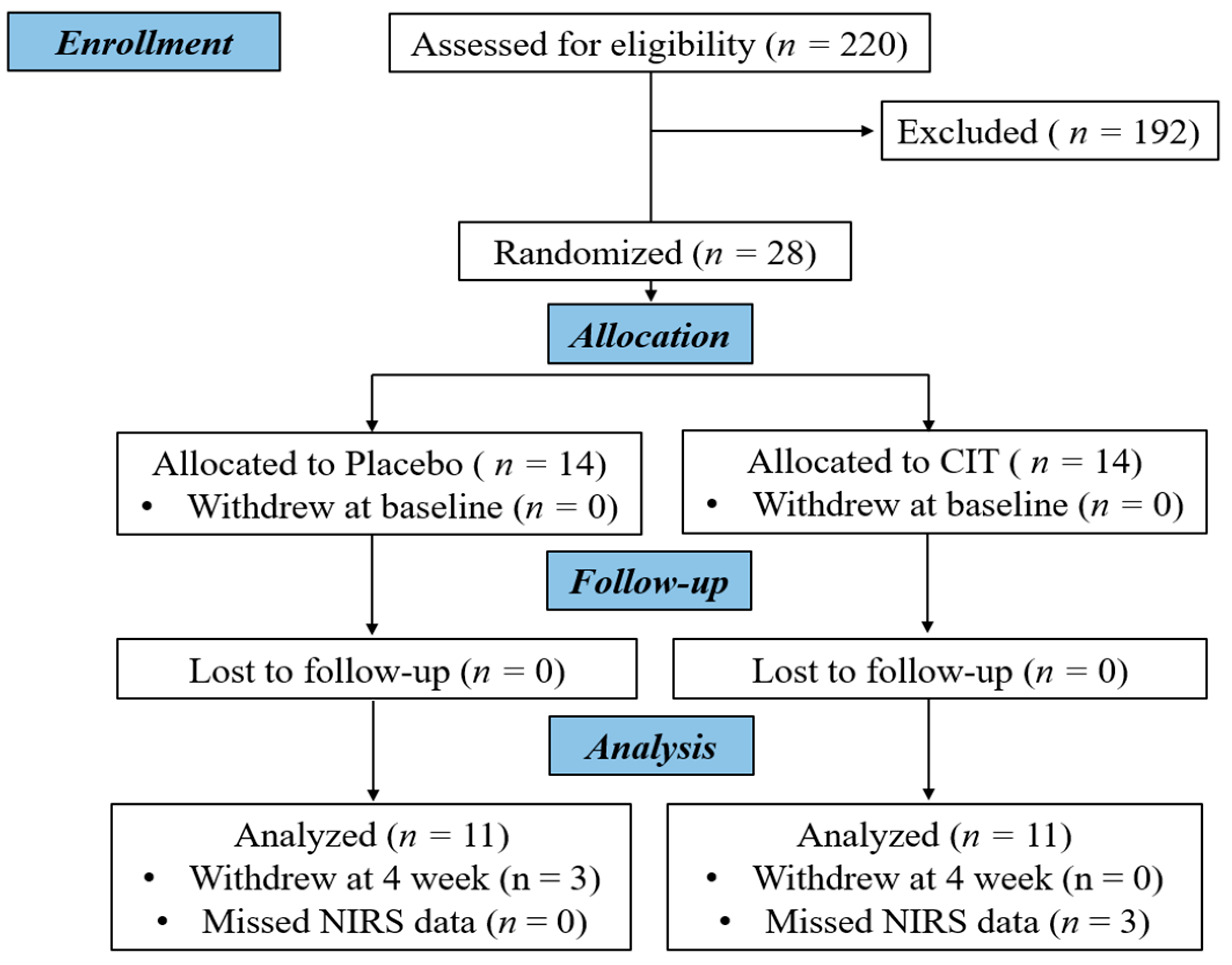
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Measurements
2.3.1. Anthropometrics
2.3.2. Forearm Muscle Strength and Dynamic Handgrip Exercise
2.3.3. Brachial and Aortic Blood Pressure and Arterial Stiffness
2.3.4. Brachial Artery Flow-Mediated Dilation (FMD) and Hemodynamics
2.3.5. Muscle Oxygenation
2.4. Statistical Analysis
3. Results
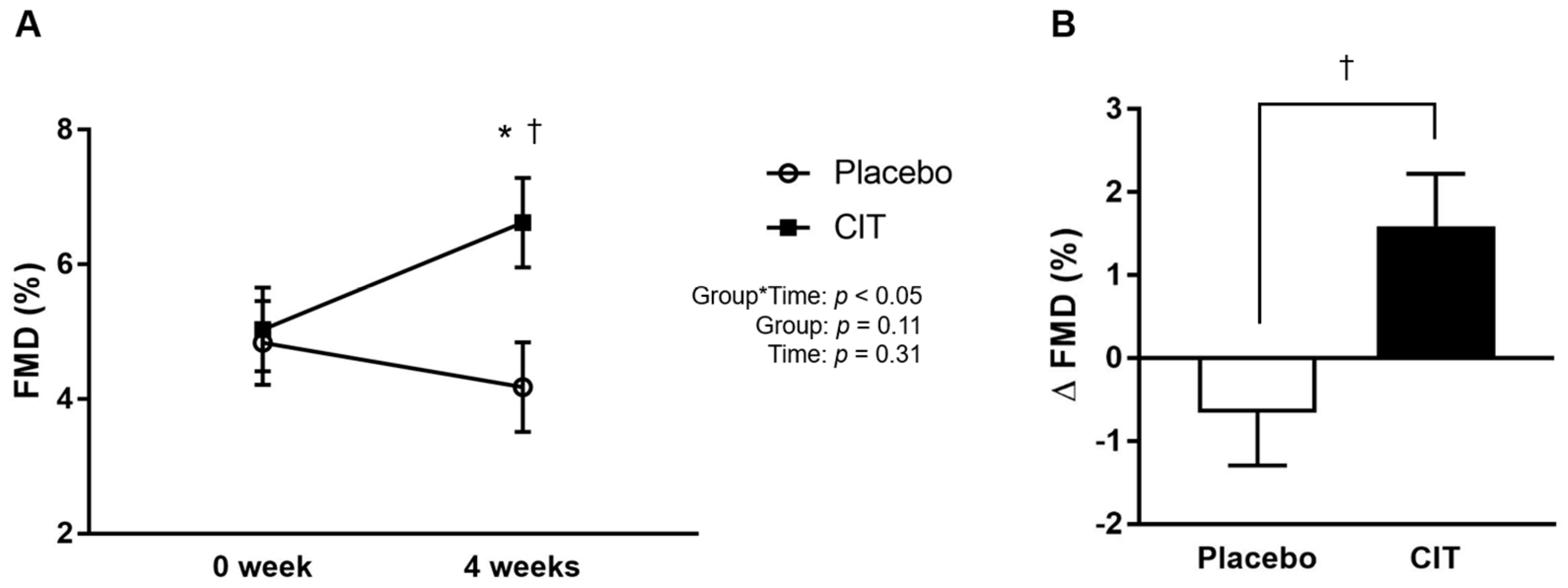
3.1. Effects of Supplementations on Blood Pressure, Endothelial Function, and Arterial Stiffness
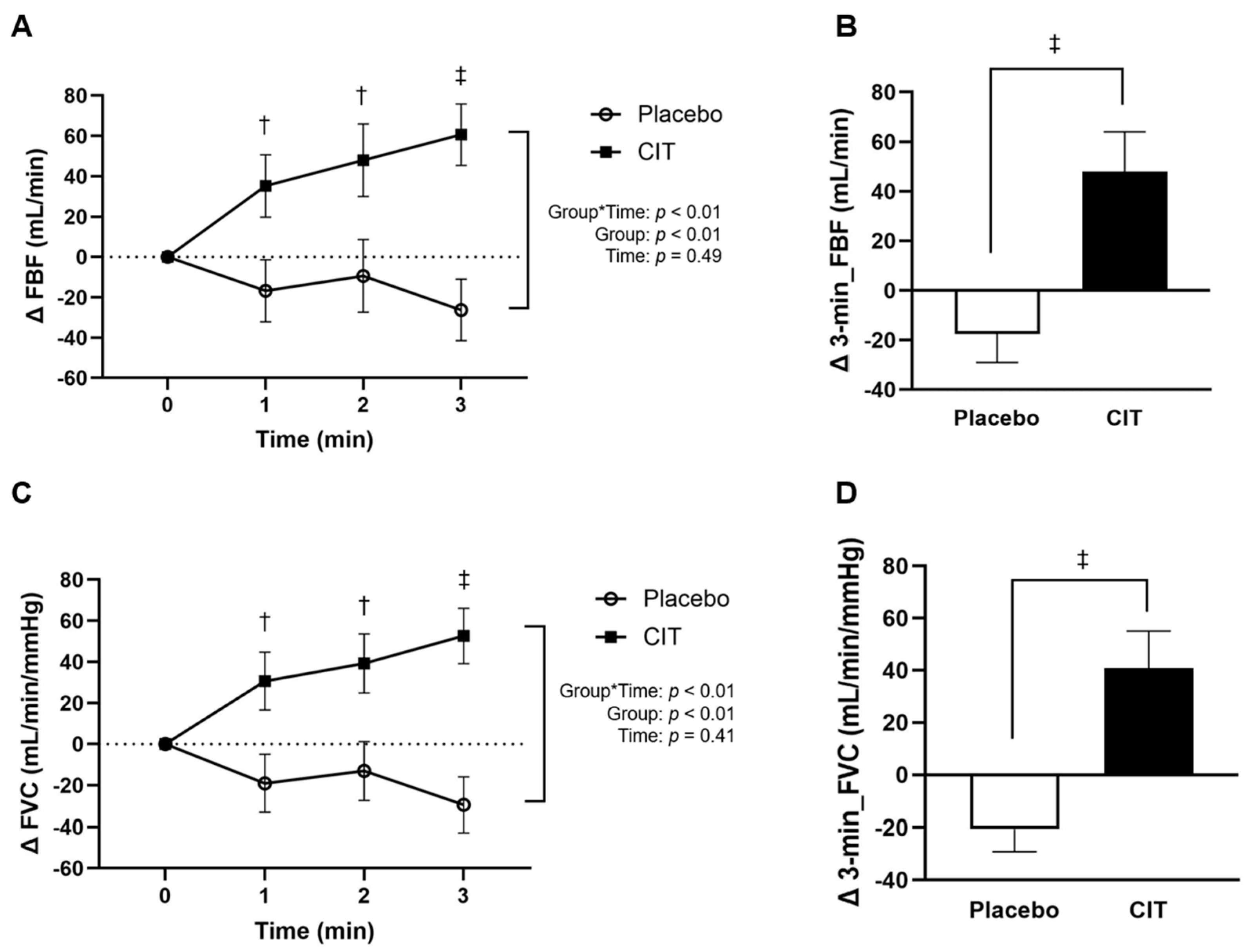
3.2. Effects of Supplementations on Arterial Vasodilation during Exercise
3.3. Effects of Supplementations on Muscle Oxygenation Responses to Exercise
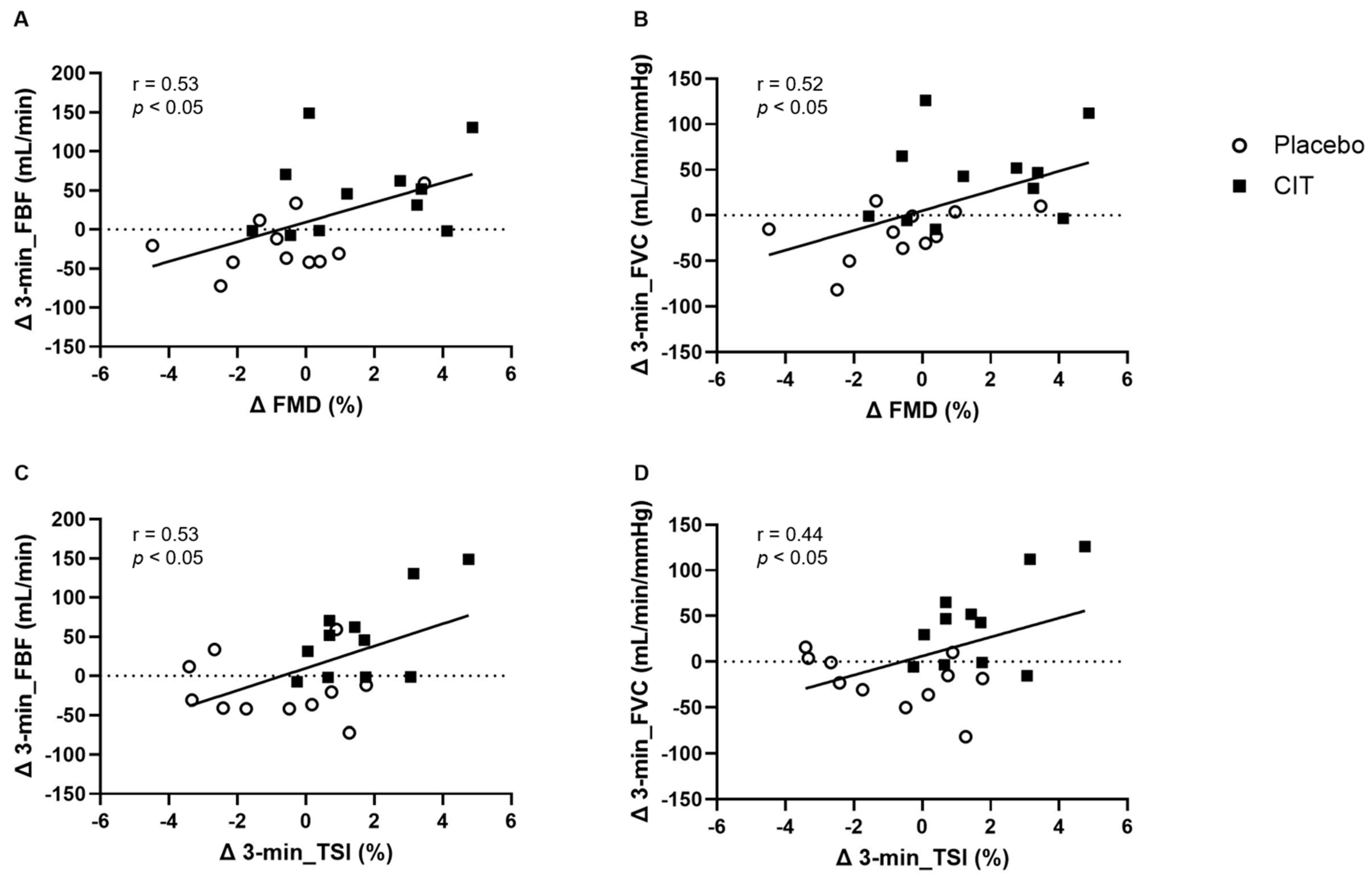
3.4. Correlations between FMD with Arterial Vasodilation and Muscle Oxygenation after CIT Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057. [Google Scholar] [CrossRef]
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric oxide in the vasculature: Where does it come from and where does it go? A quantitative perspective. Antioxid. Redox Signal. 2008, 10, 1185–1198. [Google Scholar] [CrossRef]
- Roux, E.; Bougaran, P.; Dufourcq, P.; Couffinhal, T. Fluid shear stress sensing by the endothelial layer. Front. Physiol. 2020, 11, 861. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Padilla, J.; Simmons, G.H.; Fadel, P.J.; Laughlin, M.H.; Joyner, M.J.; Casey, D.P. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: Role of nitric oxide. Hypertension 2011, 57, 484–489. [Google Scholar] [CrossRef]
- Carter, K.J.; Ward, A.T.; Kellawan, J.M.; Eldridge, M.W.; Al-Subu, A.; Walker, B.J.; Lee, J.W.; Wieben, O.; Schrage, W.G. Nitric oxide synthase inhibition in healthy adults reduces regional and total cerebral macrovascular blood flow and microvascular perfusion. J. Physiol. 2021, 599, 4973–4989. [Google Scholar] [CrossRef]
- Rizzoni, D.; De Ciuceis, C.; Salvetti, M.; Paini, A.; Rossini, C.; Agabiti-Rosei, C.; Muiesan, M.L. Interactions between macro-and micro-circulation: Are they relevant? High Blood Press. Cardiovasc. Prev. 2015, 22, 119–128. [Google Scholar] [CrossRef]
- Klawitter, J.; Hildreth, K.L.; Christians, U.; Kohrt, W.M.; Moreau, K.L. A relative L-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol. Rep. 2017, 5, e13409. [Google Scholar] [CrossRef]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef]
- James, M.A.; Tullett, J.; Hemsley, A.G.; Shore, A.C. Effects of aging and hypertension on the microcirculation. Hypertension 2006, 47, 968–974. [Google Scholar] [CrossRef]
- De Boer, M.P.; Meijer, R.I.; Wijnstok, N.J.; Jonk, A.M.; Houben, A.J.; Stehouwer, C.D.; Smulders, Y.M.; Eringa, E.C.; Serne, E.H. Microvascular dysfunction: A potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Microcirculation 2012, 19, 5–18. [Google Scholar] [CrossRef]
