Calcifediol or Corticosteroids in the Treatment of COVID-19: An Observational Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Overview of the Study
2.2. Statistical Methods
3. Results
3.1. Characteristics of the Study Population
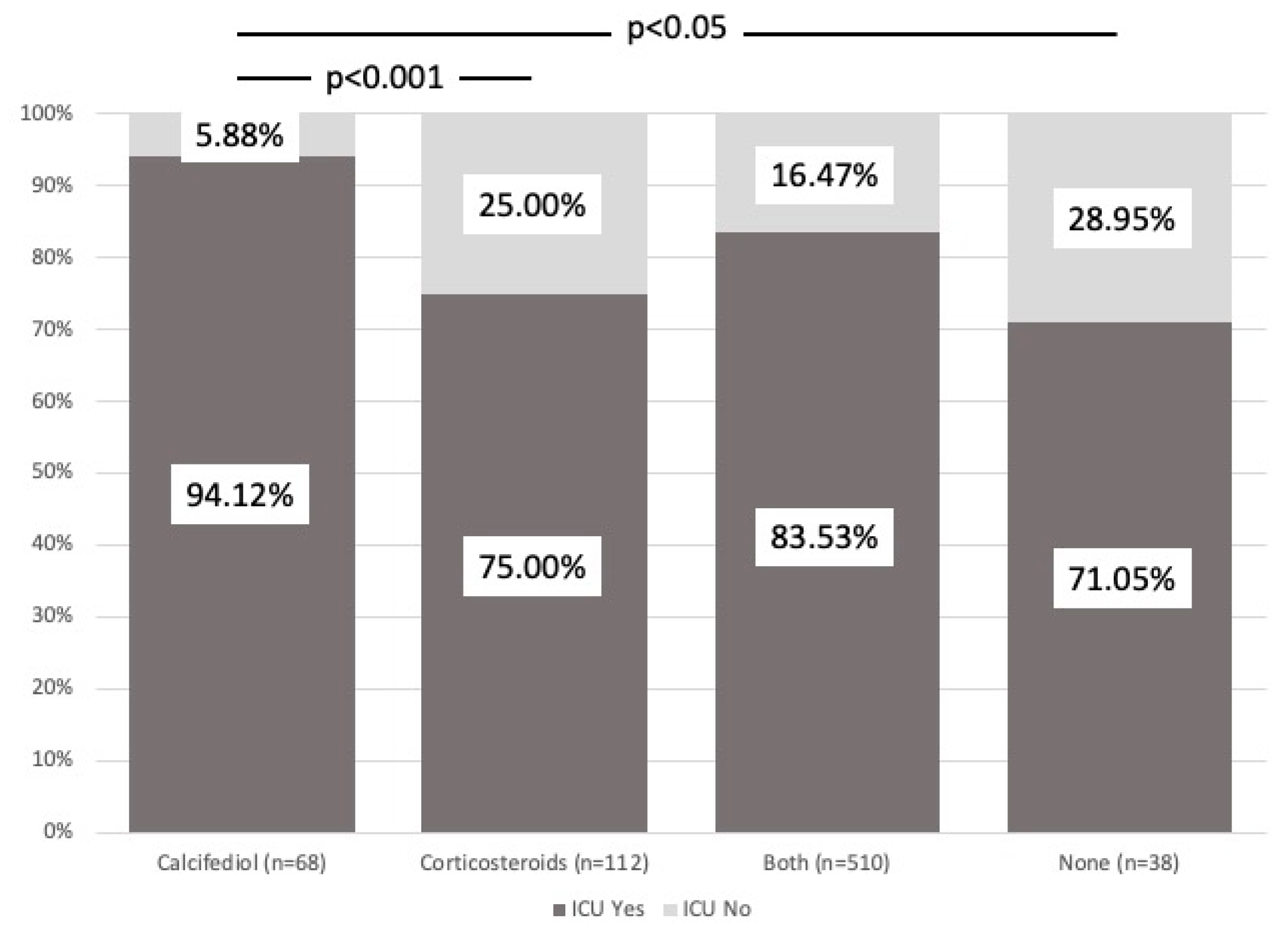
3.2. Intensive Care Unit Admission
3.3. Death
3.4. Poor Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranabhat, C.L.; Jakovljevic, M.; Kim, C.B.; Simkhada, P. COVID-19 Pandemic: An Opportunity for Universal Health Coverage. Front. Public Health 2021, 9, 673542. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA J. Am. Med. Assoc. 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X.; Li, Y.; Huang, J.; Jiang, J.; Su, N. Specific cytokines in the inflammatory cytokine storm of patients with COVID-19-associated acute respiratory distress syndrome and extrapulmonary multiple-organ dysfunction. Virol. J. 2021, 18, 117. [Google Scholar] [CrossRef]
- Rysz, S.; Al-Saadi, J.; Sjöström, A.; Farm, M.; Campoccia Jalde, F.; Plattén, M.; Eriksson, H.; Klein, M.; Vargas-Paris, R.; Nyrén, S.; et al. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat. Commun. 2021, 12, 2417. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Spiezia, L.; Boscolo, A.; Poletto, F.; Cerruti, L.; Tiberio, I.; Campello, E.; Navalesi, P.; Simioni, P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb. Haemost. 2020, 120, 998–1000. [Google Scholar] [CrossRef]
- Murakami, N.; Hayden, R.; Hills, T.; Al-Samkari, H.; Casey, J.; Del Sorbo, L.; Lawler, P.R.; Sise, M.E.; Leaf, D.E. Therapeutic advances in COVID-19. Nat. Rev. Nephrol. 2023, 19, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Nishiyama, K.; Nishimura, A.; Noda, T.; Okabe, K.; Kusakabe, T.; Kanda, Y.; Nishida, M. Drug repurposing for the treatment of COVID-19. J. Pharmacol. Sci. 2022, 149, 108–114. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Entrenas-Castillo, M.; Bouillon, R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. J. Steroid Biochem. Mol. Biol. 2020, 202, 105719. [Google Scholar] [CrossRef] [PubMed]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef] [PubMed]
- Nogues, X.; Ovejero, D.; Pineda-Moncusí, M.; Bouillon, R.; Arenas, D.; Pascual, J.; Ribes, A.; Guerri-Fernandez, R.; Villar-Garcia, J.; Rial, A.; et al. Calcifediol treatment and COVID-19–Related outcomes. J. Clin. Endocrinol. Metab. 2021, 106, E4017–E4027. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Diaz, J.F.; Limia-Perez, L.; Gomez-Huelgas, R.; Martin-Escalante, M.D.; Cortes-Rodriguez, B.; Zambrana-Garcia, J.L.; Entrenas-Castillo, M.; Perez-Caballero, A.I.; López-Carmona, M.D.; Garcia-Alegria, J.; et al. Calcifediol treatment and hospital mortality due to COVID-19: A cohort study. Nutrients 2021, 13, 1760. [Google Scholar] [CrossRef] [PubMed]
