Prevalence of Metabolic Syndrome Based on Activity Type and Dietary Habits in Extremely Low-Income Individuals
Abstract
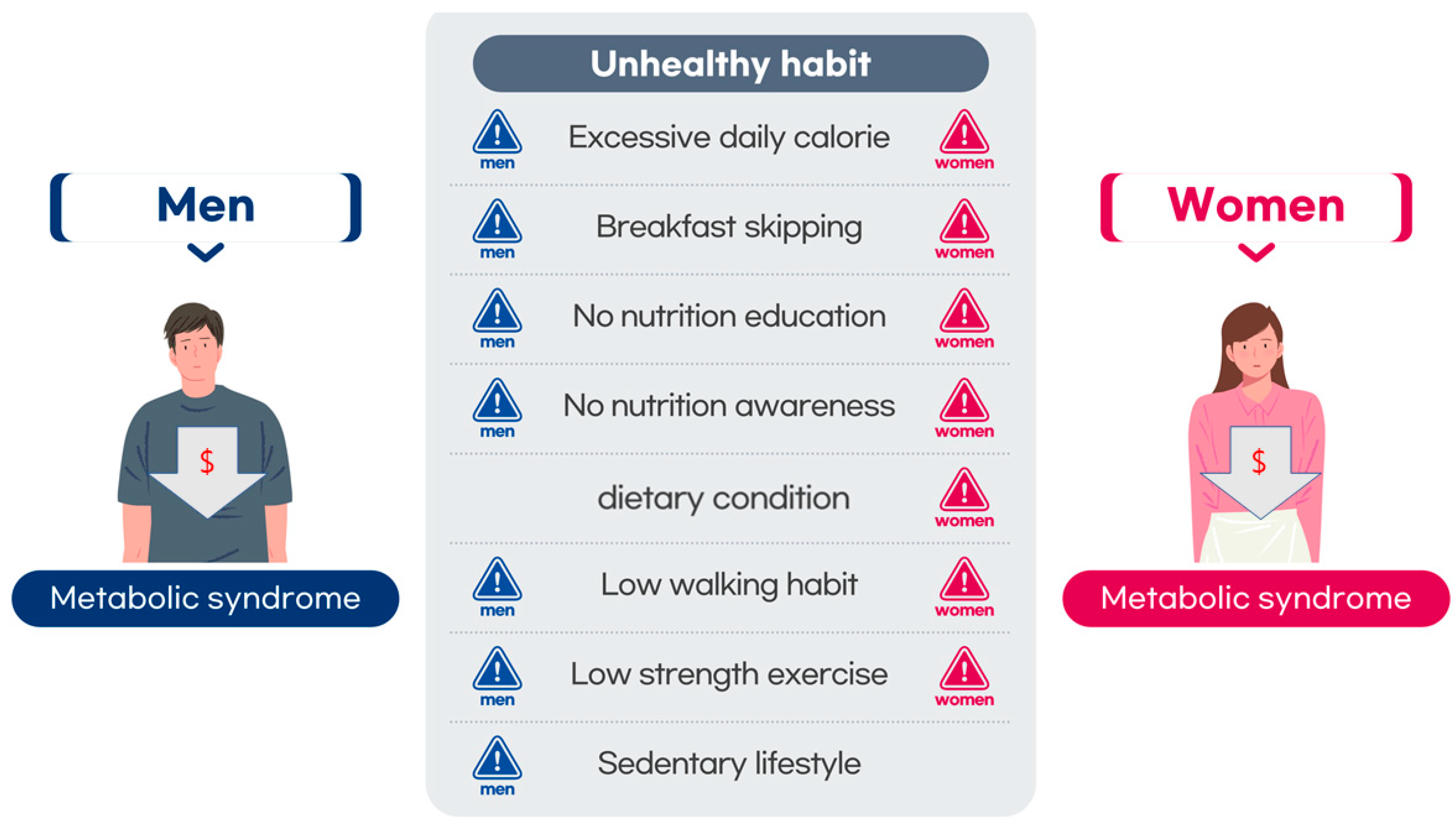
1. Introduction
2. Materials and Methods
2.1. Data and Study Population
2.2. Metabolic Syndrome
2.3. Activity Type
2.4. Dietary Pattern
2.5. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Sociodemographic Characteristics
3.3. Multiple Regression Analysis and Metabolic Syndrome
3.4. Association of Metabolic Syndrome and Dietary Pattern
3.5. Association between Metabolic Syndrome and Activity Type
3.6. Comparison of Nutrient Intake between Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossi, J.L.S.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2021, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Shin, M.-J.; Després, J.-P.; Eckel, R.H.; Tuomilehto, J.; Lim, S. 20-year trends in metabolic syndrome among Korean adults from 2001 to 2020. JACC Asia 2023, 3, 491–502. [Google Scholar] [CrossRef]
- Liang, X.; Or, B.; Tsoi, M.F.; Cheung, C.L.; Cheung, B.M. Prevalence of metabolic syndrome in the United States National Health and Nutrition Examination Survey 2011-18. Postgrad. Med. J. 2023, 99, 985–992. [Google Scholar] [CrossRef]
- Kookna, J.C.; Acharya, J. Metabolic syndrome following stroke in patients admitted in Hospital. NeuroQuantology 2022, 20, 6211. [Google Scholar]
- Tamura, Y.; Omura, T.; Toyoshima, K.; Araki, A. Nutrition management in older adults with diabetes: A review on the importance of shifting prevention strategies from metabolic syndrome to frailty. Nutrients 2020, 12, 3367. [Google Scholar] [CrossRef]
- Masrouri, S.; Moazzeni, S.S.; Cheraghloo, N.; Azizi, F.; Hadaegh, F. The clinical value of metabolic syndrome and its components with respect to sudden cardiac death using different definitions: Two decades of follow-up from the Tehran Lipid and Glucose Study. Cardiovasc. Diabetol. 2022, 21, 269. [Google Scholar] [CrossRef]
- Chen, M.-S.; Chiu, C.-H.; Chen, S.-H. Risk assessment of metabolic syndrome prevalence involving sedentary occupations and socioeconomic status. BMJ Open 2021, 11, e042802. [Google Scholar] [CrossRef] [PubMed]
- Blanquet, M.; Legrand, A.; Pélissier, A.; Mourgues, C. Socio-economics status and metabolic syndrome: A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Lago, S.; Cantarero, D.; Rivera, B.; Pascual, M.; Blázquez-Fernández, C.; Casal, B.; Reyes, F. Socioeconomic status, health inequalities and non-communicable diseases: A systematic review. J. Public Health 2018, 26, 1–14. [Google Scholar] [CrossRef]
- Nam, J.; Park, H. The 2015 welfare reform of the National basic livelihood security system in South Korea: Effects on economic outcomes. Int. J. Soc. Welf. 2020, 29, 219–232. [Google Scholar] [CrossRef]
- Moffitt, R.A. The deserving poor, the family, and the US welfare system. Demography 2015, 52, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Quinn, E.; Kinoshita, S. An Overview of Public Charge and Benefits; Immigrant Legal Resource Center: San Francisco, CA, USA, 2020; Volume 19, pp. 1–7. [Google Scholar]
