Design and Validation of MEDOC, a Tool to Assess the Combined Adherence to Mediterranean and Western Dietary Patterns
Abstract
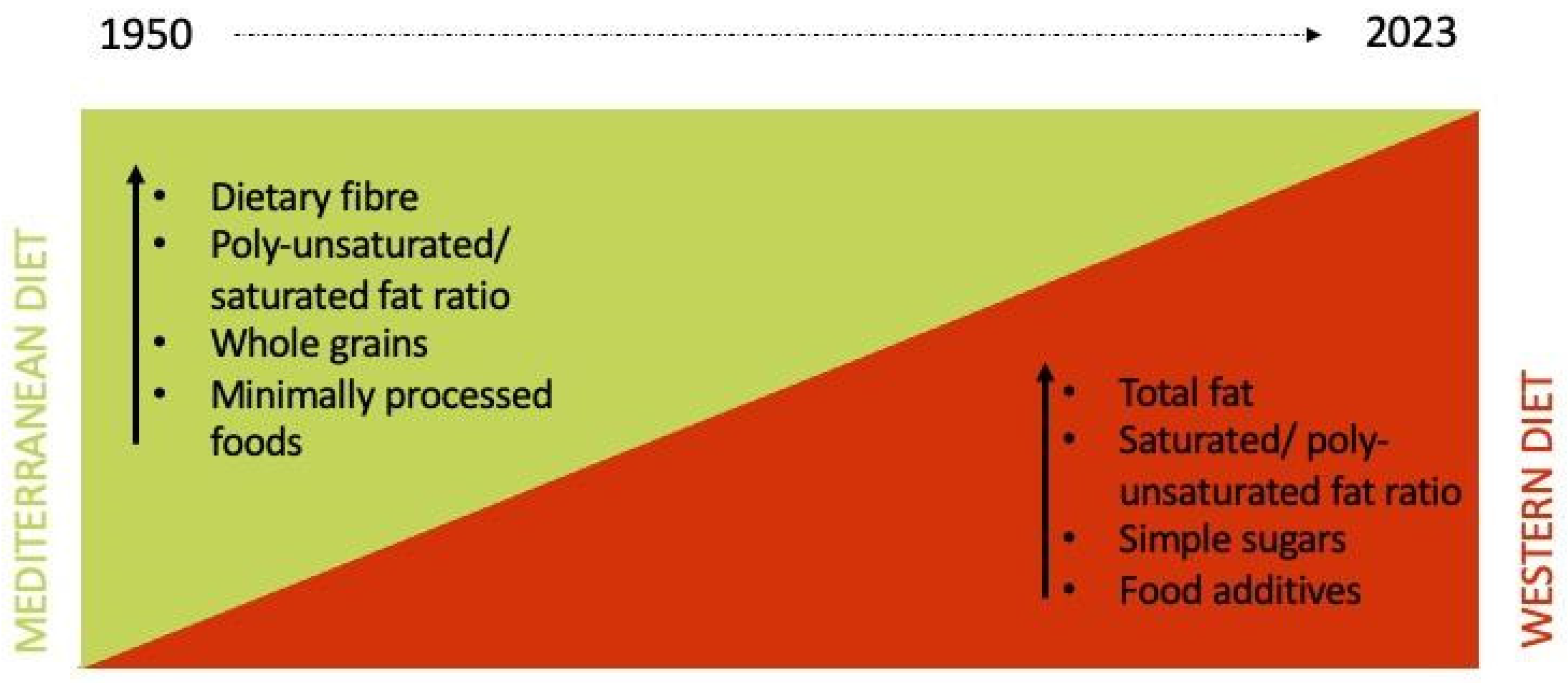
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample
2.3. MEDOC Questionnaire Development
2.4. MEDOC Score
2.5. Reproducibility and Validity Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Sample
3.2. MEDOC FFQ Showed Good Test–Retest Reliability
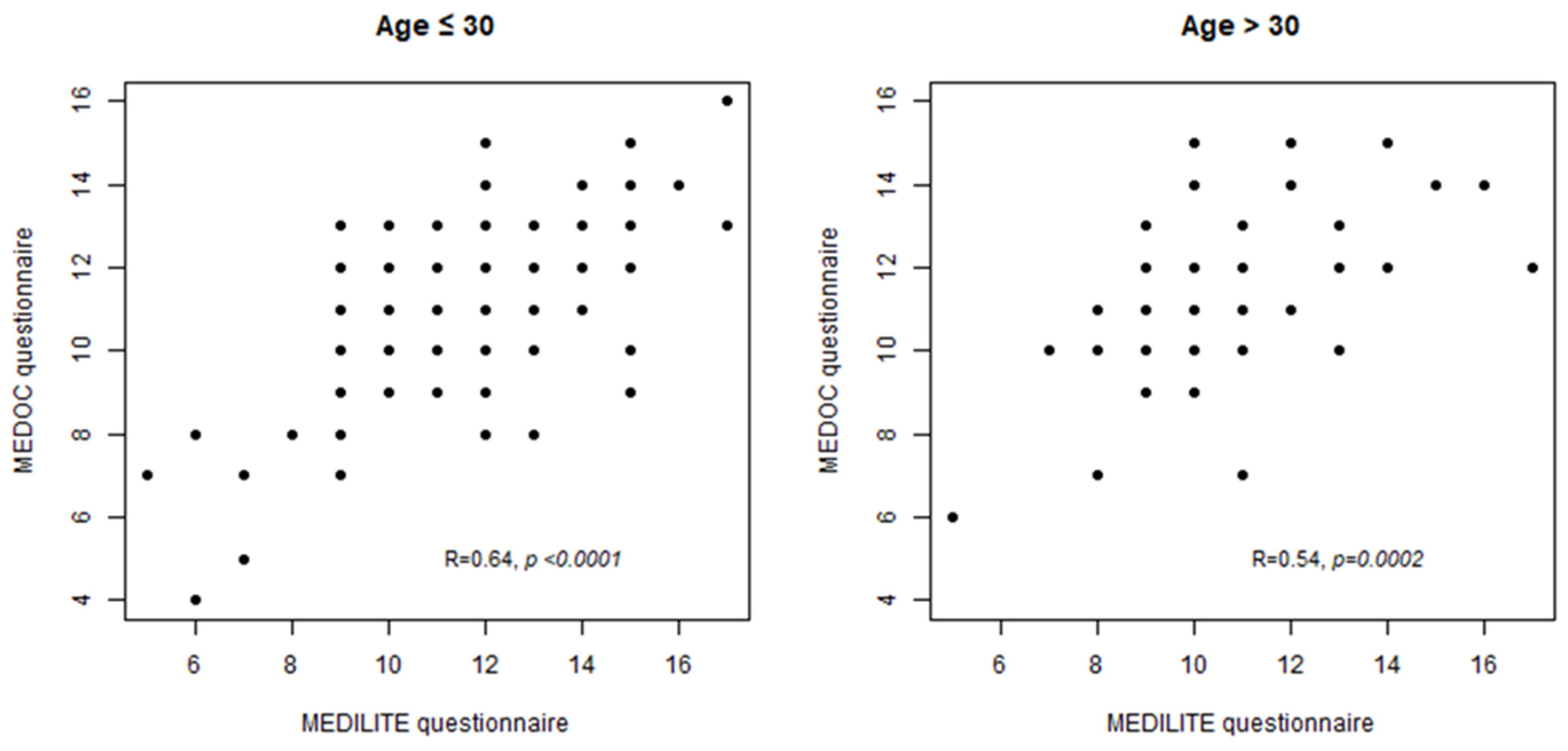
3.3. MEDILITE Scores Calculated on MEDOC and MEDILITE FFQ Show a Good Correlation
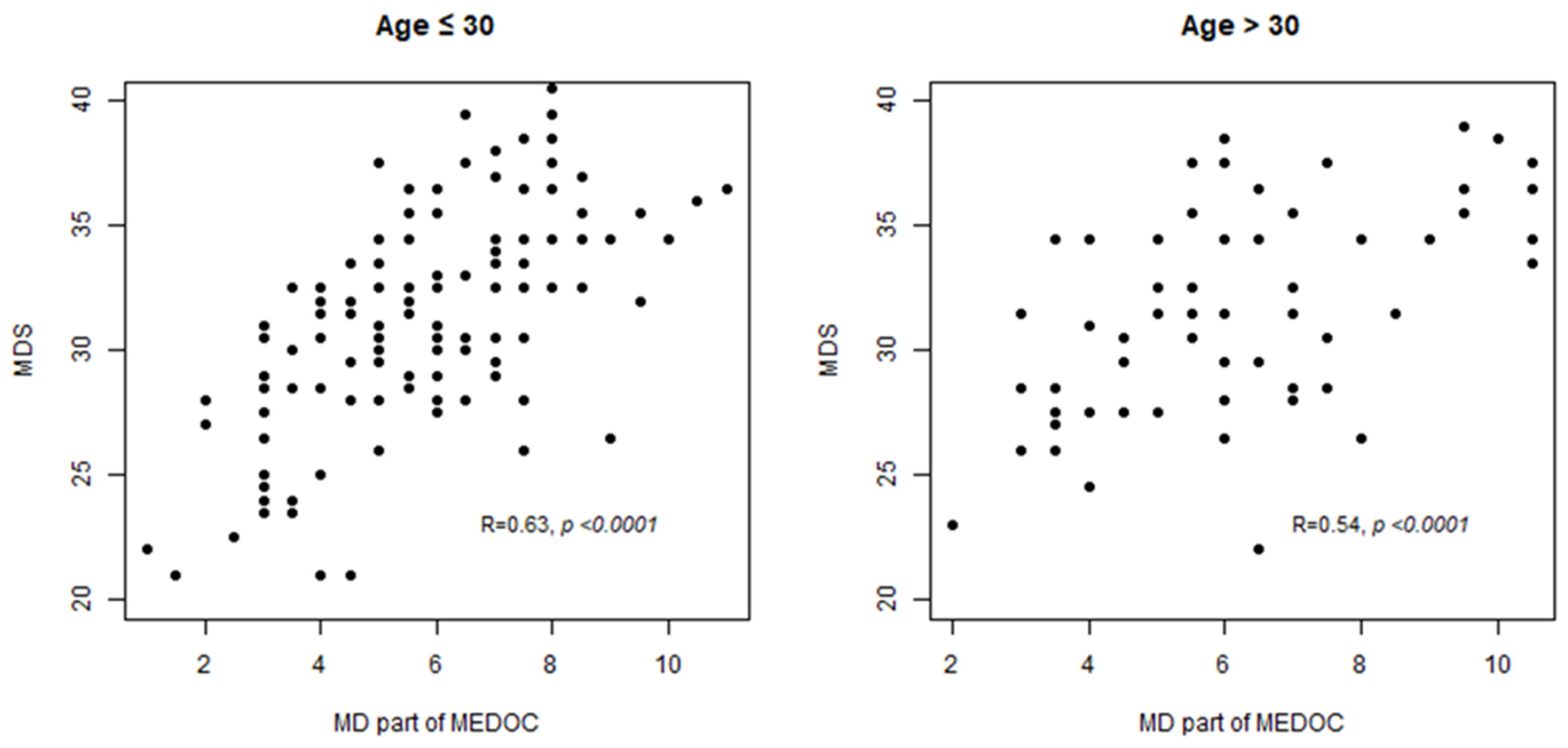
3.4. MD Score Estimated with MEDOC Correlates with MDS Score Used as Reference
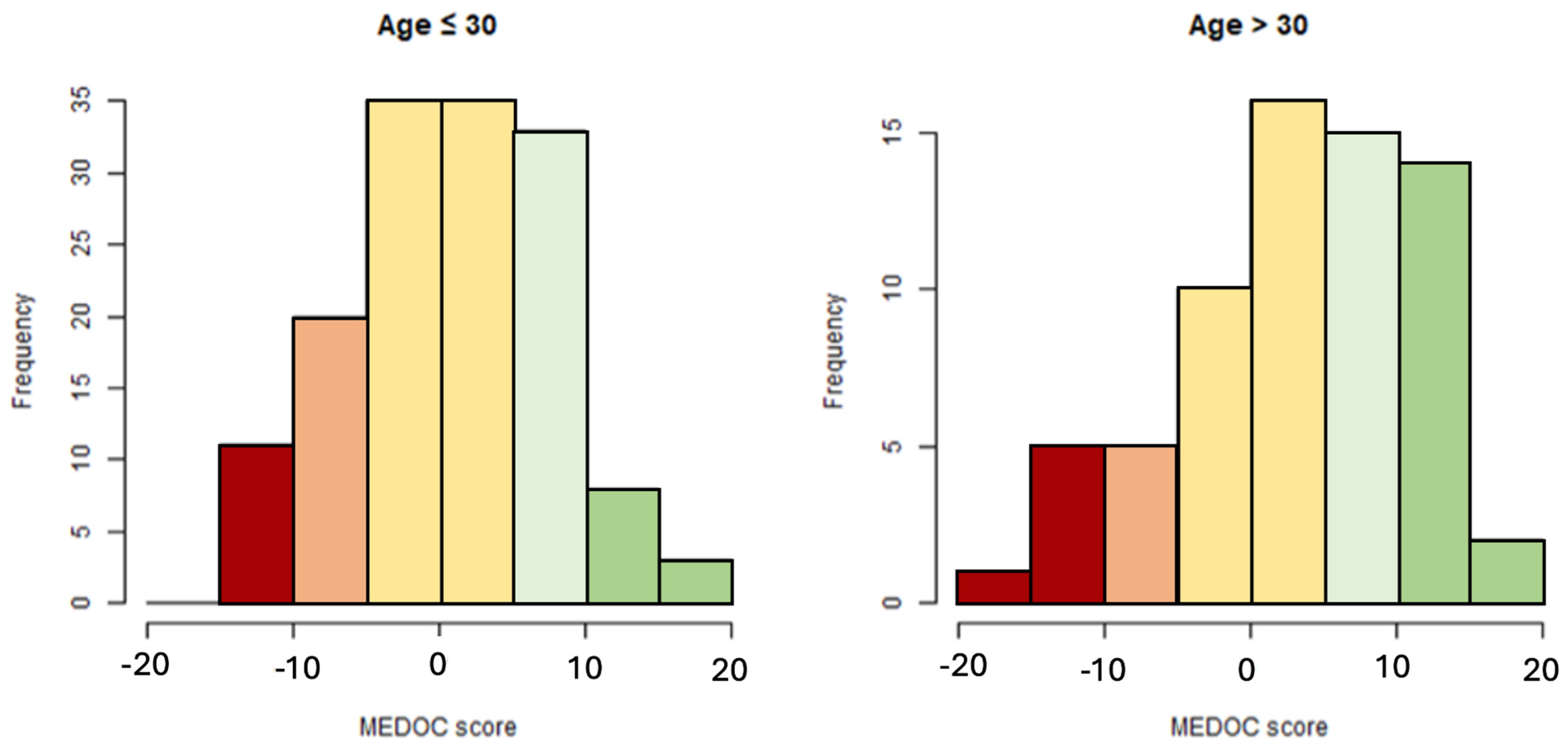
3.5. Distribution of MEDOC Score Calculated on Study Sample
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzucca, C.B.; Raineri, D.; Cappellano, G.; Chiocchetti, A. How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done. Nutrients 2021, 13, 3956. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Ceriello, A.; Giugliano, D. Prevention and Control of Type 2 Diabetes by Mediterranean Diet: A Systematic Review. Diabetes Res. Clin. Pract. 2010, 89, 97–102. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The Obesity Transition: Stages of the Global Epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M. Nutrition Transition, Diet Change, and Its Implications. Encycl. Human Nutr. 2012, 3–4, 320–328. [Google Scholar] [CrossRef]
- Grigg, D. The Nutritional Transition in Western Europe. J. Hist. Geogr. 1995, 21, 247. [Google Scholar] [CrossRef]
- Scali, J.; Richard, A.; Gerber, M. Diet Profiles in a Population Sample from Mediterranean Southern France. Public Health Nutr. 2001, 4, 173–182. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Pyramid Today. Science and Cultural Updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today 2017, 52, 208. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary Patterns: A Mediterranean Diet Score and Its Relation to Clinical and Biological Markers of Cardiovascular Disease Risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- De Matteis, C.; Crudele, L.; Battaglia, S.; Loconte, T.; Rotondo, A.; Ferrulli, R.; Gadaleta, R.M.; Piazzolla, G.; Suppressa, P.; Sabbà, C.; et al. Identification of a Novel Score for Adherence to the Mediterranean Diet That Is Inversely Associated with Visceral Adiposity and Cardiovascular Risk: The Chrono Med Diet Score (CMDS). Nutrients 2023, 15, 1910. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, Youth and the Mediterranean Diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in Children and Adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a Literature-Based Adherence Score to Mediterranean Diet: The MEDI-LITE Score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, Validation and Utilisation of Food-Frequency Questionnaires—A Review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Tomaino, L.; Dernini, S.; Berry, E.M.; Lairon, D.; de la Cruz, J.N.; Bach-Faig, A.; Donini, L.M.; Medina, F.X.; Belahsen, R.; et al. Updating the Mediterranean Diet Pyramid towards Sustainability: Focus on Environmental Concerns. Int. J. Environ. Res. Public Health 2020, 17, 8758. [Google Scholar] [CrossRef]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Slavatore, M.; Fornaro, M.; Bhugra, D. Mediterranean Diet and Its Benefits on Health and Mental Health: A Literature Review. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 156–164. [Google Scholar] [CrossRef]
- Keys, A.; Keys, M. How to Eat Well and Stay Well the Mediterranean Way; Doubleday & Co.: New York, NY, USA, 1975; 488p. [Google Scholar]
- Schmidhuber, J.; Shetty, P. The Nutrition Transition to 2030. Why Developing Countries Are Likely to Bear the Major Burden. Acta Agric. Scand. Sect. C 2005, 2, 150–166. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sánchez-Villegas, A. The Emerging Role of Mediterranean Diets in Cardiovascular Epidemiology: Monounsaturated Fats, Olive Oil, Red Wine or the Whole Pattern? Eur. J. Epidemiol. 2004, 19, 9–13. [Google Scholar] [CrossRef]
- Odermatt, A. The Western-Style Diet: A Major Risk Factor for Impaired Kidney Function and Chronic Kidney Disease. Am. J. Physiol. Ren. Physiol. 2011, 301, F919–F931. [Google Scholar] [CrossRef]
- Oikonomou, E.; Psaltopoulou, T.; Georgiopoulos, G.; Siasos, G.; Kokkou, E.; Antonopoulos, A.; Vogiatzi, G.; Tsalamandris, S.; Gennimata, V.; Papanikolaou, A.; et al. Western Dietary Pattern Is Associated with Severe Coronary Artery Disease. Angiology 2018, 69, 339–346. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Villani, A.; Sultana, J.; Doecke, J.; Mantzioris, E. Differences in the Interpretation of a Modernized Mediterranean Diet Prescribed in Intervention Studies for the Management of Type 2 Diabetes: How Closely Does This Align with a Traditional Mediterranean Diet? Eur. J. Nutr. 2019, 58, 1369–1380. [Google Scholar] [CrossRef]
- Radd-Vagenas, S.; Kouris-Blazos, A.; Singh, M.F.; Flood, V.M. Evolution of Mediterranean Diets and Cuisine: Concepts and Definitions. Asia Pac. J. Clin. Nutr. 2017, 26, 749–763. [Google Scholar] [CrossRef]
- Cohen, D.A.; Story, M. Mitigating the Health Risks of Dining Out: The Need for Standardized Portion Sizes in Restaurants. Am. J. Public Health 2014, 104, 586. [Google Scholar] [CrossRef]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef]
- Maldonado-Pereira, L.; Barnaba, C.; de Los Campos, G.; Medina-Meza, I.G. Evaluation of the Nutritional Quality of Ultra-Processed Foods (Ready to Eat + Fast Food): Fatty Acids, Sugar, and Sodium. J. Food Sci. 2022, 87, 3659–3676. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Y.; Li, F.; Shao, Y.; Sun, Z.; Zhong, C.; Fan, P.; Li, Z.; Zhang, M.; Li, X.; et al. Development and Validation of a Photographic Atlas of Food Portions for Accurate Quantification of Dietary Intakes in China. J. Human Nutr. Diet. 2021, 34, 604–615. [Google Scholar] [CrossRef]
