Dietary Phosphorus Levels Influence Protein-Derived Uremic Toxin Production in Nephrectomized Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Analytical Methods
2.2.1. Blood Urea Nitrogen (BUN)
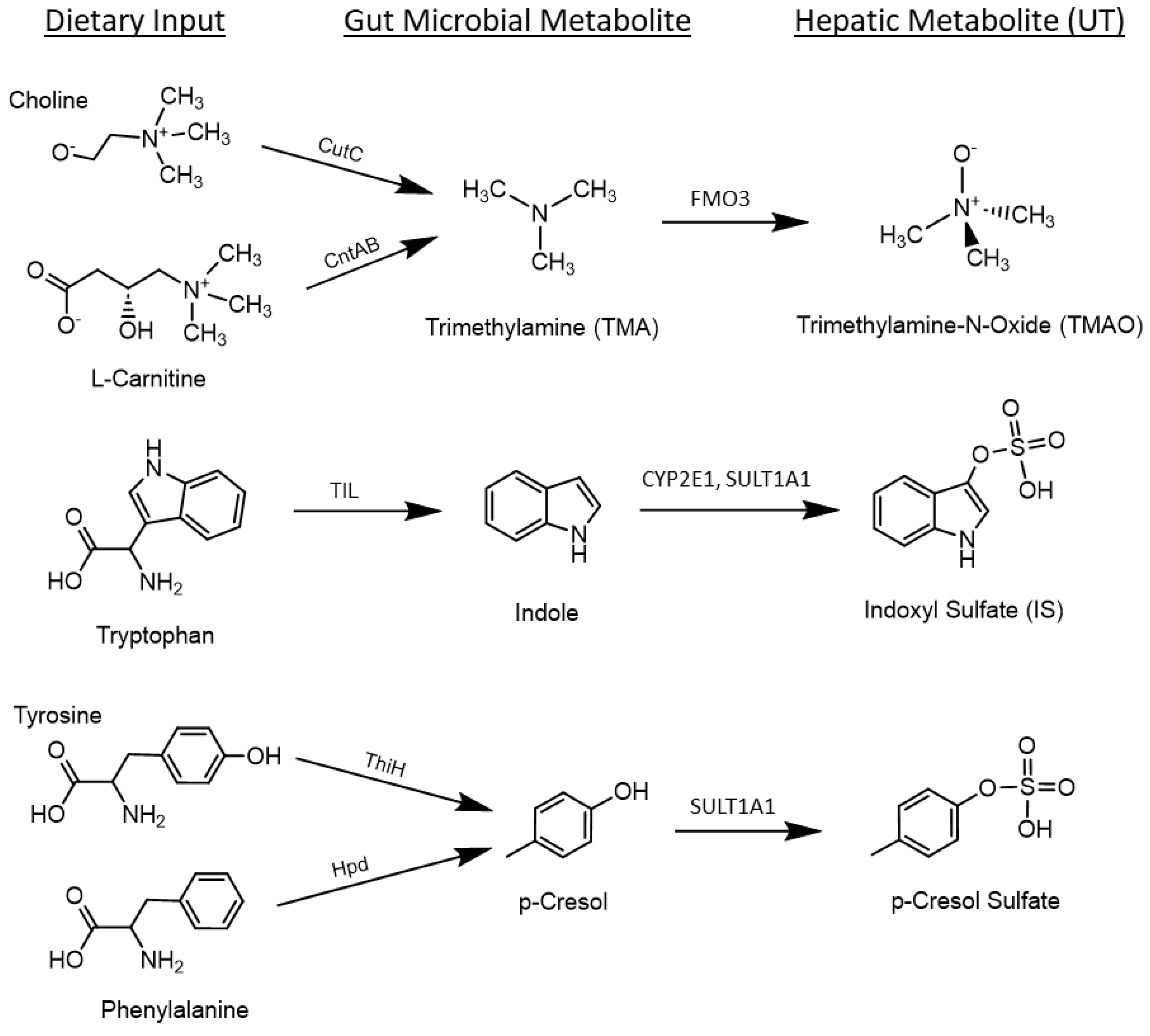
2.2.2. Uremic Toxins (UT)
2.3. Statistics
3. Results
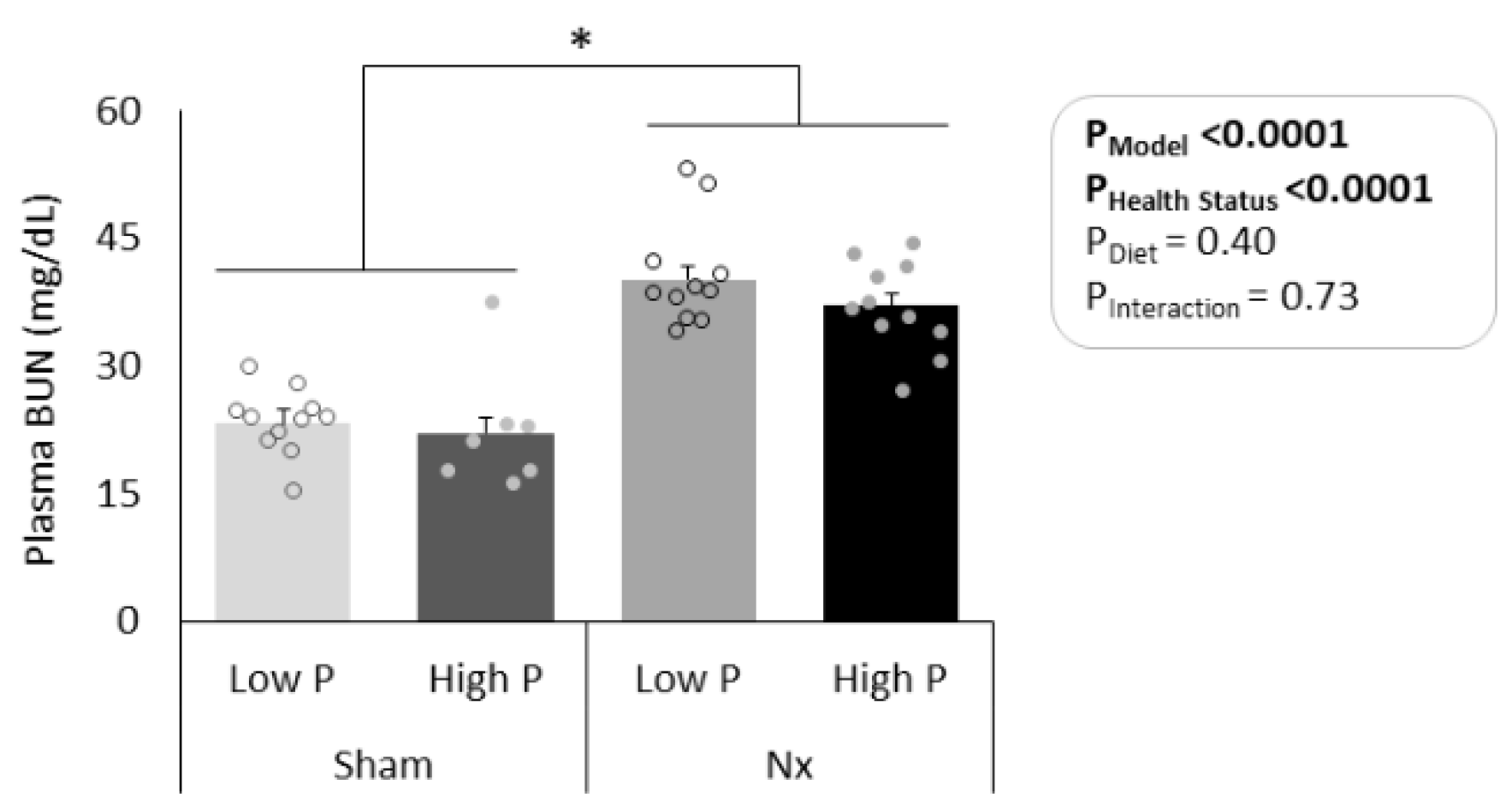
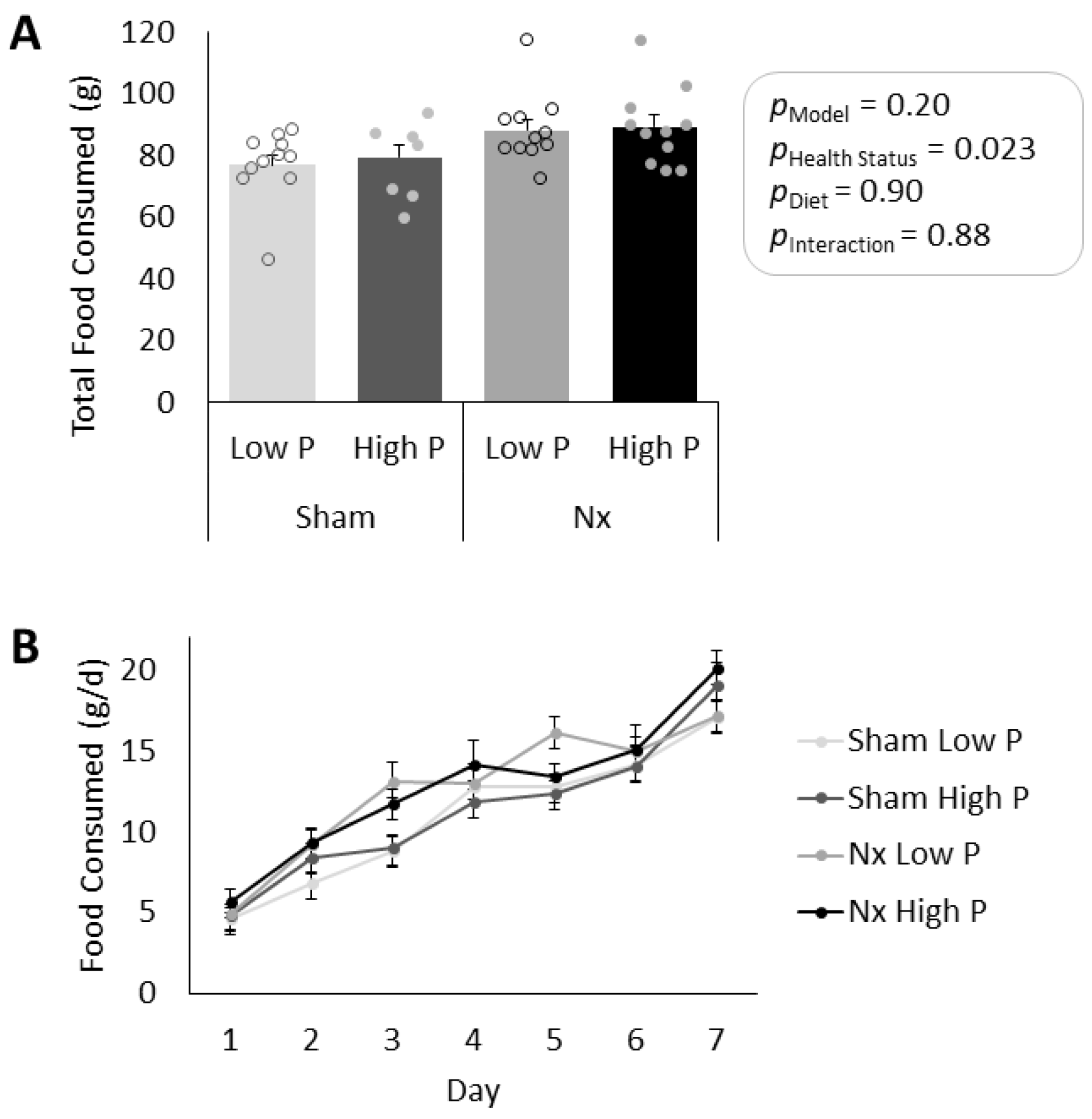
3.1. Model Verification and Food Consumption