- Hellsten, Y.; Nyberg, M.; Jensen, L.; Mortensen, S. Vasodilator interactions in skeletal muscle blood flow regulation. J. Physiol. 2012, 590, 6297–6305. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef]
- Saltin, B.; Mortensen, S.P. Inefficient functional sympatholysis is an overlooked cause of malperfusion in contracting skeletal muscle. J. Physiol. 2012, 590, 6269–6275. [Google Scholar] [CrossRef]
- Hearon, C.M., Jr.; Dinenno, F.A. Regulation of skeletal muscle blood flow during exercise in ageing humans. J. Physiol. 2016, 594, 2261–2273. [Google Scholar] [CrossRef]
- Jones, S.; Chiesa, S.T.; Chaturvedi, N.; Hughes, A.D. Recent developments in near-infrared spectroscopy (NIRS) for the assessment of local skeletal muscle microvascular function and capacity to utilise oxygen. Artery Res. 2016, 16, 25–33. [Google Scholar] [CrossRef]
- Jones, S.; Tillin, T.; Williams, S.; Rapala, A.; Chaturvedi, N.; Hughes, A.D. Skeletal Muscle Tissue Saturation Changes Measured Using Near Infrared Spectroscopy During Exercise Are Associated with Post-Occlusive Reactive Hyperaemia. Front. Physiol. 2022, 13, 1379. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef]
- Gravelle, B.M.; Murias, J.M.; Spencer, M.D.; Paterson, D.H.; Kowalchuk, J.M. Adjustments of pulmonary O2 uptake and muscle deoxygenation during ramp incremental exercise and constant-load moderate-intensity exercise in young and older adults. J. Appl. Physiol. 2012, 113, 1466–1475. [Google Scholar] [CrossRef]
- Ilkka, H.; Bengt, S.; Jukka, K.; Sipilä, H.T.; Vesa, O.; Pirjo, N.; Juhani, K.; Kari, K.; Ylva, H. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: A pet study with nitric oxide and cyclooxygenase inhibition. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H1510–H1517. [Google Scholar] [CrossRef]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef]
- Buerk, D.G. Nitric oxide regulation of microvascular oxygen. Antioxid. Redox Signal. 2007, 9, 829–843. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Böger, R.H.; Frölich, J.C. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef]
- Huang, J.; Ladeiras, D.; Yu, Y.; Ming, X.-F.; Yang, Z. Detrimental effects of chronic L-arginine rich food on aging kidney. Front. Pharmacol. 2021, 11, 582155. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. L-citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Moinard, C.; Maccario, J.; Walrand, S.; Lasserre, V.; Marc, J.; Boirie, Y.; Cynober, L. Arginine behaviour after arginine or citrulline administration in older subjects. Br. J. Nutr. 2016, 115, 399–404. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef]
