Vitamin D Metabolites in Mother–Infant Dyads and Associated Clinical Outcomes in a Population of Nigerian Women
Abstract
1. Introduction
1.1. Vitamin D Physiology
1.2. Effects of Vitamin D on the Immune System and Infections
1.3. Vitamin D Metabolites and Associated Outcomes
2. Materials and Methods
2.1. Recruitment
2.2. Ethical Approval
2.3. Sample and Data Collection
2.4. Biochemical Analysis
2.5. Clinical Outcome
2.6. Growth Outcome
2.7. Statistical Analysis
3. Results
3.1. Vitamin D Metabolites and Associated Newborn Outcomes
3.2. Vitamin D Metabolites and Associated Maternal Risk Factors and Outcomes
3.3. Comparison of Mother–Infant Dyad Categories of 25(OH)D Levels
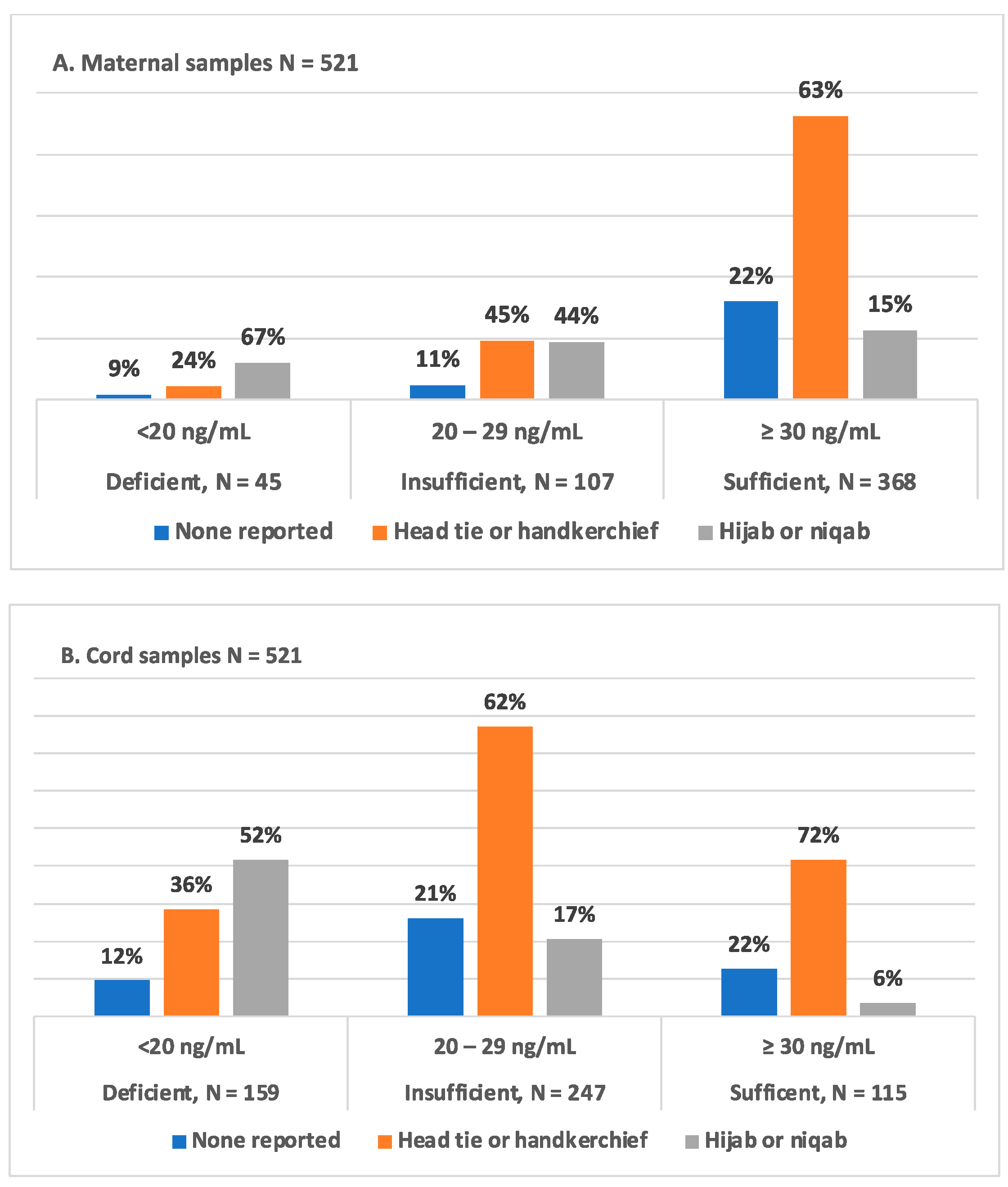
3.4. Type of Maternal Head Covering and 25(OH)D Status in Mother–Infant Dyads
3.5. Correlations Associated with Mother–Infant Dyad 25(OH)D and Metabolite Levels
4. Discussion
4.1. Primary Outcome
4.2. Vitamin D and Metabolite Status
4.3. Birth Anthropometrics
4.4. Maternal Risk Factors and Newborn Outcomes
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nigeria Country Profile—Key Demographic Indicators Nigeria. Available online: https://data.unicef.org/country/nga/ (accessed on 12 August 2023).
- Munoz, F.M. Current Challenges and Achievements in Maternal Immunization Research. Front. Immunol. 2018, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Piso, B.; Reinsperger, I.; Winkler, R. Recommendations from international clinical guidelines for routine antenatal infection screening: Does evidence matter? Int. J. Evid. Based Healthc. 2014, 12, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Gray, T.K.; Lowe, W.; Lester, G.E. Vitamin D and pregnancy: The maternal-fetal metabolism of Vitamin D. Endocr. Rev. 1981, 2, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Berry, A.; Thoene, M.; Wagner, J.; Lyden, E.; Jones, G.; Kaufmann, M.; Van Ormer, M.; Hanson, C. Randomized trial of two doses of vitamin D3 in preterm infants <32 weeks: Dose impact on achieving desired serum 25(OH)D3 in a NICU population. PLoS ONE 2017, 12, e0185950. [Google Scholar]
- Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Mao, D.; Yuen, L.-Y.; Ho, C.-S.; Wang, C.-C.; Tam, C.H.-T.; Chan, M.H.-M.; Lowe, W.L.; Ma, R.C.-W.; Tam, W.-H. Maternal and Neonatal 3-epi-25-hydroxyvitamin D Concentration and Factors Influencing Their Concentrations. J. Endocr. Soc. 2022, 6, bvab170. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J.; Taylor, R.L.; Reddy, G.S.; Grebe, S.K.G. C-3 Epimers can account for a significant proportion of total circulating 25-Hydroxyvitamin D in infants, complicating accurate measurement and interpretation of Vitamin D status. J. Clin. Endocrinol. Metab. 