- Faggiano, F.; Vineis, P.; Cravanzola, D.; Pisani, P.; Xompero, G.; Riboli, E.; Kaaks, R. Validation of a Method for the Estimation of Food Portion Size. Epidemiology 1992, 3, 379–382. [Google Scholar] [CrossRef]
- Hunter, D.J.; Sampson, L.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.; Willett, W.C. Variability in Portion Sizes of Commonly Consumed Foods among a Population of Women in the United States. Am. J. Epidemiol. 1988, 127, 1240–1249. [Google Scholar] [CrossRef]

| Food Items | Cut-Off | Points | |
|---|---|---|---|
| Fruits/Vegetables | ≥5 servings/day | 1.5 | Seasonal |
| ≥5 servings/day | 1 | Not seasonal | |
| <5 servings/day | −1 | Seasonal | |
| <5 servings/day | −1.5 | Not seasonal | |
| Cereals | 3–6 servings/day | 1.5 | Non-refined |
| 3–6 servings/day | 1 | Refined | |
| <3 o >6 servings/day | −1 | Non-refined | |
| <3 o >6 servings/day | −1.5 | Refined | |
| Olive oil | Regular/Frequent consumption | 1 | |
| Occasional consumption | −1 | ||
| Dairy | 2 servings/day | 1.5 | Skimmed/partially skimmed |
| 2 servings/day | 1 | Full fat | |
| <2 o >2 servings/day | −1 | Skimmed/partially skimmed | |
| <2 o >2 servings/day | −1.5 | Full fat | |
| Eggs | 2–4 servings/week | 1 | |
| <2 o >4 servings/week | −1 | ||
| Legumes | ≥2 servings/week | 1 | |
| <2 servings/week | −1 | ||
| Fish | ≥2 servings/week | 1.5 | Bought by local producer |
| ≥2 servings/week | 1 | Bought at supermarket | |
| <2 servings/week | −1 | Bought by local producer | |
| <2 servings/week | −1.5 | Bought at supermarket | |
| White meat | 2 servings/week | 1.5 | Bought at supermarket |
| 2 servings/week | 1 | Bought at supermarket | |
| <2 o >2 servings/week | −1 | Brought at supermarke | |
| <2 o >2 servings/week | −1.5 | Bought at supermarket | |
| Red meat | <2 servings/week | 1.5 | Bought by local producer |
| <2 servings/week | 1 | Bought at supermarket | |
| >1 servings/week | −1 | Bought by local producer | |
| >1 servings/week | −1.5 | Bought at supermarket | |
| Processed meat | ≤1 servings/week | 1 | |
| >1 servings/week | −1 | ||
| Sweets/cakes/pastries | ≤2 servings/week | 1.5 | Homemade |
| ≤2 servings/week | 1 | Packed | |
| >2 servings/week | −1 | Homemade | |
| >2 servings/week | −1.5 | Packed | |
| Breakfast with croissant and cappuccino | No breakfast or >1 times week | −1 | |
| 0–1 times week | 1 | ||
| Time dedicated to meals | <30 min | −1 | |
| ≥30 min | 1 | ||
| Nibbling | Yes | −1 | |
| No | 1 | ||
| Adding spices instead of salt | No | −1 | |
| Yes | 1 | ||
| Dining out/takeaway | ≥4 times/week | −1 | |
| <4 times/week | 1 | ||