- Maharaj, A.; Fischer, S.M.; Dillon, K.N.; Kang, Y.; Martinez, M.A.; Figueroa, A. Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women. Nutrients 2022, 14, 4396. [Google Scholar] [CrossRef]
- Ratchford, S.M.; Bunsawat, K.; Alpenglow, J.K.; Zhao, J.; Wright, J.B.; Ryan, J.J.; Wray, D.W. Improved Vascular Function and Functional Capacity Following L-Citrulline Administration in Patients with HFpEF: A Single-arm, Open-label Pilot Study. J. Appl. Physiol. 2022, 134, 328–338. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar]
- Kang, Y.; Maharaj, A.; Dillon, K.N.; Fischer, S.M.; Figueroa, A. Menopause influences aortic pulse pressure and pressure wave responses to metaboreflex activation in women. Menopause 2022, 29, 1423–1429. [Google Scholar] [CrossRef]
- Fischer, S.M.; Maharaj, A.; Kang, Y.; Dillon, K.N.; Martinez, M.A.; Figueroa, A. Endothelial and exercise vasodilation are reduced in postmenopausal females with obesity versus lean and overweight. Int. J. Obes. 2024, 1–8. [Google Scholar] [CrossRef]
- Dipla, K.; Triantafyllou, A.; Koletsos, N.; Papadopoulos, S.; Sachpekidis, V.; Vrabas, I.S.; Gkaliagkousi, E.; Zafeiridis, A.; Douma, S. Impaired muscle oxygenation and elevated exercise blood pressure in hypertensive patients: Links with vascular stiffness. Hypertension 2017, 70, 444–451. [Google Scholar] [CrossRef]
- Richards, J.C.; Racine, M.L.; Hearon, C.M.; Kunkel, M.; Luckasen, G.J.; Larson, D.G.; Allen, J.D.; Dinenno, F.A. Acute ingestion of dietary nitrate increases muscle blood flow via local vasodilation during handgrip exercise in young adults. Physiol. Rep. 2018, 6, e13572. [Google Scholar] [CrossRef]
- Papadopoulos, S.; Dipla, K.; Triantafyllou, A.; Nikolaidis, M.G.; Kyparos, A.; Touplikioti, P.; Vrabas, I.S.; Zafeiridis, A. Beetroot increases muscle performance and oxygenation during sustained isometric exercise, but does not alter muscle oxidative efficiency and microvascular reactivity at rest. J. Am. Coll. Nutr. 2018, 37, 361–372. [Google Scholar] [CrossRef]
- Yanes, L.L.; Reckelhoff, J.F. Postmenopausal Hypertension. Am. J. Hypertens. 2011, 24, 740–749. [Google Scholar] [CrossRef]
- Lima, R.; Wofford, M.; Reckelhoff, J.F. Hypertension in postmenopausal women. Curr. Hypertens. Rep. 2012, 14, 254–260. [Google Scholar] [CrossRef]
- Coutinho, T.; Borlaug, B.A.; Pellikka, P.A.; Turner, S.T.; Kullo, I.J. Sex differences in arterial stiffness and ventricular-arterial interactions. J. Am. Coll. Cardiol. 2013, 61, 96–103. [Google Scholar] [CrossRef]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef]
- Kang, Y.; Dillon, K.N.; Martinez, M.A.; Maharaj, A.; Fischer, S.M.; Figueroa, A. Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women. Nutrients 2023, 15, 74. [Google Scholar] [CrossRef]