- Jo, J.Y. Korea’s national basic livelihood programme and social development. In Social Policy and Poverty in East Asia; Routledge: London, UK, 2009; pp. 95–112. [Google Scholar]
- Kang, S.-W.; Yang, J.-H.; Shin, W.-C.; Kim, Y.-J.; Choi, M.-H. Influence of Residence Area and Basic Livelihood Conditions on the Prevalence and Diagnosis Experience of Osteoporosis in Postmenopausal Women Aged over 50 Years: Evaluation Using Korea National Health and Nutrition Examination Survey Data. Int. J. Environ. Res. Public Health 2021, 18, 9478. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Shim, J. Predictors of Depression among Individuals Receiving the Basic Livelihood Security Program Benefits in Korea: A Study Based on the Sixth and Seventh Korea National Health and Nutrition Examination Survey (2013–2018). Int. J. Environ. Res. Public Health 2022, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Alipour, P.; Azizi, Z.; Raparelli, V.; Norris, C.M.; Kautzky-Willer, A.; Kublickiene, K.; Herrero, M.T.; El Emam, K.; Vollenweider, P.; Preisig, M. Role of sex and gender-related variables in development of metabolic syndrome: A prospective cohort study. Eur. J. Intern. Med. 2024, 121, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Valsta, L.M.; Tapanainen, H.; Kortetmäki, T.; Sares-Jäske, L.; Paalanen, L.; Kaartinen, N.E.; Haario, P.; Kaljonen, M. Disparities in nutritional adequacy of diets between different socioeconomic groups of Finnish adults. Nutrients 2022, 14, 1347. [Google Scholar] [CrossRef] [PubMed]
- Khang, Y.-H.; Cho, H.-J. Socioeconomic inequality in cigarette smoking: Trends by gender, age, and socioeconomic position in South Korea, 1989–2003. Prev. Med. 2006, 42, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Kang, D.R.; Kim, J.Y.; Koh, K.K. Metabolic syndrome fact sheet 2021: Executive report. CardioMetabolic Syndr. J. 2021, 1, 125–134. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Zimmet, P.; Caterson, I.; Chunming, C.; Ikeda, Y.; Khalid, A.; Kim, Y. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; World Health Organization: Sydney, Australia, 2000. [Google Scholar]
- Craig, C.; Marshall, A.; Sjostrom, M.; Bauman, A.; Lee, P.; Macfarlane, D.; Lam, T.; Stewart, S. International physical activity questionnaire-short form. J. Am. Coll. Health 2017, 65, 492–501. [Google Scholar]
- Yun, S.H.; Shim, J.-S.; Kweon, S.; Oh, K. Development of a food frequency questionnaire for the Korea National Health and Nutrition Examination Survey: Data from the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV). Korean J. Nutr. 2013, 46, 186–196. [Google Scholar] [CrossRef]
- Jung, H. Validation of Food Frequency Questionnaire for Korea National Health and Nutrition Examination Survey; Korea Center for Diease Control and Prevention: Osong, Republic of Korea, 2010. [Google Scholar]