2006, 91, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.W.; Al-Shehri, M.A. Cord blood 25(OH)D levels and the subsequent risk of lower respiratory tract infections in early childhood: The Ulm birth cohort. Eur. J. Epidemiol. 2014, 29, 585–594. [Google Scholar]
- Karatekin, G.; Kaya, A.; Salihoğlu, Ö.; Balci, H.; Nuhoğlu, A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur. J. Clin. Nutr. 2009, 63, 473–477. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F. Pregnancy, Breastfeeding, and Vitamin D. Int. J. Mol. Sci. 2023, 24, 11881. [Google Scholar] [CrossRef]
- Bailey, D.; Veljkovic, K.; Yazdanpanah, M.; Adeli, K. Analytical measurement and clinical relevance of vitamin D(3) C3-epimer. Clin. Biochem. 2013, 46, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Parva, N.R.; Tadepalli, S.; Singh, P.; Qian, A.; Joshi, R.; Kandala, H.; Nookala, V.K.; Cheriyath, P. Prevalence of Vitamin D Deficiency and Associated Risk Factors in the US Population (2011–2012). Cureus 2018, 10, e2741. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, R.; Hovind, P.H.; Jensen, L.T.; Schwarz, P. Determinants of vitamin D status in young adults: Influence of lifestyle, sociodemographic and anthropometric factors. BMC Public Health 2016, 16, 385. [Google Scholar] [CrossRef] [PubMed]
- El Rifai, N.M.; Moety, G.A.F.A.; Gaafar, H.M.; Hamed, D.A. Vitamin D deficiency in Egyptian mothers and their neonates and possible related factors. J. Matern. Fetal. Neonatal Med. 2014, 27, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Mogire, R.M.; Mutua, A.; Kimita, W.; Kamau, A.; Bejon, P.; Pettifor, J.M.; Adeyemo, A.; Williams, T.N.; Atkinson, S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e134–e142. [Google Scholar] [CrossRef] [PubMed]
- Bitew, Z.W.; Worku, T.; Alemu, A. Effects of vitamin D on neonatal sepsis: A systematic review and meta-analysis. Food Sci. Nutr. 2021, 9, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Akeredolu, F.D.; Akuse, R.M.; Mado, S.M.; Yusuf, R. Relationship Between Serum Vitamin D Levels and Acute Pneumonia in Children Aged 1-59 Months in Nigeria. J. Trop. Pediatr. 2021, 67, fmaa101. [Google Scholar] [CrossRef] [PubMed]