- Figueroa, A.; Jaime, S.J.; Johnson, S.A.; Alvarez-Alvarado, S.; Campbell, J.C.; Feresin, R.G.; Elam, M.L.; Arjmandi, B.H. Impact of age on aortic wave reflection responses to metaboreflex activation and its relationship with leg lean mass in post-menopausal women. Exp. Gerontol. 2015, 70, 119–124. [Google Scholar] [CrossRef]
- Jaime, S.J.; Nagel, J.; Maharaj, A.; Fischer, S.M.; Schwab, E.; Martinson, C.; Radtke, K.; Mikat, R.P.; Figueroa, A. L-Citrulline supplementation attenuates aortic pulse pressure and wave reflection responses to cold stress in older adults. Exp. Gerontol. 2022, 159, 111685. [Google Scholar] [CrossRef]
- Rowell, L.B. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): Cycles of revision and new vision. J. Appl. Physiol. 2004, 97, 384–392. [Google Scholar] [CrossRef]
- Bockman, E.L. Blood flow and oxygen consumption in active soleus and gracilis muscles in cats. Am. J. Physiol.-Heart Circ. Physiol. 1983, 244, H546–H551. [Google Scholar] [CrossRef]
- Clifford, P.S.; Hellsten, Y. Vasodilatory mechanisms in contracting skeletal muscle. J. Appl. Physiol. 2004, 97, 393–403. [Google Scholar] [CrossRef]
- Dinenno, F.A.; Jones, P.P.; Seals, D.R.; Tanaka, H. Limb blood flow and vascular conductance are reduced with age in healthy humans: Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 1999, 100, 164–170. [Google Scholar] [CrossRef]
- Schrage, W.G.; Eisenach, J.H.; Joyner, M.J. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J. Physiol. 2007, 579, 227–236. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Bernini, G.; Magagna, A.; Salvetti, A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001, 38, 274–279. [Google Scholar] [CrossRef]
- Gonzales, J.U.; Fischer, S.M.; Maharaj, A.; Vellers, H.; Anderson, T.; Karnjanapiboonwong, A.; Subbiah, S.; Kellawan, J.M.; Figueroa, A. Response of exercise-onset vasodilator kinetics to L-citrulline supplementation during different phases of the menstrual cycle. Physiol. Rep. 2020, 8, e14536. [Google Scholar] [CrossRef]
- Gonzales, J.U.; Raymond, A.; Ashley, J.; Kim, Y. Does l-citrulline supplementation improve exercise blood flow in older adults? Exp. Physiol. 2017, 102, 1661–1671. [Google Scholar] [CrossRef]
- Newcomer, S.C.; Leuenberger, U.A.; Hogeman, C.S.; Handly, B.D.; Proctor, D.N. Different vasodilator responses of human arms and legs. J. Physiol. 2004, 556, 1001–1011. [Google Scholar] [CrossRef]
- Choi, H.-M.; Stebbins, C.L.; Nho, H.; Kim, K.-A.; Kim, C.; Kim, J.-K. Skeletal muscle metaboreflex is enhanced in postmenopausal women. Eur. J. Appl. Physiol. 2012, 112, 2671–2678. [Google Scholar] [CrossRef]
- Trinity, J.D.; Layec, G.; Hart, C.R.; Richardson, R.S. Sex-specific impact of aging on the blood pressure response to exercise. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H95–H104. [Google Scholar] [CrossRef]