- Rodrigues, M.C.; Maciel, E.d.S.; Quaresma, F.R.P.; Sesti, L.F.C.; Paiva, L.d.S.; Macedo Junior, H.; Araújo, F.A.d.; Fonseca, F.L.A.; Adami, F. Prevalence and factors associated with metabolic syndrome in a vulnerable population in northern Brazil: A cross-sectional study. J. Hum. Growth Dev. 2021, 31, 291–301. [Google Scholar] [CrossRef]
- Bao, J.; Wang, L.; Hu, P.; Liu, J.; Tu, J.; Wang, J.; Li, J.; Ning, X. Burden of metabolic syndrome among a low-income population in China: A population-based cross-sectional study. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Neubeck, L.; Atherton, I. Impact of area deprivation on the cardiac mortality in the UK between1991 and 2010: Evidence from a population-based longitudinal study. Eur. J. Cardiovasc. Nurs. 2021, 20, 436–444. [Google Scholar] [CrossRef]
- Rahman, S.; Mirza, A.-S.; Wathington, D.; Green, S.; Mayers, Y.; Iranmanesh, E.; Woodard, L. Chronic disease and socioeconomic factors among uninsured patients: A retrospective study. Chronic Illn. 2021, 17, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Vilhjalmsson, R. Family income and insufficient medical care: A prospective study of alternative explanations. Scand. J. Public Health 2021, 49, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.; Davenport, L. Barriers to health care access for low income families: A review of literature. J. Community Health Nurs. 2018, 35, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Gesteiro, E.; García-Carro, A.; Aparicio-Ugarriza, R.; González-Gross, M. Eating out of home: Influence on nutrition, health, and policies: A scoping review. Nutrients 2022, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Rong, S.; Sun, Y.; Liu, B.; Wu, Y.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Association between frequency of eating away-from-home meals and risk of all-cause and cause-specific mortality. J. Acad. Nutr. Diet. 2021, 121, 1741–1749.e1741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Kim, Y.; Lee, J. Prevalence of Metabolic Syndrome Based on the Dietary Habits and Physical Activity of Korean Women Cancer Survivors. Foods 2023, 12, 3554. [Google Scholar] [CrossRef]
- Thuita, A.W.; Kiage, B.N.; Onyango, A.N.; Makokha, A.O. Effect of a nutrition education programme on the metabolic syndrome in type 2 diabetes mellitus patients at a level 5 Hospital in Kenya: “A randomized controlled trial”. BMC Nutr. 2020, 6, 30. [Google Scholar] [CrossRef]
- Wang, D.; Stewart, D. The implementation and effectiveness of school-based nutrition promotion programmes using a health-promoting schools approach: A systematic review. Public Health Nutr. 2013, 16, 1082–1100. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bea, W.; Lee, K.; Han, J.; Kim, S.; Kim, M.; Na, W.; Sohn, C. Effect of the telephone-delivered nutrition education on dietary intake and biochemical parameters in subjects with metabolic syndrome. Clin. Nutr. Res. 2013, 2, 115. [Google Scholar] [CrossRef] [PubMed]