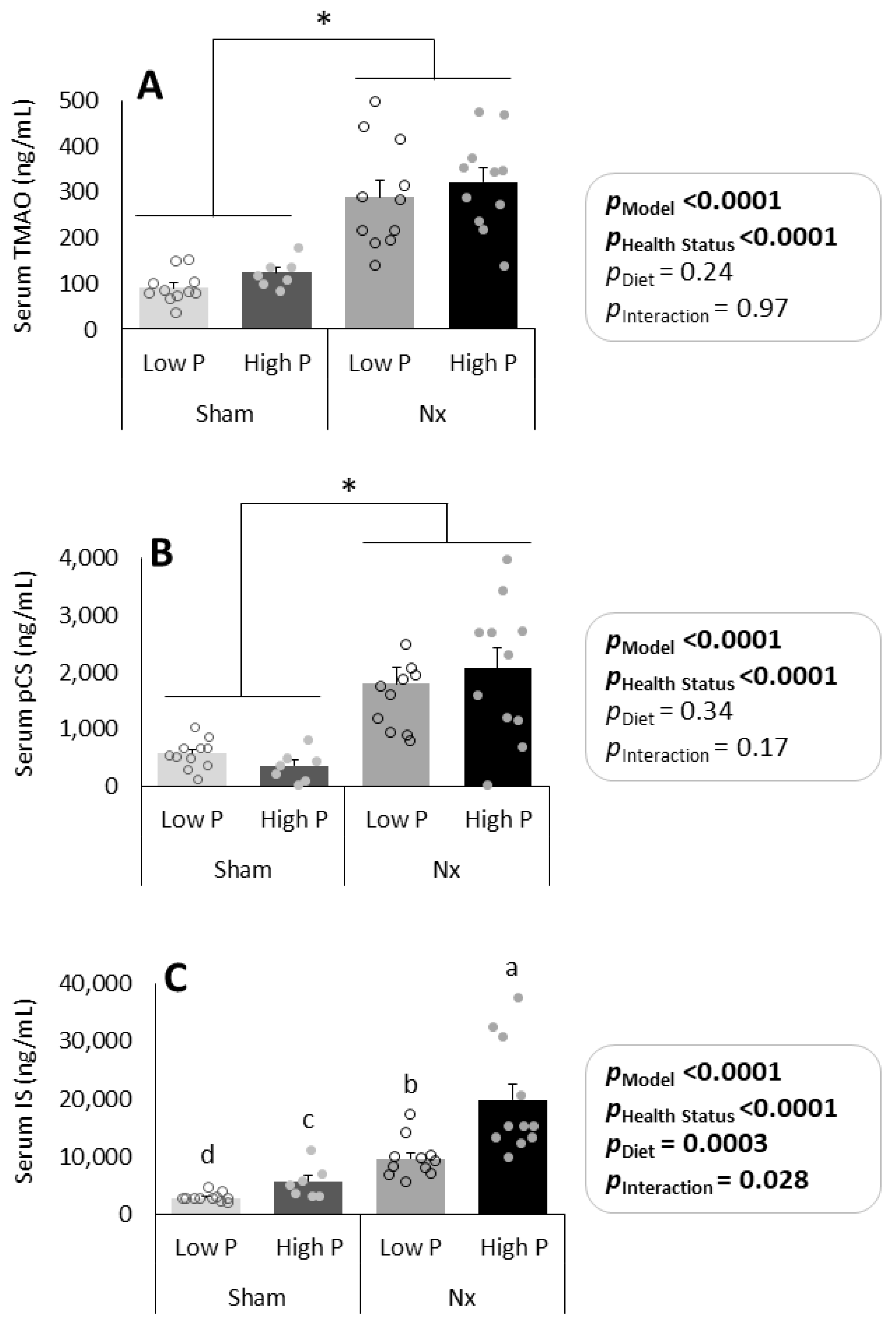
3.2. Effects of Dietary Phosphorus Intake and CKD on Serum UT
3.3. Effects of a Change from Baseline Diet Macronutrient Composition and Source on Serum UT
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chronic Kidney Disease in the United States, 2021. Chronic Kidney Disease Initiative. Available online: https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html (accessed on 2 May 2024).
