The Relation of Serum Vitamin C Concentrations with Alzheimer’s Disease Mortality in a National Cohort of Community-Dwelling Elderly Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Vitamin C
2.3. AD Mortality
2.4. Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Dehghani, F.; Ramezan, M.; Gannaban, R.B.; Haque, Z.F.; Rahimi, F.; Abbasi, S.; Shin, A.C. Revisiting the Role of Vitamins and Minerals in Alzheimer’s Disease. Antioxidants 2023, 12, 415. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Travica, N.; Ried, K.; Sali, A.; Scholey, A.; Hudson, I.; Pipingas, A. Vitamin C Status and Cognitive Function: A Systematic Review. Nutrients 2017, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef]
- Hamid, M.; Mansoor, S.; Amber, S.; Zahid, S. A quantitative meta-analysis of vitamin C in the pathophysiology of Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 970263. [Google Scholar] [CrossRef] [PubMed]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-Pinto, A.; Acuña, A.I.; Beltrán, F.A.; Torres-Díaz, L.; Castro, M.A. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef]
- Huang, J.; May, J.M. Ascorbic acid protects SH-SY5Y neuroblastoma cells from apoptosis and death induced by beta-amyloid. Brain Res. 2006, 1097, 52–58. [Google Scholar] [CrossRef]
- Dixit, S.; Bernardo, A.; Walker, J.M.; Kennard, J.A.; Kim, G.Y.; Kessler, E.S.; Harrison, F.E. Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice. ACS Chem. Neurosci. 2015, 6, 570–581. [Google Scholar] [CrossRef]
- Consoli, D.C.; Brady, L.J.; Bowman, A.B.; Calipari, E.S.; Harrison, F.E. Ascorbate deficiency decreases dopamine release in gulo−/− and APP/PSEN1 mice. J. Neurochem. 2021, 157, 656–665. [Google Scholar] [CrossRef]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Wu, X.; Acheampong, K.; Liu, A. Dopamine and Dopamine Receptors in Alzheimer’s Disease: A Systematic Review and Network Meta-Analysis. Front. Aging Neurosci. 2019, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- De Nuccio, F.; Cianciulli, A.; Porro, C.; Kashyrina, M.; Ruggiero, M.; Calvello, R.; Miraglia, A.; Nicolardi, G.; Lofrumento, D.D.; Panaro, M.A. Inflammatory Response Modulation by Vitamin C in an MPTP Mouse Model of Parkinson’s Disease. Biology 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Abd El-Fattah, A.I.; Abu-Elfotuh, K.; Elariny, H.A. Natural antioxidants enhance the power of physical and mental activities versus risk factors inducing progression of Alzheimer’s disease in rats. Int. Immunopharmacol. 2021, 96, 107729. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yang, Q.; Zhang, Y.; Li, J.; Zhang, L.; Zhao, H. Meta-analysis of vitamin C, vitamin E and β-carotene levels in the plasma of Alzheimer’s disease patients. Wei Sheng Yan Jiu 2018, 47, 648–654. [Google Scholar] [PubMed]
- Williams, D.M.; Hägg, S.; Pedersen, N.L. Circulating antioxidants and Alzheimer disease prevention: A Mendelian randomization study. Am. J. Clin. Nutr. 2019, 109, 90–98. [Google Scholar] [CrossRef]
- Chen, L.; Sun, X.; Wang, Z.; Lu, Y.; Chen, M.; He, Y.; Xu, H.; Zheng, L. The impact of plasma vitamin C levels on the risk of cardiovascular diseases and Alzheimer’s disease: A Mendelian randomization study. Clin. Nutr. 2021, 40, 5327–5334. [Google Scholar] [CrossRef]
- Heo, J.H.; Hyon, L.; Lee, K.M. The possible role of antioxidant vitamin C in Alzheimer’s disease treatment and prevention. Am. J. Alzheimer’s Dis. Other Dement. 2013, 28, 120–125. [Google Scholar] [CrossRef]
- Boothby, L.A.; Doering, P.L. Vitamin C and vitamin E for Alzheimer’s disease. Ann. Pharmacother. 2005, 39, 2073–2080. [Google Scholar] [CrossRef]
- Harrison, F.E. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 29, 711–726. [Google Scholar] [CrossRef]
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010; Vital and Health Statistics; National Center for Health Statistics: Hyattsville, MA, USA, 2013; pp. 1–37. [Google Scholar]
- Gunter, E.W.; Lewis, B.G.; Koncikowski, S.M. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994; National Health and Nutrition Examination Survey (U.S.): Hyattsville, MD, USA, 1996. [Google Scholar]
- Centers for Disease Control and Prevention. Serum Vitamin C (Ascorbic Acid) Laboratory Procedure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/labmethods/VIC-J-MET-508.pdf (accessed on 27 February 2024).