- Shahar, S.; Adznam, S.N.A.; Lee, L.K.; Yusof, N.A.M.; Salleh, M.; Mohamed Sakian, N.I. A nutrition education intervention for anthropometric and biochemical profiles of rural older Malays with metabolic syndrome. Public Health Nurs. 2013, 30, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Mirmiran, P.; Momenan, A.A.; Hadaegh, F.; Moeini, A.H.; Hosseini, F.; Zahediasl, S.; Ghanbarian, A.; Hosseinpanah, F. The effect of community-based education for lifestyle intervention on the prevalence of metabolic syndrome and its components: Tehran lipid and glucose study. Int. J. Endocrinol. Metab. 2013, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Z.; Huang, H.; He, Y.; Yu, Y.; Chen, G.; Liu, L.; Wang, B.; Li, Q.; Lai, W. Malnutrition in patients with coronary artery disease: Prevalence and mortality in a 46,485 Chinese cohort study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Katsuura-Kamano, S.; Arisawa, K.; Uemura, H.; Van Nguyen, T.; Takezaki, T.; Ibusuki, R.; Suzuki, S.; Otani, T.; Okada, R.; Kubo, Y. Association of skipping breakfast and short sleep duration with the prevalence of metabolic syndrome in the general Japanese population: Baseline data from the Japan Multi-Institutional Collaborative cohort study. Prev. Med. Rep. 2021, 24, 101613. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Cascales, R.; Sánchez-SanSegundo, M.; Ruiz-Robledillo, N.; Albaladejo-Blázquez, N.; Laguna-Pérez, A.; Zaragoza-Martí, A. Eat or skip breakfast? The important role of breakfast quality for health-related quality of life, stress and depression in Spanish adolescents. Int. J. Environ. Res. Public Health 2018, 15, 1781. [Google Scholar] [CrossRef] [PubMed]
- Jing, B.; Yun, W.; Fan, Z.X.; Ouyang, Y.F.; Zhang, B.; Wang, Z.H.; Du, S.F.; Wang, H.J. Associations of sedentary time and physical activity with metabolic syndrome among chinese adults: Results from the china health and nutrition survey. Biomed. Environ. Sci. 2021, 34, 963–975. [Google Scholar]
- Tian, D.; Meng, J. Exercise for prevention and relief of cardiovascular disease: Prognoses, mechanisms, and approaches. Oxidative Med. Cell. Longev. 2019, 2019, 3756750. [Google Scholar] [CrossRef]
- Nagata, J.M.; Vittinghoff, E.; Gabriel, K.P.; Garber, A.K.; Moran, A.E.; Rana, J.S.; Reis, J.P.; Sidney, S.; Bibbins-Domingo, K. Moderate-to-vigorous intensity physical activity from young adulthood to middle age and metabolic disease: A 30-year population-based cohort study. Br. J. Sports Med. 2022, 56, 847–853. [Google Scholar] [CrossRef]
- Ballin, M.; Nordström, P.; Nordström, A. Associations of light, moderate to vigorous, and Total physical activity with the prevalence of metabolic syndrome in 4,652 community-dwelling 70-year-olds: A population-based cross-sectional study. J. Aging Phys. Act. 2021, 29, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, K.; Brussaard, J.; Kruizinga, A.; Telman, J.; Löwik, M. Socio-economic status, dietary intake and 10 y trends: The Dutch National Food Consumption Survey. Eur. J. Clin. Nutr. 2003, 57, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, B.M.; Milliron, B.