- Wenner, M.M.; Greaney, J.L.; Matthews, E.L.; McGinty, S.; Kaur, J.; Vongpatanasin, W.; Fadel, P.J. Influence of Age and Estradiol on Sympathetic Nerve Activity Responses to Exercise in Women. Med. Sci. Sports Exerc. 2022, 54, 408–416. [Google Scholar] [CrossRef]
- Martinez, M.A.; Dillon, K.N.; Kang, Y.; Maharaj, A.; Fischer, S.M.; Figueroa, A. Endothelial dysfunction influences augmented aortic hemodynamic responses to metaboreflex activation in postmenopausal women. Eur. J. Appl. Physiol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Schrage, W.G.; Joyner, M.J.; Dinenno, F.A. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J. Physiol. 2004, 557, 599–611. [Google Scholar] [CrossRef]
- Mortensen, S.P.; González-Alonso, J.; Damsgaard, R.; Saltin, B.; Hellsten, Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J. Physiol. 2007, 581, 853–861. [Google Scholar] [CrossRef]
- Gates, P.E.; Strain, W.D.; Shore, A.C. Human endothelial function and microvascular ageing. Exp. Physiol. 2009, 94, 311–316. [Google Scholar] [CrossRef]
- Jin, K. A Microcirculatory Theory of Aging. Aging Dis. 2019, 10, 676–683. [Google Scholar] [CrossRef]
- Nyberg, M.; Jensen, L.G.; Thaning, P.; Hellsten, Y.; Mortensen, S.P. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: Effect of high-intensity aerobic training. J. Physiol. 2012, 590, 1481–1494. [Google Scholar] [CrossRef]
- Goodman, J.; McLaughlin, P.; Plyley, M.; Holloway, R.; Fell, D.; Logan, A.; Liu, P. Impaired cardiopulmonary response to exercise in moderate hypertension. Can. J. Cardiol. 1992, 8, 363–371. [Google Scholar]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide Biol. Chem. 2016, 59, 10–20. [Google Scholar] [CrossRef]
- Horiuchi, M.; Endo, J.; Dobashi, S.; Handa, Y.; Kiuchi, M.; Koyama, K. Muscle oxygenation profiles between active and inactive muscles with nitrate supplementation under hypoxic exercise. Physiol. Rep. 2017, 5, e13475. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.G.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Jones, A.M. Acute L-arginine supplementation reduces the O2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J. Appl. Physiol. 2010, 109, 1394–1403. [Google Scholar] [CrossRef]
- Masschelein, E.; Van Thienen, R.; Wang, X.; Van Schepdael, A.; Thomis, M.; Hespel, P. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J. Appl. Physiol. 2012, 113, 736–745. [Google Scholar] [CrossRef]
- Moreau, K.L.; Hildreth, K.L.; Klawitter, J.; Blatchford, P.; Kohrt, W.M. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience 2020, 42, 1699–1714. [Google Scholar] [CrossRef]