- National Center for Health Statistics (US). Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–1994; Series 1: Programs and Collection Procedures. Vital and Health Statistics; National Center for Health Statistics: Hyattsville, MA, USA, 1994; pp. 1–407. [Google Scholar]
- Kant, A.K. Nature of Dietary Reporting by Adults in the Third National Health and Nutrition Examination Survey, 1988–1994. J. Am. Coll. Nutr. 2002, 21, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Briefel, R.R.; Sempos, C.T.; McDowell, M.A.; Chien, S.; Alaimo, K. Dietary methods research in the third National Health and Nutrition Examination Survey: Underreporting of energy intake. Am. J. Clin. Nutr. 1997, 65, 1203s–1209s. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Melzer, D. Vitamin D and cognitive impairment in the elderly U.S. population. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.M.; Borrell, L.N.; Papapanou, P.N.; Elkind, M.S.; Scarmeas, N.; Wright, C.B. Periodontitis is associated with cognitive impairment among older adults: Analysis of NHANES-III. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Hampl, J.S.; Taylor, C.A.; Johnston, C.S. Vitamin C deficiency and depletion in the United States: The Third National Health and Nutrition Examination Survey, 1988 to 1994. Am. J. Public Health 2004, 94, 870–875. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mary Louie, E.H.; Chen, L.; Spiegelman, D. The SAS LGTPHCURV9 Macro. Available online: https://www.hsph.harvard.edu/donna-spiegelman/software/lgtphcurv9/ (accessed on 3 March 2024).
- Noguchi-Shinohara, M.; Abe, C.; Yuki-Nozaki, S.; Dohmoto, C.; Mori, A.; Hayashi, K.; Shibata, S.; Ikeda, Y.; Sakai, K.; Iwasa, K.; et al. Higher Blood Vitamin C Levels are Associated with Reduction of Apolipoprotein E E4-related Risks of Cognitive Decline in Women: The Nakajima Study. J. Alzheimer’s Dis. 2018, 63, 1289–1297. [Google Scholar] [CrossRef]
- Lanyau-Domínguez, Y.; Macías-Matos, C.; Jesús, J.; María, G.; Suárez-Medina, R.; Eugenia, M.; Noriega-Fernández, L.; Guerra-Hernández, M.; Calvo-Rodríguez, M.; Sánchez-Gil, Y.; et al. Levels of Vitamins and Homocysteine in Older Adults with Alzheimer Disease or Mild Cognitive Impairment in Cuba. MEDICC Rev. 2020, 22, 40–47. [Google Scholar] [CrossRef]
- Rivière, S.; Birlouez-Aragon, I.; Nourhashémi, F.; Vellas, B. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int. J. Geriatr. Psychiatry 1998, 13, 749–754. [Google Scholar] [CrossRef]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimer’s Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef] [PubMed]
- von Arnim, C.A.; Herbolsheimer, F.; Nikolaus, T.; Peter, R.; Biesalski, H.K.; Ludolph, A.C.; Riepe, M.; Nagel, G. Dietary antioxidants and dementia in a population-based case-control study among older people in South Germany. J. Alzheimer’s Dis. 2012, 31, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Mann, U.; Arlt, S.; Ujeyl, A.; Lührs, C.; Müller-Thomsen, T.; Beisiegel, U. Influence of vitamin E and C supplementation on lipoprotein oxidation in patients with Alzheimer’s disease. Free Radic. Biol. Med. 2001, 31, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch. Neurol. 2004, 61, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, M.J.; Geerlings, M.I.; Ruitenberg, A.; van Swieten, J.C.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002, 287, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Raffaitin, C.; Letenneur, L.; Berr, C.; Tzourio, C.; Dartigues, J.F.; Alpérovitch, A. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology 2007, 69, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Holland, T.M.; Wang, Y.; Bennett, D.A.; Morris, M.C. Association of Strawberries and Anthocyanidin Intake with Alzheimer’s Dementia Risk. Nutrients 2019, 11, 3060. [Google Scholar] [CrossRef]
- Masaki, K.H.; Losonczy, K.G.; Izmirlian, G.; Foley, D.J.; Ross, G.W.; Petrovitch, H.; Havlik, R.; White, L.R. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 2000, 54, 1265–1272. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Kawasaki, Y.; Yamanaka, M.; Kawakami, N.; Katsuyama, Y.; Yoshida, H.; Kim, K.; Shiosaki, E.; Sonoda, A.; et al. Peripheral Vitamin C Levels in Alzheimer’s Disease: A Cross-Sectional Study. J. Nutr. Sci. Vitaminol. 2016, 62, 432–436. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Wilson, R.S.; Scherr, P.A. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 2002, 287, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Ulstein, I.; Bøhmer, T. Normal Vitamin Levels and Nutritional Indices in Alzheimer’s Disease Patients with Mild Cognitive Impairment or Dementia with Normal Body Mass Indexes. J. Alzheimer’s Dis. 2017, 55, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.X.; Shea, S.; Mayeux, R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch. Neurol. 2003, 60, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G.; et al. Antioxidants for Alzheimer disease: A randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012, 69, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Hu, Y.; Zhang, H.; Wang, T.; Han, Z.; Gao, S.; Wang, L.; Liu, G. Mendelian randomization to evaluate the effect of plasma vitamin C levels on the risk of Alzheimer’s disease. Genes Nutr. 2021, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Xu, W.; Gaglioti, A.H.; Holt, J.B.; Croft, J.B.; Mack, D.; McGuire, L.C. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s Dement. 2019, 15, 17–24. [Google Scholar] [CrossRef]
- Office of Dietary Supplement; National Institute of Health. Vitamin C: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/#en59 (accessed on 3 March 2024).