-J.; Woolf, K.; Johnson, T.J.; Pagoto, S.L.; Schneider, K.L.; Whited, M.C.; Ventrelle, J.C. Socioeconomic status, energy cost, and nutrient content of supermarket food purchases. Am. J. Prev. Med. 2012, 42, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Bo, Y.; Zhao, L.; Li, Y.; Ju, L.; Fang, H.; Piao, W.; Yu, D.; Lao, X. Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017. Nutrients 2021, 13, 4475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D. Epidemiological features of cardiovascular disease in Asia. JACC Asia 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, N.; Shuster, S.M. Health and health care of sexual and gender minorities. J. Health Soc. Behav. 2021, 62, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yu, J.; Chen, R.; Gao, J.; Ding, R.; Fu, Y.; Zhang, L.; Hu, D. Socioeconomic status and metabolic syndrome in the general population of China: A cross-sectional study. BMC Public Health 2012, 12, 921. [Google Scholar] [CrossRef] [PubMed]
- Messiah, S.E.; Xie, L.; Kapti, E.G.; Chandrasekhar, A.; Srikanth, N.; Hill, K.; Williams, S.; Reid, A.; Mathew, M.S.; Barlow, S.E. Prevalence of the metabolic syndrome by household food insecurity status in the United States adolescent population, 2001–2020: A cross-sectional study. Am. J. Clin. Nutr. 2023, 119, 354–361. [Google Scholar] [CrossRef]
- Choi, M.; Han, J.; Kim, Y.; Chung, J. The relationship between metabolic syndrome and smoking and alcohol experiences in adolescents from low-income households. Children 2021, 8, 812. [Google Scholar] [CrossRef]
| Variables | Men | p | Women | p | ||
|---|---|---|---|---|---|---|
| Non-BLS (n = 13,926, 94.1%) | BLS (n = 877, 5.9%) | Non-BLS (n = 18,911, 93.2%) | BLS (n = 1388, 6.8%) | |||
| Age, years | 55.6 ± 14.2 | 60.5 ± 13.2 | <0.001 | 54.5 ± 13.8 | 61.2 ± 13.6 | <0.001 |
| Waistline, cm | 88.8 ± 8.8 | 90.2 ± 10.0 | 0.177 | 82.4 ± 9.6 | 85.7 ± 10.4 | <0.001 |
| Systolic blood pressure, mmHg | 122.1 ± 15.1 | 124.0 ± 16.7 | <0.001 | 118.1 ± 17.6 | 123.3 ± 18.3 | <0.001 |
| Diastolic blood pressure, mmHg | 77.7 ± 10.3 | 76.1 ± 10.9 | <0.001 | 73.8 ± 9.4 | 74.1 ± 9.8 | 0.244 |
| Triglyceride, mg/dL | 159.6 ± 126.9 | 172 ± 158.3 | 0.006 | 116.1 ± 78 | 128.3 ± 79.6 | <0.001 |
| HDLC, mg/dL | 47.5 ± 11.6 | 46.6 ± 12.2 | 0.019 | 55.2 ± 13.2 | 52.5 ± 12.7 | <0.001 |
| Glucose, mg/dL | 105.8 ± 25.6 | 112.5 ± 36.4 | <0.001 | 99.6 ± 21.5 | 105.1 ± 27.9 | <0.001 |
| Income, thousand Won | 4471.3 ± 3229.2 | 1675.6 ± 1678.5 | <0.001 | 4361.5 ± 3261.1 | 1683.6 ± 1705.8 | <0.001 |
| MS, n (%) | 5684 (40.8%) | 411 (46.9%) | <0.001 | 5947 (31.4%) | 658 (47.7%) | <0.001 |
| MS waistline, n (%) | 6378 (45.8%) | 430 (49.0%) | 0.175 | 5743 (30.4%) | 625 (45.0%) | <0.001 |
| MS blood pressure, n (%) | 7253 (52.1%) | 525 (59.9%) | <0.001 | 7420 (39.2%) | 780 (56.2%) | <0.001 |
| MS triglyceride, n (%) | 6657 (47.8%) | 445 (50.7%) | 0.091 | 6562 (34.7%) | 621 (44.7%) | <0.001 |