| Bread | Weekly frequency of loaf bread > fresh bread | −1 | |
| Weekly frequency of loaf bread ≤ fresh bread | 1 | ||
| Bread substitutes | >1 times/week | −1 | |
| ≤1 times/week | 1 | ||
| Fast food | >0 times/week | −1 | |
| 0 times/week | 1 | ||
| Salted snacks | >0 times/week | −1 | |
| 0 times/week | 1 | ||
| Sodas | >0 times/week | −1 | |
| 0 times/week | 1 | ||
| Ready-to-eat meals/Frozen food | >1 times/week | −1 | |
| ≤1 times/week | 1 |
| Age ≤ 30 N = 145 | Age > 30 N = 68 | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (years) | 22.91 (2.25) | 56.33 (17.43) |
| Height (cm) | 168.85 (7.78) | 166.67 (9.16) |
| Weight (Kg) | 62.36 (11.50) | 64.72 (11.78) |
| BMI (Kg/m2) | 21.82 (3.35) | 23.18 (3.23) |
| N (%) | N (%) | |
| Sex | ||
| Females | 109 (75.17) | 44 (64.71) |
| Males | 36 (24.83) | 24 (35.29) |
| Education level | ||
| High school or lower | 99 (68.28) | 31 (45.59) |
| Bachelor degree | 32 (22.07) | 18 (26.47) |
| Master degree | 10 (6.90) | 8 (11.76) |
| Post-lauream | 4 (2.76) | 11 (16.18) |
| Special diet | ||
| No | 125 (87.41) | 54 (85.71) |
| Vegetarian | 6 (4.20) | 1 (1.59) |
| Vegetarian with fish consumption | 3 (2.10) | 3 (4.76) |
| Vegan | 1 (0.70) | 0 (0.00) |
| Others | 8 (5.59) | 5 (7.94) |
| BMI classes | ||
| Underweight (<18.5 kg/m2) | 20 (13.89) | 2 (3.13) |
| Healthy weight (18.5–24.9 kg/m2) | 104 (72.22) | 42 (65.63) |
| Overweight (25–29.9 kg/m2) | 17 (11.91) | 20 (31.25) |
| Obese (≥30 kg/m2) | 3 (2.08) | 0 (0.00) |
| (a) | Age ≤ 30 Years N = 145 | ||||||
|---|---|---|---|---|---|---|---|
| T0 Weekly Frequency | T1 Weekly Frequency | ||||||
| Food Items | N | Mean (SD) | Median (Q1–Q3) | Mean (SD) | Median (Q1–Q3) | R (95%CI) | ICC |
| Vegetables and fruits | |||||||
| Medium/large sized fruit | 145 | 7.17 (4.77) | 7 (4;10) | 6.08 (4.12) | 5 (3;7) | 0.82 (0.75;0.86) | 0.79 |
| Small sized fruit | 145 | 4.14 (3.2) | 4 (2;6) | 3.49 (3.07) | 3 (1;5) | 0.61 (0.50;0.71) | 0.60 |
| Dried fruit | 145 | 2.71 (2.55) | 2 (1;4) | 2.64 (2.47) | 2 (1;4) | 0.68 (0.58;0.76) | 0.68 |
| Cooked vegetables | 145 | 7.53 (4.26) | 7 (5;10) | 6.85 (4.33) | 6 (4;10) | 0.69 (0.60;0.77) | 0.68 |
| Raw vegetables | 145 | 4.43 (3.61) | 4 (2;6) | 3.55 (2.98) | 3 (2;5) | 0.64 (0.54;0.73) | 0.61 |
| Salad | 145 | 2.73 (2.79) | 2 (1;4) | 2.56 (2.59) | 2 (1;3) | 0.85 (0.80;0.89) | 0.85 |
| Potatoes | 145 | 1.56 (1.45) | 1 (1;2) | 1.5 (1.26) | 1 (1;2) | 0.51 (0.38;0.62) | 0.51 |
| French fries | 145 | 0.87 (1.2) | 1 (0;1) | 0.92 (0.98) | 1 (0;1) | 0.60 (0.49;0.70) | 0.59 |
| Cereals | |||||||
| Breakfast cereals | 145 | 2.12 (2.73) | 1 (0;4) | 2.01 (2.66) | 1 (0;3) | 0.78 (0.70;0.83) | 0.78 |
| Loaf bread | 145 | 2.65 (2.98) | 2 (1;3) | 2.35 (2.18) | 2 (1;3) | 0.68 (0.59;0.76) | 0.65 |
| Fresh bread | 145 | 4.32 (3.86) | 4 (1;6) | 3.96 (3.35) | 3 (2;5) | 0.86 (0.81;0.89) | 0.84 |