- Shimba, A.; Ikuta, K. Glucocorticoids Regulate Circadian Rhythm of Innate and Adaptive Immunity. Front. Immunol. 2020, 11, 545780. [Google Scholar] [CrossRef] [PubMed]
- Dagens, A.; Sigfrid, L.; Cai, E.; Lipworth, S.; Cheung, V.; Harris, E.; Bannister, P.; Rigby, I.; Horby, P. Scope, quality, and inclusivity of clinical guidelines produced early in the COVID-19 pandemic: Rapid review. BMJ 2020, 369, m1936. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; RECOVERY Collaborative Group; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Update to living WHO guideline on drugs for COVID-19. BMJ 2021, 372, n860. [CrossRef]
- Liu, J.; Zhang, S.; Dong, X.; Li, Z.; Xu, Q.; Feng, H.; Cai, J.; Huang, S.; Guo, J.; Zhang, L.; et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J. Clin. Investig. 2020, 130, 6417–6428. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, W.; Chen, P.; Guo, J.; Liu, R.; Wen, P.; Li, K.; Lu, Y.; Ma, T.; Li, X.; et al. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0249481. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Spannella, F.; Giulietti, F.; Di Pentima, C.; Giordano, P.; Giacometti, A. Possible harm from glucocorticoid drugs misuse in the early phase of SARS-CoV-2 infection: A narrative review of the evidence. Intern. Emerg. Med. 2022, 17, 329–338. [Google Scholar] [CrossRef]
- Efird, J.T.; Anderson, E.J.; Jindal, C.; Suzuki, A. Interaction of Vitamin D and Corticosteroid Use in Hospitalized COVID-19 Patients: A Potential Explanation for Inconsistent Findings in the Literature. Curr. Pharm. Des. 2022, 28, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, R.M.; Almaghraby, A.; Shaaban, R.; Kamal, A.; Beshir, H.; Moursi, A.; Ramadan, A.; Taha, S.H.N. A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci. Rep. 2020, 10, 22139. [Google Scholar] [CrossRef] [PubMed]
- Luis, M.; Talía, E.; David, S.; Laura, A.; María, E.; Barrio, I.; José, M. Documento Técnico Manejo Clínico del COVID-19: Atención Hospitalaria. 2020. Available online: https://www.sanidad.gob.es/areas/alertasEmergenciasSanitarias/alertasActuales/nCov/documentos/Protocolo_manejo_clinico_ah_COVID-19.pdf (accessed on 30 March 2024).