- Molina, P.; Gavela, E.; Vizcaino, B.; Huarte, E.; Carrero, J.J. Optimizing diet to slow CKD progression. Front. Med. 2021, 8, 654250. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.J.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Wiese, G.N.; Biruete, A.; Moorthi, R.N.; Moe, S.M.; Lindemann, S.R.; Hill Gallant, K.M. Plant-based diets, the gut microbiota, and trimethylamine N-oxide production in chronic kidney disease: Therapeutic potential and methodological considerations. J. Ren. Nutr. 2021, 31, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Zhao, Y.Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 2016, 31, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. CKD impairs barrier function and alters microbial flora of the intestine: A major link to inflammation and uremic toxicity. Curr. Opin. Nephrol. Hypertens. 2012, 21, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.T.; Singh, P.; Nigam, S.K. Gut-derived uremic toxin handling in vivo requires OAT-mediated tubular secretion in chronic kidney disease. JCI Insight 2020, 5, e133817. [Google Scholar] [CrossRef] [PubMed]
- Graboski, A.L.; Redinbo, M.R. Gut-derived protein-bound uremic toxins. Toxins 2020, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Armani, R.G.; Ramezani, A.; Yasir, A.; Sharama, S.; Canziani, M.E.F.; Raj, D.S. Gut microbiome in chronic kidney disease. Curr. Hypertens. Rep. 2017, 19, 29. [Google Scholar] [CrossRef]
- Ellis, R.J.; Small, D.M.; Vesey, D.A.; Johnson, D.W.; Francis, R.; Vitetta, L.; Gobe, G.C.; Morais, C. Indoxyl sulphate and kidney disease: Causes, consequences and interventions. Nephrology 2016, 21, 170–177. [Google Scholar] [CrossRef]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic toxins in the progression of chronic kidney disease and cardiovascular disease: Mechanisms and therapeutic targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Overview of uremic toxins. In Uremic Toxins and Organ Failure; Saito, H., Abe, E., Eds.; Springer: Singapore, 2020; pp. 1–15. [Google Scholar]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Simo, C.; Garcia-Canas, V. Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development. Food Funct. 2020, 11, 6745–6776. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Hostetter, T.H. Uremic solutes from colon microbes. Kidney Int. 2012, 81, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Favero, C.; Carriazo, S.; Cuarental, L.; Fernandez-Prado, R.; Gomá-Garcés, E.; Perez-Gomez, M.V.; Ortiz, A.; Fernandez-Fernandez, B.; Sanchez-Niño, M.D. Phosphate, microbiota and CKD. Nutrients 2021, 13, 1273. [Google Scholar] [CrossRef]
- Biruete, A.; Hill Gallant, K.M.; Lindemann, S.R.; Wiese, G.N.; Chen, N.X.; Moe, S.M. Phosphate binders and nonphosphate effects in the gastrointestinal tract. J. Ren. Nutr. 2020, 30, 4–10. [Google Scholar] [CrossRef]
- Ye, G.; Yang, W.; Bi, Z.; Huang, L.; Liu, F. Effects of a high-phosphorus diet on the gut microbiota in CKD rats. Ren. Fail. 2021, 43, 1577–1587. [Google Scholar] [CrossRef]
- Burstad, K.M.; Cladis, D.P.; Vorland, C.J.; Wastney, M.E.; Biruete, A.; Dominguez, J.M.; D O, K.; Chen, N.X.; Moe, S.M.; Gallant, K.M.H. Acute high diestary phosphorus following low phosphorus diet acclimation does not enhance intestinal fractional phosphorus absorption in nephrectomized male rats. JBMR Plus 2022, e10698. [Google Scholar] [CrossRef]
- Giral, H.; Caldas, Y.; Sutherland, E.; Wilson, P.; Breusegem, S.; Barry, N.; Blaine, J.; Jiang, T.; Wang, X.X.; Levi, M. Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am. J. Physiol. Ren. Physiol. 2009, 297, F1466–F1475. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frolich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Hill Gallant, K.M. Effects of Low and High Dietary Phosphorus and Acute High Dietary Phosphorus on Phosphorus Absorption in Nephrectomized Male Rats. 2020. Available online: https://www.animalstudyregistry.org/asr_web/index.action (accessed on 4 June 2024).