| Bread substitutes | 144 | 3.32 (3.32) | 3 (1;5) | 2.61 (2.49) | 2 (1;4) | 0.75 (0.67;0.82) | 0.70 |
| Focaccia bread | 145 | 1.79 (1.27) | 2 (1;2) | 1.86 (1.5) | 2 (1;3) | 0.65 (0.55;0.74) | 0.64 |
| Pizza | 145 | 1.06 (0.57) | 1 (1;1) | 1.08 (0.5) | 1 (1;1) | 0.49 (0.36;0.61) | 0.49 |
| Rice and other cereals | 145 | 3.22 (2.41) | 3 (2;4) | 2.86 (2.02) | 2 (1;4) | 0.72 (0.64;0.79) | 0.71 |
| Pasta | 145 | 5.55 (2.91) | 5 (3;7) | 5.41 (2.94) | 5 (3;7) | 0.78 (0.70;0.84) | 0.78 |
| Cookies | 145 | 3.27 (2.64) | 3 (1;5) | 2.92 (2.76) | 3 (1;4) | 0.72 (0.63;0.79) | 0.72 |
| Sweets/cakes/pastries | 145 | 2.9 (3.04) | 2 (1;4) | 2.34 (2.33) | 2 (1;3) | 0.79 (0.72;0.85) | 0.75 |
| Dairy products | |||||||
| Cow milk | 145 | 3.02 (3.36) | 2 (0;7) | 3.03 (3.22) | 2 (0;6) | 0.85 (0.80;0.89) | 0.85 |
| Milk products | 145 | 2.52 (2.67) | 2 (0;4) | 2.39 (2.63) | 2 (0;4) | 0.83 (0.78;0.88) | 0.83 |
| Fresh cheese | 145 | 2.3 (1.81) | 2 (1;3) | 2.02 (1.54) | 2 (1;3) | 0.74 (0.66;0.81) | 0.73 |
| Seasoned cheese | 145 | 1.78 (1.85) | 1 (0;3) | 1.51 (1.56) | 1 (0;2) | 0.77 (0.70;0.83) | 0.75 |
| Meat and Fish | |||||||
| White meat | 145 | 2.94 (2.23) | 2 (1;4) | 2.77 (2.03) | 2 (2;4) | 0.82 (0.75;0.86) | 0.81 |
| Red meat | 145 | 2.18 (1.64) | 2 (1;3) | 2.07 (1.74) | 2 (1;3) | 0.74 (0.65;0.81) | 0.74 |
| Ultra-processed food | 145 | 2.68 (2.38) | 2 (1;4) | 2.34 (1.98) | 2 (1;3) | 0.80 (0.73;0.85) | 0.78 |
| Fresh fish | 145 | 1.31 (1.2) | 1 (0;2) | 1.27 (1.1) | 1 (0;2) | 0.79 (0.73;0.85) | 0.79 |
| Tinned fish | 145 | 1.43 (1.34) | 1 (0;2) | 1.28 (1.22) | 1 (0;2) | 0.77 (0.70;0.83) | 0.77 |
| Eggs | 144 | 1.81 (1.43) | 2 (1;2) | 1.87 (1.76) | 2 (1;2) | 0.58 (0.46;0.68) | 0.57 |
| Legumes | 145 | 2.45 (2.23) | 2 (1;4) | 2.29 (2.22) | 2 (1;3) | 0.79 (0.71;0.84) | 0.78 |
| Drinks | |||||||
| Soda | 145 | 1.37 (2.24) | 1 (0;2) | 1.08 (1.67) | 1 (0;2) | 0.80 (0.73;0.85) | 0.76 |
| Wine | 144 | 0.96 (1.11) | 1 (0;1) | 0.95 (1.17) | 1 (0;1.5) | 0.64 (0.54;0.73) | 0.65 |
| Beer | 144 | 0.6 (1.17) | 0 (0;1) | 0.51 (0.78) | 0 (0;1) | 0.79 (0.72;0.84) | 0.72 |
| Cocktail | 145 | 0.7 (0.72) | 1 (0;1) | 0.73 (0.91) | 1 (0;1) | 0.59 (0.47;0.68) | 0.57 |
| Spirits | 145 | 0.26 (0.67) | 0 (0;0) | 0.18 (0.51) | 0 (0;0) | 0.62 (0.51;0.71) | 0.59 |
| Unhealthy foods | |||||||
| Fast food | 145 | 0.51 (0.71) | 0 (0;1) | 0.48 (0.64) | 0 (0;1) | 0.71 (0.62;0.79) | 0.71 |
| Salted snack | 144 | 1.06 (1.34) | 1 (0;1.5) | 1.06 (1.11) | 1 (0;2) | 0.58 (0.47;0.68) | 0.58 |
| Frozen foods | 144 | 0.94 (1.32) | 0 (0;1) | 0.86 (1.11) | 1 (0;1) | 0.56 (0.43;0.66) | 0.55 |
| Ready-to-eat-meals | 145 | 1.01 (1.54) | 0 (0;2) | 0.89 (1.32) | 0 (0;1) | 0.59 (0.47;0.69) | 0.58 |
| Breakfast with croissant and cappuccino | 123 | 1.29 (1.98) | 1 (0;1) | 1.27 (1.86) | 1 (0;1) | 0.81 (0.73;0.86) | 0.82 |
| (b) | Age > 30 Years N = 68 | ||||||
| T0 Weekly Frequency | T1 Weekly Frequency | ||||||