- Adinma, J.I.B.-D.; Ahaneku, J.E.; Adinma, E.D.; Ugboaja, J.O.; Okolie, V.; Adinma-Obiajulu, N.D.; Edet, M.M. Vitamin D and associated factors, among pregnant women in southeastern Nigeria. J. Obstet. Gynaecol. 2022, 42, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Sowah, D.; Fan, X.; Dennett, L.; Hagtvedt, R.; Straube, S. Vitamin D levels and deficiency with different occupations: A systematic review. BMC Public Health 2017, 17, 519. [Google Scholar] [CrossRef]
- Forrest, K.Y.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Owie, E.; Afolabi, B.B. Vitamin D deficiency in pregnant women and newborns in Lagos, Nigeria. J. Obstet. Gynaecol. 2018, 38, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.A.; Okunade, K.S.; Okojie, O.E. Maternal serum vitamin D levels and preterm delivery among low-risk parturients in Lagos, Nigeria. Int. J. Gynecol. Obstet. 2019, 144, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Gbadegesin, A.; Sobande, A.; Adedeji, O.; Disu, E.; Korede, O.; Dosunmu, A.; Shakunle, A. Maternal serum vitamin D levels and pregnancy outcomes: From Lagos, Nigeria. J. Obstet. Gynaecol. 2017, 37, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Elechi, H.; Oduwole, A.; Idris, H.; Faruk, M.; Alhaji, M. Vitamin D and bone mineral status of newborn-maternal pair delivering at a tertiary hospital in Nigeria. Niger. J. Clin. Pract. 2021, 24, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.; Perumal, N.; Yazdanpanah, M.; Al Mahmud, A.; Baqui, A.H.; Adeli, K.; Roth, D.E. Maternal-fetal-infant dynamics of the C3-epimer of 25-hydroxyvitamin D. Clin. Biochem. 2014, 47, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Jones, G.; Lyden, E.; Kaufmann, M.; Armas, L.; Anderson-Berry, A. Vitamin D metabolism in the premature newborn: A randomized trial. Clin. Nutr. 2016, 35, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Ritter, C.; Slatopolsky, E.; Muralidharan, K.R.; Okamura, W.H.; Reddy, G.S. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J. Cell. Biochem. 1999, 73, 106–113. [Google Scholar] [CrossRef]
- Rehan, V.K.; Torday, J.S.; Peleg, S.; Gennaro, L.; Vouros, P.; Padbury, J.; Rao, D.S.; Reddy, G.S. 1α,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1α,25-dihydroxy vitamin D3: Production and biological activity studies in pulmonary alveolar type II cells. Mol. Genet. Metab. 2002, 76, 46–56. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Goldfarb, D.M.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of paediatric and neonatal sepsis: A systematic review. Lancet Respir. Med. 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Pathirana, J.; Muñoz, F.M.; Abbing-Karahagopian, V.; Bhat, N.; Harris, T.; Kapoor, A.; Keene, D.L.; Mangili, A.; Padula, M.A.; Pande, S.L.; et al. Neonatal death: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2016, 34, 6027–6037. [Google Scholar] [CrossRef]