- Marks, J.; Churchill, L.J.; Srai, S.K.; Biber, J.; Murer, H.; Jaeger, P.; Debnam, E.; Unwin, R. Intestinal phosphate absorption in a model of chronic renal failure. Kidney Int. 2007, 72, 166–173. [Google Scholar] [CrossRef]
- Yu, Y.H.; Kim, S.W.; Park, D.K.; Song, H.Y.; Kim, D.S.; Gil, H.W. Altered emotional phenotypes in chronic kidney disease following 5/6 nephrectomy. Brain Sci. 2021, 11, 882. [Google Scholar] [CrossRef]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.B.; Nolin, T.D.; et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. 2016, 27, 305–313. [Google Scholar] [CrossRef]
- Lin, C.N.; Wu, I.W.; Huang, Y.F.; Peng, S.Y.; Huang, Y.C.; Ning, H.C. Measuring serum total and free indoxyl sulfate and p-cresyl sulfate in chronic kidney disease using UPLC-MS/MS. J. Food Drug Anal. 2019, 27, 502–509. [Google Scholar] [CrossRef]
- Cotterill, O.J.; Glauert, J.; Froning, G.W. Nutrient composition of commercially spray-dried egg products. Poult. Sci. 1978, 57, 439–442. [Google Scholar] [CrossRef]
- Rehault-Godbert, S.; Guyot, N.; Nys, Y. The golden egg: Nutritional value, bioactivities, and emerging benefits for human health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Egg, White, Dried. USDA FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/323793/nutrients (accessed on 11 August 2022).
- Lee, J.H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cladis, D.P.; Burstad, K.M.; Biruete, A.; Jannasch, A.H.; Cooper, B.R.; Hill Gallant, K.M. Dietary Phosphorus Levels Influence Protein-Derived Uremic Toxin Production in Nephrectomized Male Rats. Nutrients 2024, 16, 1807. https://doi.org/10.3390/nu16121807
Cladis DP, Burstad KM, Biruete A, Jannasch AH, Cooper BR, Hill Gallant KM. Dietary Phosphorus Levels Influence Protein-Derived Uremic Toxin Production in Nephrectomized Male Rats. Nutrients. 2024; 16(12):1807. https://doi.org/10.3390/nu16121807
Chicago/Turabian StyleCladis, Dennis P., Kendal M. Burstad, Annabel Biruete, Amber H. Jannasch, Bruce R. Cooper, and Kathleen M. Hill Gallant. 2024. "Dietary Phosphorus Levels Influence Protein-Derived Uremic Toxin Production in Nephrectomized Male Rats" Nutrients 16, no. 12: 1807. https://doi.org/10.3390/nu16121807
APA StyleCladis, D. P., Burstad, K. M., Biruete, A., Jannasch, A. H., Cooper, B. R., & Hill Gallant, K. M. (2024). Dietary Phosphorus Levels Influence Protein-Derived Uremic Toxin Production in Nephrectomized Male Rats. Nutrients, 16(12), 1807. https://doi.org/10.3390/nu16121807