| Food Items | N | Mean (SD) | Median (Q1–Q3) | Mean (SD) | Median (Q1–Q3) | R (95%CI) | ICC |
| Vegetables and fruits | |||||||
| Medium/large sized fruit | 68 | 8.46 (5.22) | 7 (5;14) | 7.47 (4.65) | 7 (4;11.5) | 0.67 (0.51;0.78) | 0.66 |
| Small sized fruit | 68 | 4.41 (4.6) | 3 (1;6.5) | 3.56 (3.64) | 3 (1;5.5) | 0.64 (0.48;0.76) | 0.62 |
| Dried fruit | 68 | 3.56 (4.1) | 2 (1;6) | 3.21 (3.02) | 3 (0;5.5) | 0.65 (0.48;0.77) | 0.62 |
| Cooked vegetables | 68 | 7.28 (4.25) | 7 (4;10) | 6.71 (3.8) | 6 (4;9) | 0.74 (0.60;0.83) | 0.73 |
| Raw vegetables | 67 | 3.76 (3.02) | 3 (1;6) | 3.28 (2.81) | 3 (2;4) | 0.54 (0.35;0.69) | 0.54 |
| Salad | 68 | 3.76 (2.83) | 4 (2;5) | 3.57 (2.66) | 3 (2;5) | 0.78 (0.66;0.86) | 0.78 |
| Potatoes | 68 | 1.53 (1.23) | 1 (1;2) | 1.59 (1.32) | 1 (1;2) | 0.81 (0.71;0.88) | 0.81 |
| French fries | 68 | 0.49 (0.86) | 0 (0;1) | 0.49 (0.82) | 0 (0;1) | 0.60 (0.42;0.73) | 0.60 |
| Cereals | |||||||
| Breakfast cereals | 68 | 1.32 (2.03) | 0 (0;2) | 1.34 (2.02) | 0 (0;2) | 0.86 (0.78;0.91) | 0.86 |
| Loaf bread | 68 | 2.25 (3.24) | 1 (0;3.5) | 1.93 (2.54) | 1 (0;3) | 0.63 (0.47;0.76) | 0.62 |
| Fresh bread | 68 | 5.15 (4.21) | 5 (1.5;7) | 5.12 (4.57) | 4.5 (1.5;7) | 0.85 (0.77;0.90) | 0.85 |
| Bread substitutes | 68 | 3.76 (3.44) | 3 (1;5.5) | 3 (2.75) | 3 (1;4) | 0.70 (0.55;0.80) | 0.67 |
| Focaccia bread | 68 | 1.06 (1.17) | 1 (0;1) | 0.94 (1.13) | 1 (0;1) | 0.52 (0.32;0.68) | 0.52 |
| Pizza | 67 | 0.9 (0.61) | 1 (1;1) | 1.07 (1) | 1 (1;1) | 0.59 (0.40;0.72) | 0.51 |
| Rice and other cereals | 68 | 2.49 (2.2) | 2 (1;3) | 2.26 (1.66) | 2 (1;3) | 0.59 (0.40;0.72) | 0.56 |
| Pasta | 68 | 4.37 (3.07) | 4 (2;6) | 4.09 (2.39) | 4 (3;5) | 0.76 (0.63;0.84) | 0.73 |
| Cookies | 68 | 3.78 (2.78) | 4 (1;7) | 3.75 (2.69) | 4 (1.5;7) | 0.70 (0.55;0.81) | 0.71 |
| Sweets/cakes/pastries | 68 | 1.49 (2.15) | 1 (0;2) | 1.47 (2.26) | 1 (0;2) | 0.70 (0.55;0.80) | 0.70 |
| Dairy products | |||||||
| Cow milk | 68 | 3.56 (3.6) | 2.5 (0;7) | 3.37 (3.34) | 3 (0;7) | 0.80 (0.69;0.87) | 0.80 |
| Milk products | 68 | 2.62 (2.56) | 2 (0;5) | 2.59 (2.58) | 2 (0;4.5) | 0.81 (0.71;0.88) | 0.81 |
| Fresh cheese | 68 | 2.62 (2.31) | 2 (1;3.5) | 2.32 (1.86) | 2 (1;3) | 0.70 (0.56;0.81) | 0.68 |
| Seasoned cheese | 68 | 2.07 (1.93) | 2 (1;3) | 2.21 (2.05) | 2 (1;3) | 0.75 (0.62;0.84) | 0.75 |
| Meat and Fish | |||||||
| White meat | 67 | 2.46 (1.92) | 2 (1;3) | 2.31 (1.71) | 2 (1;3) | 0.85 (0.77;0.91) | 0.85 |
| Red meat | 67 | 1.73 (1.45) | 1 (1;2) | 1.69 (1.42) | 1 (1;2) | 0.68 (0.53;0.79) | 0.69 |
| Ultra-processed food | 67 | 2.1 (2.14) | 2 (1;3) | 1.99 (1.73) | 2 (1;3) | 0.80 (0.69;0.87) | 0.78 |
| Fresh fish | 68 | 1.46 (1.61) | 1 (0;2) | 1.56 (1.64) | 1 (0;2) | 0.72 (0.58;0.82) | 0.72 |
| Tinned fish | 68 | 1.24 (1.11) | 1 (1;2) | 1.18 (1.02) | 1 (1;1.5) | 0.74 (0.61;0.83) | 0.74 |