- Mingiano, C.; Picchioni, T.; Cavati, G.; Pirrotta, F.; Calabrese, M.; Nuti, R.; Gonnelli, S.; Fortini, A.; Frediani, B.; Gennari, L.; et al. Vitamin D Deficiency in COVID-19 Patients and Role of Calcifediol Supplementation. Nutrients 2023, 15, 3392. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Quesada-Gomez, J.M. Vitamin D Endocrine System and COVID-19. JBMR Plus 2021, 5, e10576. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment with 25-Hydroxyvitamin D3 (Calcifediol) Is Associated with a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients with COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Bli. Endocr. Pract. 2021, 27, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics with COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, A.; Leopold, K.; Jackson, S.; Christensen, S.; Nayfeh, T.; Mohammed, K.; Creo, A.; Tebben, P.; Kumar, S. Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis. Clin. Endocrinol. 2022, 96, 281–287. [Google Scholar] [CrossRef]
- Lugg, S.T.; Thickett, D.R. The role of vitamin D in COVID-19. In Feldman Pike’s Vitamin D; Academic Press: Cambridge, MA, USA, 2023; pp. 1091–1108. [Google Scholar] [CrossRef]
- Smolders, J.; van den Ouweland, J.; Geven, C.; Pickkers, P.; Kox, M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metab. Clin. Experimental. 2021, 115, 154434. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yang, J.; Hu, X.; Li, M.; Wang, Q.; Dancer, R.C.A.; Parekh, D.; Gao-Smith, F.; Thickett, D.R.; Jin, S. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem. Pharmacol. 2020, 177, 113955. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.M.; Whettlock, E.M.; Liu, S.; Arachchillage, D.J.; Boyton, R.J. The immunology of long covid. Nat. Rev. Immunol. 2023, 23, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Gomez, J.M.; Bouillon, R. Is calcifediol better than cholecalciferol for vitamin D supplementation? 25OHD 25-Hydroxyvitamin D 3 and 25-hydroxyvitamin D 2 combined in plasma Vitamin D Vitamin D 3 or D 2 RCT Randomized controlled trial. Osteoporos. Int. 2018, 32, 1065–1076. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Lopez-Miranda, J.; Entrenas-Castillo, M.; Casado-Díaz, A.; Nogues, Y.; Solans, X.; Mansur, J.L.; Bouillon, R. Vitamin D Endocrine System and COVID-19: Treatment with Calcifediol. Nutrients 2022, 14, 2716. [Google Scholar] [CrossRef] [PubMed]
- Ketha, H.; Thacher, T.D.; Oberhelman, S.S.; Fischer, P.R.; Singh, R.J.; Kumar, R. Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 ratio. Bone 2018, 110, 321–325. [Google Scholar] [CrossRef]
- Jenkinson, C.; Desai, R.; McLeod, M.D.; Wolf Mueller, J.; Hewison, M.; Handelsman, D.J. Circulating Conjugated and Unconjugated Vitamin D Metabolite Measurements by Liquid Chromatography Mass Spectrometry. J. Clin. Endocrinol. Metab. 2022, 107, 435–449. [Google Scholar] [CrossRef]