- Kaufmann, M.; Gallagher, J.C.; Peacock, M.; Schlingmann, K.-P.; Konrad, M.; DeLuca, H.F.; Sigueiro, R.; Lopez, B.; Mourino, A.; Maestro, M.; et al. Clinical utility of simultaneous quantitation of 25-Hydroxyvitamin D and 24,25-Dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J. Clin. Endocrinol. Metab. 2014, 99, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of Vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, C.; Xu, R.; Wang, K.; Zhang, D.; Pang, W.; Tu, W.; Chen, Y. Effects of vitamin D supplementation during pregnancy on offspring health at birth: A meta-analysis of randomized controlled trails. Clin. Nutr. 2022, 41, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, F.G.; Sadilkova, K.; Laha, T.J.; LeSourd, S.E.; Bornhorst, J.A.; Hoofnagle, A.N.; Jack, R. 3-epi-25 hydroxyvitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin. Chim. Acta 2012, 413, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ozim, C.O.; Mahendran, R.; Amalan, M.; Puthussery, S. Prevalence of human immunodeficiency virus (HIV) among pregnant women in Nigeria: A systematic review and meta-analysis. BMJ Open 2023, 13, e050164. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, D.A.; Olakunde, B.O.; Ozigbu, C.E.; Agogo, E.A.; Morka, M.; Atoba, T.; Obanubi, C.; Okorie, G.; Davies, A.; Oladimeji, O. Elimination of mother-to-child transmission of syphilis: Is it a reality in Nigeria by 2020? Scand J. Public Health 2018, 46, 794–797. [Google Scholar] [CrossRef]
- Mishal, A.A. Effects of different dress styles on Vitamin D levels in healthy young jordanian women. Osteoporos. Int. 2001, 12, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Gerges, M.E.S.; Amin, G.E.A.; Andraous, F.; Hamid, D.M.A.; Allam, M.F. Vitamin D level in a sample of Egyptian females of childbearing age attending a family medicine center. Int. J. Clin. Pract. 2021, 75, e13738. [Google Scholar] [CrossRef]
- Amegah, A.K.; Nsoh, M.; Ashley-Amegah, G.; Anaman-Togbor, J. What factors influences dietary and non-dietary vitamin D intake among pregnant women in an African population? Nutrition 2018, 50, 36–44. [Google Scholar] [CrossRef]
- Agarwal, S.; Kovilam, O.; Agrawal, D.K. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review. Crit. Rev. Food Sci. Nutr. 2018, 58, 755–769. [Google Scholar] [CrossRef]
- Best, C.M.; Pressman, E.K.; Queenan, R.A.; Cooper, E.; Vermeylen, F.; O’Brien, K.O. Gestational Age and Maternal Serum 25-hydroxyvitamin D Concentration Interact to Affect the 24,25-dihydroxyvitamin D Concentration in Pregnant Adolescents. J. Nutr. 2018, 148, 868–875. [Google Scholar] [CrossRef]