| MS HDLC, n (%) | 3561 (25.6%) | 277 (31.6%) | <0.001 | 6863 (36.3%) | 616 (44.4%) | <0.001 |
| MS glucose, n (%) | 6976 (50.1%) | 508 (57.9%) | <0.001 | 6487 (34.3%) | 634 (45.7%) | <0.001 |
| Category | Men | Women | Sex, p a and b | ||
|---|---|---|---|---|---|
| Non-BLS (n = 13,926, 94.1%) | BLS (n = 877, 5.9%) | Non-BLS (n = 18,911, 93.2%) | BLS (n = 1388, 6.8%) | ||
| MS, n (%) | p < 0.001 | p < 0.001 | |||
| Non-MS | 8242 (59.2%) | 466 (53.1%) | 12,964 (68.6%) | 730 (52.6%) | a p < 0.001 b p = 0.829 |
| MS | 5684 (40.8%) | 411 (46.9%) | 5947 (31.4%) | 658 (47.4%) | |
| Age Group | p < 0.001 | p < 0.001 | |||
| 30–39 | 2398 (17.2%) | 65 (7.4%) | 3354 (17.7%) | 108 (7.8%) | a p < 0.001 b p = 0.033 |
| 40–49 | 2749 (19.7%) | 126 (14.4%) | 4097 (21.7%) | 220 (15.9%) | |
| 50–59 | 2891 (20.8%) | 206 (23.5%) | 4296 (22.7%) | 256 (18.4%) | |
| 60–69 | 3100 (22.3%) | 221 (25.2%) | 3970 (21.0%) | 338 (24.4%) | |
| 70–80 | 2891 (20.8%) | 206 (23.5%) | 3194 (16.9%) | 466 (33.6%) | |
| BMI | p < 0.001 | p < 0.001 | |||
| ≤22.9 | 5431 (39.0%) | 270 (30.8%) | 8893 (47.0%) | 515 (37.1%) | a p < 0.001 b p = 0.205 |
| 23.0–24.9 | 3259 (23.4%) | 237 (27.0%) | 4171 (22.1%) | 300 (21.6%) | |
| ≥25.0 | 5239 (37.6%) | 370 (42.2%) | 5839 (30.9%) | 573 (41.3%) | |
| Education Status | p < 0.001 | p < 0.001 | |||
| To elementary | 2068 (14.8%) | 347 (39.6%) | 4581 (24.2%) | 699 (50.4%) | a p < 0.001 b p < 0.001 |
| To middle | 1545 (11.1%) | 159 (18.1%) | 2040 (10.8%) | 197 (14.2%) | |
| To high | 4282 (30.7%) | 243 (27.7%) | 5709 (30.2%) | 352 (25.4%) | |
| Above college | 6031 (43.3%) | 128 (14.6%) | 6581 (34.8%) | 140 (10.1%) | |
| Occupation | p < 0.001 | p < 0.001 | |||
| Yes | 10,575 (75.9%) | 361 (41.2%) | 9896 (52.3%) | 539 (38.8%) | a p < 0.001 b p = 0.271 |
| No | 3351 (24.1%) | 516 (58.8%) | 9015 (47.7%) | 849 (61.2%) | |
| Marital Status | p < 0.001 | p < 0.001 | |||
| With spouse | 11,768 (84.5%) | 472 (53.8%) | 14,730 (77.9%) | 537 (38.7%) | a p < 0.001 b p < 0.001 |
| Not married | 1319 (9.5%) | 166 (18.9%) | 897 (4.7%) | 81 (5.8%) | |
| Death of spouse | 315 (2.3%) | 57 (6.5%) | 2417 (12.8%) | 474 (34.1%) | |
| Divorce | 524 (3.8%) | 182 (20.8%) | 867 (4.6%) | 296 (21.3%) | |
| Residence Region | p = 0.015 | p < 0.001 | |||
| City | 10,905 (78.3%) | 656 (74.8%) | 15,260 (80.7%) | 1063 (76.6%) | a p < 0.001 b p = 0.338 |
| Rural | 3021 (21.7%) | 221 (25.2%) | 3651 (19.3%) | 325 (23.4%) | |
| Smoking History | p < 0.001 | p < 0.001 | |||
| Never | 2882 (20.7%) | 150 (17.1%) | 17,141 (90.6%) | 1129 (81.3%) | a p < 0.001 b p < 0.001 |
| Past | 6705 (48.1%) | 369 (42.1%) | 1050 (5.6%) | 112 (8.1%) | |
| Current | 4339 (31.2%) | 358 (40.8%) | 720 (3.8%) | 147 (10.6%) | |
| Alcohol Frequency | p < 0.001 | p < 0.001 | |||
| None or 1–2 per month | 5481 (39.4%) | 455 (51.9%) | 13,573 (71.8%) | 1086 (78.2%) | a p < 0.001 b p < 0.001 |
| 1 per week | 3430 (24.6%) | 130 (14.8%) | 3314 (17.5%) | 162 (11.7%) | |
| >2 per week | 5015 (36.0%) | 292 (33.3%) | 2024 (10.7%) | 140 (10.1%) | |