- Lauer, A.A.; Grimm, H.S.; Apel, B.; Golobrodska, N.; Kruse, L.; Ratanski, E.; Schulten, N.; Schwarze, L.; Slawik, T.; Sperlich, S.; et al. Mechanistic Link between Vitamin B12 and Alzheimer’s Disease. Biomolecules 2022, 12, 129. [Google Scholar] [CrossRef]
- Lee, D.H.; Folsom, A.R.; Harnack, L.; Halliwell, B.; Jacobs, D.R., Jr. Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am. J. Clin. Nutr. 2004, 80, 1194–1200. [Google Scholar] [CrossRef]
- Jia, J.; Ning, Y.; Chen, M.; Wang, S.; Yang, H.; Li, F.; Ding, J.; Li, Y.; Zhao, B.; Lyu, J.; et al. Biomarker Changes during 20 Years Preceding Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 712–722. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. NHANES I Epidemiologic Follow-Up Survey (NHEFS) Calibration Sample for NDI Matching Methodology. Available online: http://www.cdc.gov/nchs/data/datalinkage/mort_calibration_study.pdf (accessed on 3 March 2024).

| Serum Vitamin C (mg/dL) | ||||
|---|---|---|---|---|
| Characteristics | Tertile 1 (<0.56) | Tertile 2 (0.56–0.98) | Tertile 3 (>0.98) | p Values |
| Age, years | 69.5 (7.2) | 69.5 (7.10) | 70.6 (7.4) | 0.017 |
| Sex, male, % | 53.7 | 49.5 | 29.7 | <0.001 |
| Race/ethnicity, % | <0.001 | |||
| Non-Hispanic White | 78.6 | 83.7 | 91.4 | |
| Non-Hispanic Black | 13.3 | 7.7 | 3.4 | |
| Mexican American | 3.3 | 2.2 | 1.5 | |
| Other | 4.8 | 6.4 | 3.8 | |