- Hanson, C.; Anderson-Berry, A.; Lyden, E.; Kaufmann, M.; Wu, A.; Elliott, E.; Lee, J.; Jones, G. Dynamics of Vitamin D Metabolism in Maternal–Fetal Dyads. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 486–490. [Google Scholar] [CrossRef]
- Oyedele, O.K. Disparities and barriers of health facility delivery following optimal and suboptimal pregnancy care in Nigeria: Evidence of home births from cross-sectional surveys. BMC Women’s Health 2023, 23, 194. [Google Scholar] [CrossRef]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; Dijck-Brouwer, D.J.; Muskiet, F.A. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br. J. Nutr. 2012, 108, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Vitamin D and pregnancy: Skeletal effects, nonskeletal effects, and birth outcomes. Calcif. Tissue Int. 2013, 92, 128–139. [Google Scholar] [CrossRef] [PubMed]
- El-Khateeb, M.; Khader, Y.; Batieha, A.; Jaddou, H.; Hyassat, D.; Khawaja, N.; Abujbara, M.; Ajlouni, K. Vitamin D deficiency and associated factors in Jordan. SAGE Open Med. 2019, 7, 2050312119876151. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after Pregnancy: Effect on Neonates and Children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef]
- Karras, S.N.; Fakhoury, H.; Muscogiuri, G.; Grant, W.B.; Ouweland, J.M.v.D.; Colao, A.M.; Kotsa, K. Maternal vitamin D levels during pregnancy and neonatal health: Evidence to date and clinical implications. Ther. Adv. Musculoskelet. Dis. 2016, 8, 124–135. [Google Scholar] [CrossRef]

| Maternal Characteristics | ||
|---|---|---|
| Continuous variables | N | Mean (SD) |
| Age (years) | 535 | 30 (5) |
| BMI (Kg/m2) | 535 | 30 (5.34) |
| Hemoglobin (g/dL) | 426 | 11.4 (1.1) |
| Categorical variables | N (%) | |
| Education level | ||
| Grade 1–12 | 208 (47.5) | |
| Tertiary | 309 (49.5) | |
| Employed | ||
| Yes | 341 (64.2) | |
| No | 190 (35.8) | |
| Type of head covering | ||
| None reported | 96 (18.4) | |
| Hijab or niqab | 131 (25.3) | |
| Head tie or handkerchief | 294 (56.4) | |
| HIV status | ||
| Yes | 22 (4.3) | |
| No | 487 (95.7) | |
| Malaria status | ||
| Yes | 21 (3.9) | |
| No | 62 (11.6) | |
| Unknown | 453 (84.5) | |
| Mode of delivery | ||
| Vaginal | 398 (74.3) | |
| Caeserean section | 138 (25.7) | |
| Newborn Characteristics | ||
|---|---|---|
| Continuous variable | N | Mean (SD) |
| Gestational age (weeks) | 536 | 38.4 (2.2) |
| Birth anthroprometrics | ||