- Castillo-Peinado, L.d.l.S.; Calderón-Santiago, M.; Sánchez-Cano, R.L.; Quesada-Gómez, J.M.; Bouillon, R.; Priego-Capote, F. Determination of vitamin D3 conjugated metabolites: A complementary view on hydroxylated metabolites. Analyst 2023, 148, 654–664. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Marcellini, A.; Swieboda, D.; Guedán, A.; Farrow, S.N.; Casolari, P.; Contoli, M.; Johnston, S.L.; Papi, A.; Solari, R. Glucocorticoids impair type I IFN signalling and enhance rhinovirus replication. Eur. J. Pharmacol. 2021, 893, 173839. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.N.; Gunnarsson, H.I.; Yi, Z.; Gudmundsdottir, S.; Sigurjonsson, O.E.; Agerberth, B.; Gudmundsson, G.H. Glucocorticoid dexamethasone down-regulates basal and vitamin D3 induced cathelicidin expression in human monocytes and bronchial epithelial cell line. Immunobiology 2016, 221, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Clyne, C.D.; Chapman, K.E. Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. J. Endocrinol. 2020, 247, R45–R62. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, E.; Rezig, A.O.M.; Bruzzese, D.; Ialenti, A.; Cicala, C.; Cleland, J.G.F.; Guzik, T.J.; Maffia, P.; Pellicori, P. Systemic administration of glucocorticoids, cardiovascular complications and mortality in patients hospitalised with COVID-19, SARS, MERS or influenza: A systematic review and meta-analysis of randomised trials. Pharmacol. Res. 2022, 176, 106053. [Google Scholar] [CrossRef] [PubMed]
- Efird, J.T.; Anderson, E.J.; Jindal, C.; Redding, T.S.; Thompson, A.D.; Press, A.M.; Upchurch, J.; Williams, C.D.; Choi, Y.M.; Suzuki, A. The interaction of Vitamin D and corticosteroids: A mortality analysis of 26,508 veterans who tested positive for SARS-CoV-2. Int. J. Environ. Res. Public Health 2022, 19, 447. [Google Scholar] [CrossRef]
- Loucera, C.; Peña-Chilet, M.; Esteban-Medina, M.; Muñoyerro-Muñiz, D.; Villegas, R.; Lopez-Miranda, J.; Rodriguez-Baño, J.; Túnez, I.; Bouillon, R.; Dopazo, J.; et al. Real world evidence of calcifediol or vitamin D prescription and mortality rate of COVID-19 in a retrospective cohort of hospitalized Andalusian patients. Sci. Rep. 2021, 11, 23380. [Google Scholar] [CrossRef]


| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Calcifediol n = 68 | Corticosteroids n = 112 | Both n = 510 | None n = 38 | Total n = 728 | p | |
| Outbreak | 1 2 3 | 63 1 4 | 5 94 13 | 7 254 249 | 31 7 0 | 106 356 266 | <0.001 |
| Female Male | 32 36 | 46 66 | 186 324 | 16 22 | 280 448 | 0.319 | |
| Age | 52.38 ± 10.47 | 52.74 ± 11.02 | 52.72 ± 9.99 | 52.39 ± 12.61 | 52.68 ± 10.33 | 0.916 | |
| Treatment | |||||
|---|---|---|---|---|---|
| Variable | Calcifediol n = 68 | Corticosteroids n = 112 | Both n = 510 | None n = 38 | p |
| Lung disease * | 10 (8.13%) | 17 (13.82%) | 92 (74.80%) | 4 (3.25%) | 0.564 |
| Renal impairment ** | 0 (0%) | 1 (10%) | 8 (80%) | 1 (10%) | 0.688 |
| Diabetes * | 3 (3%) | 15 (15%) | 76 (76%) | 6 (6%) | 0.126 |
| High blood pressure * | 15 (5.88%) | 37 (14.51%) | 189 (74.12%) | 14 (5.49%) | 0.102 |
| Thyroid disease ** | 8 (14.04%) | 9 (15.79%) | 38 (66.67%) | 2 (3.51%) | 0.570 |