- Higashi, Y.; Kihara, Y.; Noma, K. Endothelial dysfunction and hypertension in aging. Hypertens. Res. 2012, 35, 1039–1047. [Google Scholar] [CrossRef]
- Boushel, R.; Langberg, H.; Gemmer, C.; Olesen, J.; Crameri, R.; Scheede, C.; Sander, M.; Kjær, M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J. Physiol. 2002, 543, 691–698. [Google Scholar] [CrossRef]
- Van Beekvelt, M.; Borghuis, M.; Van Engelen, B.; Wevers, R.; Colier, W. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin. Sci. 2001, 101, 21–28. [Google Scholar] [CrossRef]
- Craig, J.C.; Broxterman, R.M.; Wilcox, S.L.; Chen, C.; Barstow, T.J. Effect of adipose tissue thickness, muscle site, and sex on near-infrared spectroscopy derived total-[hemoglobin+ myoglobin]. J. Appl. Physiol. 2017, 123, 1571–1578. [Google Scholar] [CrossRef]
- Soares, R.N.; Reimer, R.A.; Doyle-Baker, P.K.; Murias, J.M. Mild obesity does not affect the forearm muscle microvascular responses to hyperglycemia. Microcirculation 2021, 28, e12669. [Google Scholar] [CrossRef]

| Characteristics | Placebo (n = 11) | CIT (n = 11) | p |
|---|---|---|---|
| Age (years) | 64 ± 5 | 61 ± 7 | 0.35 |
| Height (m) | 1.58 ± 0.09 | 1.59 ± 0.05 | 0.45 |
| Weight (kg) | 74.4 ± 15.7 | 75.0 ± 10.8 | 0.92 |
| Body mass index (kg/m2) | 30.2 ± 5.9 | 29.8 ± 4.0 | 0.85 |
| Waist circumference (cm) | 97.5 ± 18.3 | 90.8 ± 10.4 | 0.30 |
| MVC (kg) | 30 ± 7 | 30 ± 6 | 0.92 |
| Medication (n) | |||
| ARB | 1 | 2 | |
| ACE inhibitors | 0 | 1 | |
| Diuretics | 1 | 1 | |
| Calcium channel blockers | 1 | 0 | |
| Statin | 1 | 1 |
| Variables | Placebo (n = 11) | CIT (n = 11) | p | ||||
|---|---|---|---|---|---|---|---|
| 0 Week | 4 Weeks | Δ0 to 4 Weeks | 0 Week | 4 Weeks | Δ0 to 4 Weeks | ||
| Rest | |||||||
| cfPWV (m/s) | 9.2 ± 1.3 | 8.6 ± 1.6 | Δ−0.6 ± 0.8 | 9.4 ± 2.0 | 8.5 ± 1.1 * | Δ−0.9 ± 1.3 | 0.46 |
| Baseline Diameter (mm) | 3.67 ± 0.48 | 3.81 ± 0.51 | Δ0.14 ± 0.21 | 3.64 ± 0.43 | 3.60 ± 0.32 | Δ−0.04 ± 0.34 | 0.14 |
| Peak Diameter (mm) | 3.82 ± 0.32 | 3.98 ± 0.53 | Δ0.13 ± 0.18 | 3.83 ± 0.44 | 3.82 ± 0.32 | Δ−0.01 ± 0.38 | 0.31 |
| Brachial FMD (%) | 4.84 ± 1.75 | 4.18 ± 2.19 | Δ−0.7 ± 2.1 | 5.03 ± 2.34 | 6.62 ± 2.22 *† | Δ1.6 ± 2.2 | 0.02 |
| Baseline Shear Rate (s−1) | 125 ± 45 | 131 ± 36 | Δ6 ± 32 | 125 ± 46 | 169 ± 61 | Δ43 ± 164 | 0.47 |
| Peak Shear Rate (s−1) | 1028 ± 264 | 1016 ± 302 | Δ−12 ± 209 | 1055 ± 245 | 1148 ± 401 | Δ92 ± 428 | 0.78 |
| FMD/Shear RateAUC (u.a.) | 2.18 ± 1.23 | 1.82 ± 1.29 | Δ−0.36 ± 1.55 | 1.52 ± 0.83 | 2.40 ± 1.06 | Δ0.88 ± 1.35 | 0.06 |
| Brachial SBP (mmHg) | 133 ± 14 | 135 ± 15 | Δ2 ± 5 | 132 ± 12 | 128 ± 8 *† | Δ−4 ± 6 | 0.04 |
| Brachial DBP (mmHg) | 78 ± 10 | 79 ± 11 | Δ2 ± 3 | 82 ± 8 | 81 ± 7 | Δ−1 ± 4 | 0.09 |
| Brachial MAP (mmHg) | 96 ± 10 | 98 ± 11 | Δ2 ± 4 | 99 ± 8 | 97 ± 6 | Δ−2 ± 4 | 0.04 |