| Birth weight (g) | 536 | 3088 (527.2) |
| Birth length (cm) | 536 | 48.7 (4) |
| Birth head circumference (cm) | 533 | 34.5 (2.8) |
| Categorical variables | N (%) | |
| Education level | ||
| Grade 1–12 | 261 (49) | |
| Tertiary | 275 (51) | |
| Apgar at 1 min | ||
| >7 | 470 (78.8) | |
| ≤7 | 48 (14.1) | |
| Unknown | 18 (7.1) | |
| Apgar at 5 min | ||
| >7 | 500 (94.4) | |
| ≤7 | 19 (3.5) | |
| Unknown | 17 (3.2) | |
| Newborn evaluation for sepsis | ||
| Yes | 34 (6.3) | |
| No | 492 (91.8) | |
| Unknown | 10 (1.9) | |
| Newborn alive at 7 days | ||
| Yes | 450 (83.9) | |
| No | 5 (1) | |
| Unknown | 81 (15.1) | |
| Newborn alive at 28 days | ||
| Yes | 446 (83.2) | |
| No | 9 (1.7) | |
| Unknown | 81 (15.1) | |
| Maternal and newborn vitamin D and metabolites levels vs. newborn evaluation for sepsis | |||||
| Evaluation for sepsis | Yes | No | |||
| Median | IQR | Median | IQR | p value | |
| Mother (N = 525) | |||||
| 25(OH)D ng/mL | 32.82 | 15.44 | 37.09 | 16.26 | p = 0.112 |
| 24,25(OH)2D3 | 1.47 | 0.96 | 1.75 | 1.27 | p = 0.212 |
| 3-epi-25(OH)D3 ng/mL | 1.24 | 0.72 | 1.28 | 0.69 | p = 0.679 |
| 3-epi-25(OH)D3 % | 3.78 | 1.13 | 3.58 | 1.36 | p = 0.213 |
| Cord (N = 526) | |||||
| 25(OH)D ng/mL | 22.36 | 7.79 | 24 | 10.29 | p = 0.308 |
| 24,25(OH)2D3 | 0.96 | 0.65 | 1.03 | 0.77 | p = 0.529 |
| 3-epi-25(OH)D3 ng/mL | 1.34 | 0.82 | 1.3 | 0.8 | p = 0.524 |
| 3-epi-25(OH)D3 % | 6.18 | 2.16 | 5.68 | 2.12 | p = 0.036 |
| Maternal and newborn vitamin D and metabolites levels vs. newborn survival at 28 days | |||||
| Newborn survival at 28 days | Yes | No | |||
| Median | IQR | Median | IQR | p value | |
| Mother (N = 455) | |||||
| 25(OH)D ng/mL | 36.7 | 16.74 | 31.61 | 10.03 | p = 0.06 |
| 24,25(OH)2D3 | 1.73 | 1.31 | 1.36 | 0.93 | p = 0.205 |
| 3-epi-25(OH)D3 ng/mL | 1.27 | 0.71 | 1.03 | 0.57 | p = 0.212 |
| 3-epi-25(OH)D3 % | 3.57 | 1.34 | 3.76 | 1.55 | p = 0.944 |
| Cord (N = 456) | |||||
| 25(OH)D ng/mL | 23.48 | 10.44 | 23.35 | 7.15 | p = 0.574 |
| 24,25(OH)2D3 | 1.02 | 0.81 | 0.99 | 0.59 | p = 0.870 |
| 3-epi-25(OH)D3 ng/mL | 1.3 | 0.83 | 0.69 | 0.43 | p = 0.377 |
| 3-epi-25(OH)D3 % | 5.65 | 2.06 | 2.5 | 2.62 | p = 0.626 |
| Maternal and newborn vitamin D and metabolites levels vs. weight for age Z-score ≤ −2 | |||||
| Weight for age z-score ≤ −2 | Yes | No | |||
| Median | IQR | Median | IQR | p value | |
| Mother (N = 524) | |||||
| 25(OH)D ng/mL | 33.36 | 14.73 | 37.15 | 16.79 | p = 0.156 |
| 24,25(OH)2D3 | 1.76 | 1.01 | 1.74 | 1.28 | p = 0.592 |
| 3-epi-25(OH)D3 ng/mL | 1.17 | 0.7 | 1.29 | 0.7 | p = 0.698 |