| Variables | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | ß | t | p | B | SE B | ß | t | p | |
| Age | 0.004 | 0.000 | 0.116 | 9.957 | <0.001 | 0.010 | 0.000 | 0.277 | 28.014 | <0.001 |
| Education | −0.008 | 0.005 | −0.017 | −1.632 | 0.103 | −0.069 | 0.004 | −0.173 | −18.533 | <0.001 |
| Occupation | 0.029 | 0.011 | 0.025 | 2.513 | 0.012 | 0.030 | 0.006 | 0.032 | 4.777 | <0.001 |
| Marital status | 0.004 | 0.006 | 0.005 | 0.609 | 0.542 | 0.003 | 0.003 | 0.006 | 0.781 | 0.435 |
| Residence | 0.012 | 0.010 | 0.010 | 1.171 | 0.241 | 0.021 | 0.008 | 0.017 | 2.624 | 0.009 |
| Smoking | 0.029 | 0.006 | 0.041 | 4.682 | <0.001 | 0.035 | 0.007 | 0.033 | 4.896 | <0.001 |
| Alcohol | 0.041 | 0.005 | 0.073 | 8.286 | <0.001 | −0.016 | 0.005 | −0.023 | −3.398 | <0.001 |
| Variables | Classification | Men MS, Odds Ratio | Women MS, Odds Ratio | ||
|---|---|---|---|---|---|
| Non-BLS | BLS | Non-BLS | BLS | ||
| Daily calories | reference | – | 1.00 | – | 1.00 |
| G1, below RDI | 1.00 | 0.99 (0.92–1.06) | 1.00 | 1.02 (0.95–1.09) | |
| G2, above RDI | 1.17 (1.02–2.19) | 1.20 (1.04–1.76) | 1.21 (1.09–2.19) | 1.31 (1.12–2.50) | |
| Breakfast | reference | – | 1.00 | – | 1.00 |
| G1, low skipping | 1.00 | 1.10 (0.93–1.30) | 1.00 | 1.05 (0.91–1.21) | |
| G2, medium skipping | 1.07 (0.94–1.21) | 1.04 (0.91–1.19) | 0.95 (0.84–1.08) | 0.97 (0.85–1.69) | |
| G3, high skipping | 1.19 (1.03–2.00) | 1.24 (1.03–1.97) | 1.10 (1.03–2.12) | 1.11 (1.04–2.28) | |
| Eating out | reference | – | 1.00 | – | 1.00 |
| G1, low eating | 1.00 | 0.98 (0.90–1.07) | 1.00 | 0.89 (0.79–1.04) | |
| G2, medium eating | 0.96 (0.87–1.06) | 0.97 (0.88–1.08) | 0.92 (0.82–1.09) | 1.01 (0.89–1.52) | |
| G3, high eating | 1.15 (1.01–1.86) | 1.18 (0.98–1.39) | 1.11 (1.10–1.94) | 1.38 (0.84–2.57) | |
| Nutritional education | reference | – | 1.00 | – | 1.00 |
| G1, education | 1.00 | 0.82 (0.65–1.04) | 1.00 | 1.18 (0.83–1.97) | |
| G2, no education | 1.17 (0.64–2.16) | 1.11 (1.04–1.96) | 1.18 (1.01–2.08) | 1.20 (1.05–2.05) | |
| Nutritional awareness | reference | – | 1.00 | – | 1.00 |
| G1, awareness | 1.00 | 1.01 (0.82–1.24) | 1.00 | 1.02 (0.85–1.23) | |
| G2, no awareness | 1.24 (1.02–1.91) | 1.30 (1.07–1.77) | 1.11 (1.02–1.92) | 1.35 (1.14–2.59) | |
| Dietary life condition | reference | – | 1.00 | – | 1.00 |
| G1, sufficient | 1.00 | 1.08 (0.92–1.26) | 1.00 | 1.20 (0.90–1.60) | |
| G2, insufficient | 0.98 (0.76–1.10) | 0.93 (0.86–1.09) | 0.91 (0.78–1.28) | 0.94 (0.78–0.98) | |
| Variables | Classification | Men MS, Odds Ratio | Women MS, Odds Ratio | ||
|---|---|---|---|---|---|
| Non-BLS | BLS | Non-BLS | BLS | ||
| Walking | reference | – | 1.00 | – | 1.00 |
| G1, 6–7/days | 1.00 | 1.12 (0.91–1.39) | 1.00 | 1.17 (0.96–1.42) | |
| G2, 3–5/days | 1.19 (0.96–1.47) | 0.89 (0.64–1.24) | 1.21 (1.03–1.46) | 1.20 (0.92–1.56) | |
| G3, 0–2/days | 1.46 (1.17–1.83) | 1.58 (1.27–1.96) | 1.41 (1.08–1.82) | 1.47 (1.22–1.78) | |
| Strength training | reference | – | 1.00 | – | 1.00 |