| Brachial PP (mmHg) | 55 ± 13 | 55 ± 12 | Δ1 ± 5 | 50 ± 9 | 47 ± 8 | Δ−2 ± 7 | 0.21 |
| Aortic SBP (mmHg) | 127 ± 13 | 130 ± 15 | Δ3 ± 5 | 126 ± 10 | 123 ± 6 *† | Δ−4 ± 6 | 0.01 |
| Aortic DBP (mmHg) | 78 ± 11 | 79 ± 12 | Δ1 ± 4 | 83 ± 8 | 80 ± 7 * | Δ−3 ± 6 | 0.06 |
| Aortic MAP (mmHg) | 95 ± 10 | 96 ± 12 | Δ1 ± 4 | 95 ± 8 | 94 ± 6 *† | Δ−3 ± 5 | 0.02 |
| Aortic PP (mmHg) | 49 ± 12 | 51 ± 11 | Δ2 ± 3 | 43 ± 7 | 43 ± 7 | Δ0 ± 6 | 0.28 |
| Exercise | |||||||
| Δ Brachial SBP (mmHg) | 19 ± 10 | 19 ± 10 | Δ0 ± 10 | 20 ± 9 | 22 ± 7 | Δ2 ± 8 | 0.71 |
| Δ Brachial DBP (mmHg) | 6 ± 10 | 8 ± 5 | Δ2 ± 9 | 8 ± 7 | 9 ± 8 | Δ1 ± 6 | 0.73 |
| Δ Brachial MAP (mmHg) | 10 ± 10 | 11 ± 6 | Δ1 ± 8 | 12 ± 6 | 13 ± 7 | Δ1 ± 4 | 0.96 |
| Δ Brachial PP (mmHg) | 13 ± 8 | 12 ± 7 | Δ−2 ± 11 | 12 ± 10 | 13 ± 7 | Δ1 ± 11 | 0.58 |
| Variables | Placebo (n = 11) | CIT (n = 11) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 Week | 4 Weeks | 0 Week | 4 Weeks | |||||
| FBF (mL/min) | ||||||||
| Rest | 68 ± 41 | - | 107 ± 105 | - | 62 ± 18 | - | 90 ± 90 | - |
| 1 min | 150 ± 47 * | Δ82 ± 43 * | 172 ± 115 * | Δ65 ± 39 * | 127 ± 40 * | Δ65 ± 30 * | 189 ± 127 * | Δ100 ± 52 *† |
| 2 min | 176 ± 41 * | Δ107 ± 35 * | 204 ± 125 * | Δ98 ± 35 * | 149 ± 54 * | Δ87 ± 46 * | 225 ± 133 * | Δ135 ± 65 *† |
| 3 min | 200 ± 35 * | Δ132 ± 21 * | 212 ± 112 * | Δ106 ± 22 * | 177 ± 51 * | Δ115 ± 38 * | 265 ± 136 * | Δ176 ± 77 *† |
| Average over 3 min | 175 ± 38 * | Δ107 ± 30 * | 191 ± 116 * | Δ90 ± 26 * | 151 ± 44 * | Δ89 ± 33 * | 226 ± 126 * | Δ137 ± 53 *† |
| FVC (mL/min/mmHg) | ||||||||
| Rest | 68 ± 44 | 64 ± 19 | 110 ±116 | 91 ± 82 | ||||
| 1 min | 133 ± 44 * | Δ65 ± 41 * | 120 ± 43 * | Δ46 ± 36 | 156 ± 116 * | Δ56 ± 31 * | 177 ± 112 * | Δ86 ± 43 *† |
| 2 min | 155 ± 39 * | Δ87 ± 33 * | 140 ± 53 * | Δ74 ± 19 * | 184 ± 118 * | Δ76 ± 43 * | 206 ± 117 * | Δ115 ± 54 *† |
| 3 min | 176 ± 37 * | Δ108 ± 23 * | 161 ± 53 * | Δ79 ± 16 * | 189 ± 113 * | Δ97 ± 40 * | 240 ± 125 * | Δ149 ± 69 *† |
| Average over 3 min | 155 ± 38 * | Δ87 ± 30 * | 176 ± 115 * | Δ92 ± 42 * | 140 ± 47 * | Δ76 ± 35 * | 208 ± 113 * | Δ117 ± 44 *† |
| Variables | Placebo (n = 11) | CIT (n = 11) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 Week | 4 Weeks | 0 Week | 4 Weeks | |||||
| TSI (%) | ||||||||
| Rest | 61.4 ± 2.3 | - | 59.6 ± 2.7 | - | 61.8 ± 4.1 | - | 60.6 ± 3.3 | - |
| 1 min | 61.5 ± 2.5 | Δ0.18 ± 1.24 | 58.8 ± 3.8 | Δ−0.86 ± 2.27 | 61.2 ± 5.6 | Δ−0.65 ± 2.12 | 61.6 ± 3.2 | Δ0.98 ± 1.21 † |
| 2 min | 60.6 ± 2.2 | Δ−0.71 ± 1.46 | 58.0 ± 4.2 | Δ−1.64 ± 2.75 | 60.2 ± 5.7 | Δ−1.60 ± 2.66 | 60.6 ± 3.2 | Δ0.01 ± 1.96 † |
| 3 min | 60.5 ± 4.2 | Δ−0.82 ± 1.37 | 58.3 ± 4.1 | Δ−1.36 ± 2.39 | 60.4 ± 5.6 | Δ−1.46 ± 2.51 | 60.7 ± 3.2 | Δ0.13 ± 1.73 † |