| 3-epi-25(OH)D3 % | 3.6 | 1.4 | 3.59 | 1.35 | p = 0.554 |
| Cord (N = 525) | |||||
| 25(OH)D ng/mL | 22.16 | 7.25 | 24.07 | 10.29 | p = 0.139 |
| 24,25(OH)2D3 | 0.97 | 0.76 | 1.03 | 0.77 | p = 0.77 |
| 3-epi-25(OH)D3 ng/mL | 1.3 | 0.83 | 1.31 | 0.79 | p = 0.502 |
| 3-epi-25(OH)D3 % | 6.17 | 2.29 | 5.65 | 2.06 | p = 0.004 |
| Maternal and newborn vitamin D and metabolites levels vs. weight for length Z-score ≤ −3 | |||||
| Weight for for length Z-score ≤ −3 | Yes | No | |||
| Median | IQR | Median | IQR | p value | |
| Mother (N = 471) | |||||
| 25(OH)D ng/mL | 35.75 | 14.84 | 37.55 | 16 | p = 0.282 |
| 24,25(OH)2D3 | 1.7 | 1.25 | 1.75 | 1.3 | p = 0.641 |
| 3-epi-25(OH)D3 ng/mL | 1.42 | 0.87 | 1.28 | 0.65 | p = 0.358 |
| 3-epi-25(OH)D3 % | 3.76 | 2.01 | 3.58 | 1.31 | p = 0.044 |
| Cord (N = 470) | |||||
| 25(OH)D ng/mL | 24.53 | 11.69 | 23.58 | 9.93 | p = 0.795 |
| 24,25(OH)2D3 | 1.11 | 0.79 | 1.01 | 0.71 | p = 0.79 |
| 3-epi-25(OH)D3 ng/mL | 1.44 | 0.86 | 1.3 | 0.76 | p = 0.192 |
| 3-epi-25(OH)D3 % | 6.25 | 2.21 | 5.64 | 1.97 | p = 0.022 |
| Maternal newborn vitamin D and metabolites status vs. employment status | |||||
| Employment status | Yes | No | |||
| Median | IQR | Median | IQR | p value | |
| Mother (N = 521) | |||||
| 25(OH)D ng/mL | 37.65 | 15.47 | 34.97 | 17.71 | p = 0.041 |
| 24,25(OH)2D3 | 1.74 | 1.18 | 1.74 | 1.42 | p = 0.122 |
| 3-epi-25(OH)D3 ng/mL | 1.24 | 0.72 | 1.19 | 0.77 | p = 0.007 |
| 3-epi-25(OH)D3 % | 3.61 | 1.25 | 3.57 | 1.67 | p = 0.2 |
| Cord (N = 526) | |||||
| 25(OH)D ng/mL | 24.56 | 10.29 | 23.04 | 9.84 | p = 0.01 |
| 24,25(OH)2D3 | 24.56 | 0.72 | 0.98 | 0.75 | p = 0.03 |
| 3-epi-25(OH)D3 ng/mL | 1.37 | 0.76 | 1.26 | 0.88 | p = 0.005 |
| 3-epi-25(OH)D3 % | 5.78 | 2.13 | 5.66 | 2.21 | p = 0.394 |
| Maternal newborn vitamin D and metabolites status vs. education leve | |||||
| Education level | Grade 1-12 | Tertiary | |||
| Median | IQR | Median | IQR | p value | |
| Mother (N = 517) | |||||
| 25(OH)D ng/mL | 38.89 | 14.1 | 35.66 | 17.69 | p = 0.07 |
| 24,25(OH)2D3 | 1.84 | 1.03 | 1.67 | 1.36 | p = 0.092 |
| 3-epi-25(OH)D3 ng/mL | 1.4 | 0.68 | 1.21 | 0.73 | p < 0.0001 |
| 3-epi-25(OH)D3 % | 3.76 | 1.52 | 3.5 | 1.24 | p < 0.0001 |
| Cord (N = 518) | |||||
| 25(OH)D ng/mL | 24.37 | 9.83 | 23.41 | 10.52 | p = 0.332 |
| 24,25(OH)2D3 | 1.05 | 0.67 | 1 | 0.82 | p = 0.252 |
| 3-epi-25(OH)D3 ng/mL | 1.41 | 0.76 | 1.26 | 0.79 | p = 0.001 |
| 3-epi-25(OH)D3 % | 6.29 | 2.19 | 5.39 | 1.99 | p < 0.0001 |
| Maternal newborn vitamin D and metabolites status vs. mode of delivery | |||||