| Cardiovascular disease ** | 4 (6.67%) | 7 (11.67%) | 46 (76.67%) | 3 (5.00%) | 0.759 |
| Biological treatment ** | 0 (0%) | 0 (0%) | 4 (100%) | 0 (0%) | 1.00 |
| Immunosuppressive treatment ** | 7 (26.92%) | 2 (7.69%) | 17 (65.38%) | 0 (0%) | 0.026 |
| Cancer ** | 6 (18.75%) | 6 (18.75%) | 19 (59.38%) | 1 (3.12%) | 0.232 |
| Obesity/overweight * | 5 (1.27%) | 63 (15.99%) | 322 (81.73%) | 4 (1.02%) | <0.001 |
| Treatment | ||||
|---|---|---|---|---|
| Variable | Calcifediol | Corticosteroids | Both | None |
| Lymphocytes | 1198.82 (501.35) | 1021.88 (515.17) | 1071.18 (587.43) | 1120.79 (498.01) |
| Eosinophils | 20.00 (55.93) | 32.00 (24.06%) | 35.77 (77.38) | 13.89 (32.98) |
| Procalcitonin | 0.73 (4.29) | 1.43 (9.24) | 0.14 (0.25) | 4.57 (27.25) |
| IL-6 | 25.78 (67.99) | 16.18 (25.21) | 23.14 (89.99) | 15.03 (13.68) |
| IO2 | 345.17 (68.97) | 315.40 (80.64) | 320.58 (61.65) | 352.67 (84.90) |
| Treatment | |||||
|---|---|---|---|---|---|
| Drug | Calcifediol n = 68 | Corticoids n= 112 | Both n = 510 | None n = 38 | p |
| Hydroxychloroquine * | 59 | 6 | 10 | 27 | <0.001 |
| Azithromycin * | 60 | 102 | 336 | 30 | <0.001 |
| Ceftriaxone * | 55 | 100 | 362 | 31 | <0.001 |
| Calcifediol * | 68 | 0 | 510 | 0 | <0.001 |
| Glucocorticoids * | 0 | 112 | 510 | 0 | <0.001 |
| Lopinavir / Ritonavir ** | 7 | 3 | 0 | 13 | <0.001 |
| Interferon ** | 2 | 1 | 1 | 11 | <0.001 |
| Tocilizumab ** | 5 | 7 | 83 | 0 | <0.001 |
| Adalimumab ** | 0 | 5 | 16 | 0 | 0.273 |
| Immunoglobulins ** | 0 | 3 | 1 | 1 | <0.05 |
| Bevacizumab ** | 0 | 1 | 0 | 0 | 0.299 |
| Antithrombin ** | 1 | 7 | 66 | 0 | <0.001 |
| Sarilumab ** | 0 | 0 | 7 | 0 | 0.686 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Entrenas-Castillo, M.; Entrenas-Costa, L.M.; Pata, M.P.; Jurado-Gamez, B.; Muñoz-Corroto, C.; Gomez-Rebollo, C.; Mira-Padilla, E.; Bouillon, R.; Quesada-Gómez, J.M. Calcifediol or Corticosteroids in the Treatment of COVID-19: An Observational Study. Nutrients 2024, 16, 1910. https://doi.org/10.3390/nu16121910
Entrenas-Castillo M, Entrenas-Costa LM, Pata MP, Jurado-Gamez B, Muñoz-Corroto C, Gomez-Rebollo C, Mira-Padilla E, Bouillon R, Quesada-Gómez JM. Calcifediol or Corticosteroids in the Treatment of COVID-19: An Observational Study. Nutrients. 2024; 16(12):1910. https://doi.org/10.3390/nu16121910
Chicago/Turabian StyleEntrenas-Castillo, Marta, Luis Manuel Entrenas-Costa, María P. Pata, Bernabe Jurado-Gamez, Cristina Muñoz-Corroto, Cristina Gomez-Rebollo, Estefania Mira-Padilla, Roger Bouillon, and Jose Manuel Quesada-Gómez. 2024. "Calcifediol or Corticosteroids in the Treatment of COVID-19: An Observational Study" Nutrients 16, no. 12: 1910. https://doi.org/10.3390/nu16121910
APA StyleEntrenas-Castillo, M., Entrenas-Costa, L. M., Pata, M. P., Jurado-Gamez, B., Muñoz-Corroto, C., Gomez-Rebollo, C., Mira-Padilla, E., Bouillon, R., & Quesada-Gómez, J. M. (2024). Calcifediol or Corticosteroids in the Treatment of COVID-19: An Observational Study. Nutrients, 16(12), 1910. https://doi.org/10.3390/nu16121910