| Average over 3 min | 60.9 ± 2.2 | Δ−0.45 ± 1.07 | 58.4 ± 4.0 | Δ−1.29 ± 2.42 | 60.6 ± 5.6 | Δ−1.24 ± 2.38 | 61.0 ± 3.2 | Δ0.37 ± 1.62 † |
| HHb (μM) | ||||||||
| Rest | −0.60 ±0.62 | - | −0.95 ± 0.94 | - | −0.38 ± 0.49 | - | −0.51 ± 0.56 | - |
| 1 min | 0.96 ± 1.48 | Δ1.56 ± 1.70 | 1.48 ± 2.99 * | Δ2.43 ± 2.63 * | 2.01 ± 2.07 * | Δ2.39 ± 2.10 * | −0.69 ± 1.96 | Δ−0.18 ± 1.57 † |
| 2 min | 1.64 ± 1.45 * | Δ2.24 ± 1.63 * | 2.80 ± 3.73 * | Δ3.76 ± 3.68 * | 2.97 ± 2.92 * | Δ3.35 ± 2.97 * | −0.01 ± 2.48 † | Δ0.49 ± 2.13 † |
| 3 min | 2.45 ± 2.82 * | Δ3.04 ± 2.54 * | 2.80 ± 3.98 * | Δ3.75 ± 3.90 * | 2.93 ± 3.10 * | Δ3.30 ± 3.19 * | −0.06 ± 2.23 † | Δ0.45 ± 1.87 † |
| Average over 3 min | 1.68 ± 1.57 * | Δ2.28 ± 1.66 * | 2.36 ± 3.43 * | Δ3.31 ± 3.27 * | 2.64 ± 2.65 * | Δ3.02 ± 2.71 * | −0.25 ± 2.20 † | Δ0.25 ± 1.83 † |
| O2Hb (μM) | ||||||||
| Rest | 0.23 ± 0.63 | - | 0.35 ± 0.76 | - | 0.45 ± 0.47 | - | 0.70 ± 0.47 | - |
| 1 min | 1.08 ± 3.11 | Δ0.85 ± 3.07 | 0.17 ± 2.60 | Δ−0.18 ± 2.30 | 1.43 ± 4.06 | Δ0.97 ± 4.00 | 3.22 ± 2.42 *† | Δ2.52 ± 2.34 *† |
| 2 min | 1.06 ± 3.34 | Δ0.83 ± 3.44 | −0.85 ± 3.99 | Δ−1.20 ± 3.81 | 0.21 ± 5.77 | Δ−0.24 ± 5.73 | 3.45 ± 3.15 † | Δ2.75 ± 3.11 † |
| 3 min | 1.55 ± 3.77 | Δ2.32 ± 3.79 | −0.32 ± 3.75 | Δ−0.67 ± 3.51 | 0.62 ± 5.91 | Δ0.17 ± 5.93 | 3.71 ± 2.88 *† | Δ3.01 ± 2.86 *† |
| Average over 3 min | 1.56 ± 3.33 | Δ1.34 ± 3.36 | −0.34 ± 3.32 | Δ−0.69 ± 3.08 | 0.75 ± 5.17 | Δ0.30 ± 5.14 | 3.26 ± 2.79 *† | Δ2.76 ± 2.75 *† |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Dillon, K.N.; Martinez, M.A.; Maharaj, A.; Fischer, S.M.; Figueroa, A. L-Citrulline Supplementation Improves Arterial Blood Flow and Muscle Oxygenation during Handgrip Exercise in Hypertensive Postmenopausal Women. Nutrients 2024, 16, 1935. https://doi.org/10.3390/nu16121935
Kang Y, Dillon KN, Martinez MA, Maharaj A, Fischer SM, Figueroa A. L-Citrulline Supplementation Improves Arterial Blood Flow and Muscle Oxygenation during Handgrip Exercise in Hypertensive Postmenopausal Women. Nutrients. 2024; 16(12):1935. https://doi.org/10.3390/nu16121935
Chicago/Turabian StyleKang, Yejin, Katherine N. Dillon, Mauricio A. Martinez, Arun Maharaj, Stephen M. Fischer, and Arturo Figueroa. 2024. "L-Citrulline Supplementation Improves Arterial Blood Flow and Muscle Oxygenation during Handgrip Exercise in Hypertensive Postmenopausal Women" Nutrients 16, no. 12: 1935. https://doi.org/10.3390/nu16121935
APA StyleKang, Y., Dillon, K. N., Martinez, M. A., Maharaj, A., Fischer, S. M., & Figueroa, A. (2024). L-Citrulline Supplementation Improves Arterial Blood Flow and Muscle Oxygenation during Handgrip Exercise in Hypertensive Postmenopausal Women. Nutrients, 16(12), 1935. https://doi.org/10.3390/nu16121935