| Mode of delivery | Vaginal | C-section | |||
| Median | IQR | Median | IQR | p value | |
| Mother (N = 525) | |||||
| 25(OH)D ng/mL | 37.01 | 15.31 | 36.44 | 18.11 | p = 0.675 |
| 24,25(OH)2D3 | 1.77 | 1.26 | 1.59 | 1.27 | p = 0.249 |
| 3-epi-25(OH)D3 ng/mL | 1.32 | 0.69 | 1.16 | 0.7 | p = 0.013 |
| 3-epi-25(OH)D3 % | 3.68 | 1.46 | 3.45 | 1.07 | p = 0.012 |
| Cord (N = 526) | |||||
| 25(OH)D ng/mL | 23.44 | 10.47 | 24.42 | 8.83 | p = 0.717 |
| 24,25(OH)2D3 | 1.03 | 36 | 1.02 | 0.67 | p = 0.629 |
| 3-epi-25(OH)D3 ng/mL | 1.33 | 0.8 | 1.27 | 0.8 | p = 0.385 |
| 3-epi-25(OH)D3 % | 5.77 | 2.19 | 5.63 | 1.97 | p = 0.413 |
| Measurements | Mother | Newborn | r | p Value |
|---|---|---|---|---|
| N = 526 | N = 526 | |||
| 25(OH)D2, ng/mL | 2.64 ± 1.46 | 1.3 ± 0.57 | 0.91 | <0.0001 |
| 25(OH)D3, ng/mL | 34.08 ± 11.6 | 22.28 ± 8.03 | 0.7 | <0.0001 |
| 25(OH)D, ng/mL | 36.72 ± 11.6 | 24.52 ± 8.14 | 0.7 | <0.0001 |
| 3-epi-25(OH)D3, ng/mL | 1.33 ± 0.57 | 1.39 ± 0.59 | 0.84 | <0.0001 |
| 3-epi-25(OH)D3 % | 3.8 ± 1.14 | 5.9 ± 1.6 | 0.66 | <0.0001 |
| 24,25(OH)2D3 | 1.77 ± 0.90 | 1.09 ± 0.57 | 0.81 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delair, S.; Anderson-Berry, A.; Olateju, E.; Akaba, G.; Medugu, N.; Lyden, E.; Kaufmann, M.; Jones, G.; Anigilaje, E.; Thairu, Y.; et al. Vitamin D Metabolites in Mother–Infant Dyads and Associated Clinical Outcomes in a Population of Nigerian Women. Nutrients 2024, 16, 1857. https://doi.org/10.3390/nu16121857
Delair S, Anderson-Berry A, Olateju E, Akaba G, Medugu N, Lyden E, Kaufmann M, Jones G, Anigilaje E, Thairu Y, et al. Vitamin D Metabolites in Mother–Infant Dyads and Associated Clinical Outcomes in a Population of Nigerian Women. Nutrients. 2024; 16(12):1857. https://doi.org/10.3390/nu16121857
Chicago/Turabian StyleDelair, Shirley, Ann Anderson-Berry, Eyinade Olateju, Godwin Akaba, Nubwa Medugu, Elizabeth Lyden, Martin Kaufmann, Glenville Jones, Emmanuel Anigilaje, Yunusa Thairu, and et al. 2024. "Vitamin D Metabolites in Mother–Infant Dyads and Associated Clinical Outcomes in a Population of Nigerian Women" Nutrients 16, no. 12: 1857. https://doi.org/10.3390/nu16121857
APA StyleDelair, S., Anderson-Berry, A., Olateju, E., Akaba, G., Medugu, N., Lyden, E., Kaufmann, M., Jones, G., Anigilaje, E., Thairu, Y., Kocmich, N., Ajose, T., Olanipekun, G., Rezac-Elgohary, A., Obaro, S., & Hanson, C. (2024). Vitamin D Metabolites in Mother–Infant Dyads and Associated Clinical Outcomes in a Population of Nigerian Women. Nutrients, 16(12), 1857. https://doi.org/10.3390/nu16121857