| Eggs | 68 | 1.97 (1.76) | 2 (1;2) | 1.84 (1.31) | 2 (1;2) | 0.73 (0.59;0.82) | 0.70 |
| Legumes | 68 | 2.24 (2.13) | 2 (1;3) | 2.12 (1.93) | 2 (1;3) | 0.80 (0.69;0.87) | 0.80 |
| Drinks | |||||||
| Soda | 68 | 0.59 (1.26) | 0 (0;0.5) | 0.59 (1.34) | 0 (0;1) | 0.85 (0.77;0.91) | 0.85 |
| Wine | 68 | 2.47 (3.42) | 1 (0;4) | 2.25 (3.17) | 1 (0;3) | 0.86 (0.78;0.91) | 0.85 |
| Beer | 67 | 0.64 (1.08) | 0 (0;1) | 0.64 (0.9) | 0 (0;1) | 0.85 (0.76;0.90) | 0.83 |
| Cocktail | 68 | 0.35 (0.64) | 0 (0;1) | 0.35 (0.69) | 0 (0;0) | 0.80 (0.69;0.87) | 0.80 |
| Spirits | 66 | 0.55 (0.98) | 0 (0;1) | 0.53 (1.13) | 0 (0;1) | 0.88 (0.81;0.92) | 0.87 |
| Unhealthy foods | |||||||
| Fast food | 68 | 0.19 (0.47) | 0 (0;0) | 0.24 (0.67) | 0 (0;0) | 0.62 (0.45;0.75) | 0.58 |
| Salted snack | 68 | 0.82 (1.18) | 0 (0;1) | 0.81 (1.08) | 0 (0;1) | 0.80 (0.69;0.87) | 0.80 |
| Frozen foods | 68 | 0.47 (0.84) | 0 (0;1) | 0.5 (0.91) | 0 (0;1) | 0.61 (0.44;0.74) | 0.61 |
| Ready-to-eat-meals | 68 | 1.03 (2.25) | 0 (0;2) | 0.68 (1.1) | 0 (0;1) | 0.73 (0.60;0.83) | 0.57 |
| Breakfast with croissant and cappuccino | 62 | 1.89 (2.53) | 1 (0;3) | 1.08 (1.84) | 0 (0;1) | 0.39 (0.12;0.59) | 0.36 |
| Age ≤ 30 Years N = 145 | Age > 30 Years N = 68 | |||
|---|---|---|---|---|
| Food Items | Kappa (95%CI) | N | Kappa (95%CI) | N |
| Vegetables and fruits | ||||
| Medium/large sized fruit | 0.39 (0.25;0.53) | 133 | 0.28 (0.06;0.50) | 57 |
| Small sized fruit | 0.39 (0.25;0.54) | 117 | 0.32 (0.07;0.56) | 45 |
| Cooked vegetables | 0.48 (0.35;0.61) | 131 | 0.42 (0.23;0.61) | 58 |
| Raw vegetables | 0.36 (0.23;0.52) | 116 | 0.35 (0.12;0.58) | 47 |
| Salad | 0.51 (0.37;0.64) | 103 | 0.47 (0.28;0.66) | 58 |
| Potatoes | 0.44 (0.28;0.60) | 107 | 0.36 (0.07;0.64) | 52 |
| French fries | 0.54 (0.44;0.64) | 145 | 0.39 (0.16;0.62) | 68 |
| Cereals | ||||
| Breakfast cereals | 0.36 (0.14;0.55) | 62 | 0.41 (0.07;0.75) | 25 |
| Loaf bread | 0.68 (0.58;0.78) | 134 | 0.62 (0.47;0.77) | 63 |
| Fresh bread | 0.57 (0.44;0.69) | 145 | 0.40 (0.21;0.58) | 68 |
| Bread substitutes | 0.55 (0.44;0.66) | 145 | 0.34 (0.16;0.52) | 68 |
| Focaccia bread | 0.31 (0.2;0.42) | 145 | 0.32 (0.14;0.49) | 68 |
| Pizza | 0.37 (0.18;0.56) | 145 | 0.52 (0.32;0.72) | 68 |
| Rice and other cereals | 0.43 (0.29;0.57) | 135 | 0.48 (0.29;0.68) | 57 |
| Pasta | 0.50 (0.38;0.63) | 145 | 0.41 (0.25;0.56) | 68 |
| Cookies | 0.58 (0.49;0.67) | 143 | 0.49 (0.36;0.61) | 68 |
| Sweets/cakes/pastries | 0.60 (0.43;0.76) | 145 | 0.48 (0.16;0.80) | 68 |
| Dairy products | ||||
| Cow milk * | 0.39 (0.19;0.58) | 75 | 0.40 (0.06;0.75) | 30 |
| Milk products | 0.68 (0.52;0.84) | 86 | 0.45 (0.21;0.69) | 40 |
| Fresh cheese | 0.57 (0.46;0.68) | 145 | 0.35 (0.19;0.50) | 68 |
| Seasoned cheese * | 0.45 (0.25;0.65) | 95 | 0.11 (−0.25;0.46) | 46 |
| Meat and Fish | ||||
| White meat | 0.56 (0.45;0.68) | 145 | 0.49 (0.33;0.65) | 68 |