| G1, 4–7/days | 1.00 | 1.29 (0.85–1.95) | 1.00 | 1.35 (0.90–2.75) | |
| G2, 2–3/days | 1.09 (0.69–1.72) | 0.99 (0.62–1.57) | 1.26 (1.05–2.14) | 1.32 (0.89–2.88) | |
| G3, 0–1/day | 1.41 (1.18–1.70) | 1.57 (1.32–1.96) | 2.11 (1.76–2.83) | 2.16 (1.11–3.15) | |
| MHA work | reference | – | 1.00 | – | 1.00 |
| G1, hard labor | 1.00 | 1.54 (0.96–2.45) | 1.00 | 0.85 (0.53–1.38) | |
| G2, no hard labor | 1.03 (0.92–1.15) | 1.04 (0.93–1.17) | 1.05 (0.91–1.21) | 1.08 (0.94–1.25) | |
| MHA leisure | reference | – | 1.00 | – | 1.00 |
| G1, intense activity | 1.00 | 1.18 (0.84–1.65) | 1.00 | 1.20 (0.94–2.03) | |
| G2, no intense activity | 1.26 (1.17–1.45) | 1.28 (0.93–2.15) | 1.18 (1.03–1.50) | 1.24 (0.89–2.48) | |
| Sedentary | reference | – | 1.00 | – | 1.00 |
| G1, below 8.0 h | 1.00 | 1.04 (0.85–1.27) | 1.00 | 1.17 (0.97–1.41) | |
| G1, above 8.0 h | 1.20 (1.09–1.39) | 1.31 (1.09–1.69) | 1.24 (1.07–1.42) | 1.29 (0.96–1.58) | |
| Variables | Men | t | p | Women | t | p | ||
|---|---|---|---|---|---|---|---|---|
| Non-BLS | BLS | Non-BLS | BLS | |||||
| Carbohydrate, g | 323 ± 132.2 | 399.3 ± 179.2 | −16.187 | <0.001 | 320.6 ± 130.1 | 386.4 ± 171.1 | −22.742 | <0.001 |
| Fat, g | 42.5 ± 19.4 | 45.2 ± 17.3 | −2.918 | 0.004 | 40.8 ± 15.0 | 43.9 ± 16.9 | −5.355 | <0.001 |
| Unsaturated fatty acids, g | 24.8 ± 8.6 | 23.3 ± 8.7 | 4.454 | <0.001 | 24.2 ± 8.2 | 22.3 ± 8.0 | 7.109 | <0.001 |
| Saturated fatty acids, g | 14.0 ± 5.1 | 13.6 ± 4.8 | 0.966 | 0.334 | 12.9 ± 5.6 | 13.6 ± 4.9 | −2.924 | 0.003 |
| Protein, g | 79.0 ± 21.1 | 75.0 ± 26.4 | 4.299 | <0.001 | 71.9 ± 26.5 | 74.6 ± 29.8 | −4.615 | <0.001 |
| Dietary fiber, g | 29.7 ± 10.5 | 27.6 ± 10.7 | −5.177 | 0.003 | 30.5 ± 9.3 | 28.3 ± 10.9 | −8.111 | <0.001 |
| Cholesterol, mg | 220.7 ± 70.7 | 241.8 ± 78.3 | −3.008 | <0.001 | 217.8 ± 66.2 | 236.6 ± 75.5 | −4.158 | <0.001 |
| Natrium, mg | 3870.4 ± 200.1 | 4218.8 ± 242.6 | −4.914 | <0.001 | 3617 ± 196.4 | 3975.5 ± 252.1 | −8.235 | <0.001 |
| Vitamin C, mg | 68.9 ± 28.4 | 60.3 ± 28.9 | 2.814 | 0.005 | 77.2 ± 28.4 | 69.4 ± 20.0 | 4.092 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, K.; Kim, Y.; Park, Y. Prevalence of Metabolic Syndrome Based on Activity Type and Dietary Habits in Extremely Low-Income Individuals. Nutrients 2024, 16, 1677. https://doi.org/10.3390/nu16111677
Su K, Kim Y, Park Y. Prevalence of Metabolic Syndrome Based on Activity Type and Dietary Habits in Extremely Low-Income Individuals. Nutrients. 2024; 16(11):1677. https://doi.org/10.3390/nu16111677
Chicago/Turabian StyleSu, Kunxia, Yonghwan Kim, and Yoonjung Park. 2024. "Prevalence of Metabolic Syndrome Based on Activity Type and Dietary Habits in Extremely Low-Income Individuals" Nutrients 16, no. 11: 1677. https://doi.org/10.3390/nu16111677
APA StyleSu, K., Kim, Y., & Park, Y. (2024). Prevalence of Metabolic Syndrome Based on Activity Type and Dietary Habits in Extremely Low-Income Individuals. Nutrients, 16(11), 1677. https://doi.org/10.3390/nu16111677