| Red meat | 0.62 (0.51;0.73) | 145 | 0.58 (0.41;0.74) | 68 |
| Ultra-processed food | 0.53 (0.38;0.68) | 113 | 0.10 (−0.09;0.42) | 48 |
| Fresh fish | 0.34 (0.17;0.50) | 91 | 0.38 (0.15;0.61) | 42 |
| Tinned fish | 0.52 (0.37;0.66) | 93 | 0.42 (0.19;0.65) | 46 |
| Eggs | 0.89 (0.81;0.96) | 116 | 0.70 (0.49;0.92) | 59 |
| Legumes | 0.51 (0.37;0.64) | 108 | 0.51 (0.30;0.72) | 51 |
| Drinks | ||||
| Soda | 0.66 (0.55;0.77) | 145 | 0.59 (0.41;0.77) | 68 |
| Wine | 0.68 (0.6;0.75) | 141 | 0.83 (0.75;0.91) | 64 |
| Beer | 0.66 (0.56;0.76) | 139 | 0.62 (0.37;0.86) | 67 |
| Cocktail | 0.59 (0.49;0.68) | 139 | 0.76 (0.67;0.85) | 66 |
| Unhealthy foods | ||||
| Fast food | −0.17 (−0.33;−0.02) | 54 | 0.85 (0.570;1.00) | 7 |
| Salted snack | 0.63 (0.44;0.82) | 65 | 0.38 (0.03;0.72) | 25 |
| MEDOC Score | ||||
|---|---|---|---|---|
| Age ≤ 30 N = 120 | Age > 30 N = 53 | |||
| Median (Q1–Q3) | Min;Max | Median (Q1–Q3) | Min;Max | |
| All subjects | 1.50 (−4.00;6.00) | −14.5;19.0 | 3.75 (−1.00;10.00) | −16.0;16.0 |
| Sex | ||||
| Females | 1.00 (−4.00;5.50) | −14.5;19.0 | 5.25 (−0.50;10.75) | −16.0;16.0 |
| Males | 1.50 (−3.50;6.75) | −11;10.5 | 1.75 (−3.25;6.75) | −12.5;13.5 |
| Education level | ||||
| High school or lower | 1.00 (−4.50;6.50) | −14.5;19.0 | 6.00(1.00;10.00) | −10.5;16.0 |
| Bachelor degree | 1.25 (−2.50;4.75) | −10.5;10.5 | 0.50 (−4.50;2.50) | −12.5;16.0 |
| Master degree | 0.75 (−5.50;6.50) | −7.5;12.0 | 13.50 (10.25;14.75) | −16.0;16.0 |
| Post-lauream | 2.25 (−4.75;6.00) | −11.0;19.0 | 3.50 (−7.50;6.50) | −11.5;11.0 |
| BMI | ||||
| ≤median * | 1.50 (−4.50;6.50) | −14.5;14.0 | 3.50 (−0.75;10.50) | −16.0;16.0 |
| >median | 0.50 (−3.50;5.76) | −11.0;19.0 | 4.25 (−2.00;9.00) | −12.5;16.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzucca, C.B.; Scotti, L.; Raineri, D.; Cappellano, G.; Chiocchetti, A. Design and Validation of MEDOC, a Tool to Assess the Combined Adherence to Mediterranean and Western Dietary Patterns. Nutrients 2024, 16, 1745. https://doi.org/10.3390/nu16111745
Mazzucca CB, Scotti L, Raineri D, Cappellano G, Chiocchetti A. Design and Validation of MEDOC, a Tool to Assess the Combined Adherence to Mediterranean and Western Dietary Patterns. Nutrients. 2024; 16(11):1745. https://doi.org/10.3390/nu16111745
Chicago/Turabian StyleMazzucca, Camilla Barbero, Lorenza Scotti, Davide Raineri, Giuseppe Cappellano, and Annalisa Chiocchetti. 2024. "Design and Validation of MEDOC, a Tool to Assess the Combined Adherence to Mediterranean and Western Dietary Patterns" Nutrients 16, no. 11: 1745. https://doi.org/10.3390/nu16111745
APA StyleMazzucca, C. B., Scotti, L., Raineri, D., Cappellano, G., & Chiocchetti, A. (2024). Design and Validation of MEDOC, a Tool to Assess the Combined Adherence to Mediterranean and Western Dietary Patterns. Nutrients, 16(11), 1745. https://doi.org/10.3390/nu16111745