| Some college degree or higher, % | 19.2 | 32.6 | 32.3 | <0.001 |
| Low income, % | 34.1 | 25.3 | 22.6 | <0.001 |
| Marital status, married, % | 59.7 | 63.4 | 62.7 | 0.124 |
| Systolic blood pressure, mmHg | 140 (19) | 138 (19) | 138 (19) | 0.046 |
| Smoking status, current, % | 25.8 | 10.8 | 11.5 | <0.001 |
| Alcohol intake, past year, % | 42.0 | 47.9 | 42.3 | 0.060 |
| Body mass index, kg/m2 | 27.7 (5.3) | 27.7 (5.1) | 26.1 (4.7) | <0.001 |
| Obese, % | 28.0 | 28.1 | 19.2 | <0.001 |
| Sedentary lifestyle, % | 22.5 | 19.3 | 15.4 | <0.001 |
| Total cholesterol, mg/dL | 224 (46) | 224 (45) | 226 (42) | 0.300 |
| Diet quality, Healthy Eating Index | 61.3 (13.2) | 69.3 (12.7) | 72.8 (12.3) | <0.001 |
| Dietary vitamin C intake, mg | 65.1 (62.8) | 112.7 (94.4) | 135.1 (108.4) | <0.001 |
| Vitamin C supplement intake, % | 6.2 | 19.4 | 42.5 | <0.001 |
| Prevalent health conditions, % | ||||
| Hypertension | 53.6 | 53.2 | 48.9 | 0.079 |
| Diabetes | 11.5 | 11.1 | 5.9 | <0.001 |
| Cancer | 18.3 | 17.7 | 23.0 | 0.009 |
| Cardiovascular disease | 22.3 | 16.3 | 13.7 | <0.001 |
| Serum Vitamin C (mg/dL) | ||||
|---|---|---|---|---|
| Tertile 1 (<0.56) | Tertile 2 (0.56–0.98) | Tertile 3 (>0.98) | p Value | |
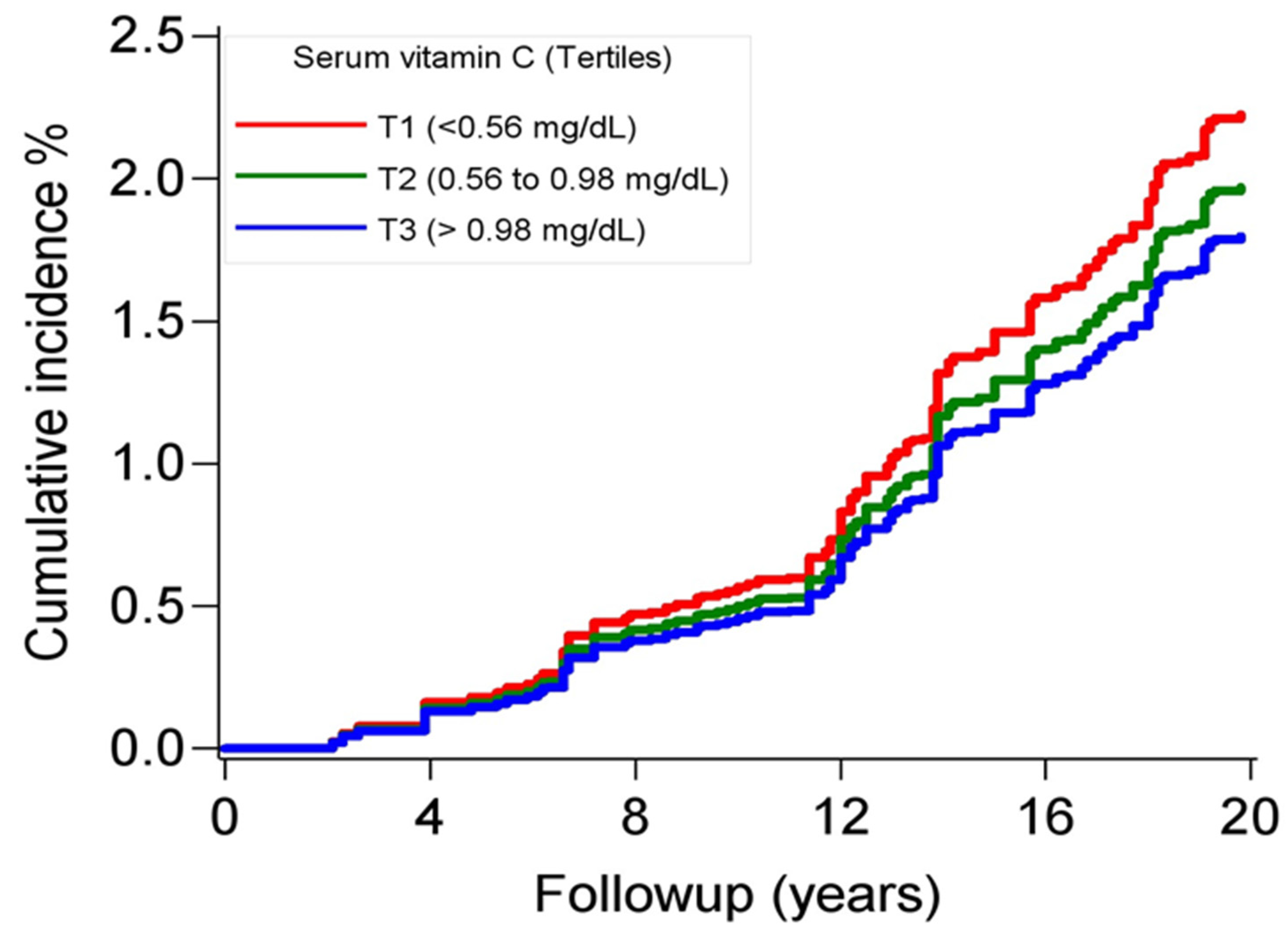
| Incidence, per 1000 | 2.9 (2.2–3.7) | 2.3 (1.7–3.0) | 2.2 (1.6–2.9) | |
| Model 1 | 1 (referent) | 0.72 (0.36–1.44) | 0.51 (0.31–0.84) | 0.028 |
| Model 2 | 1 (referent) | 0.66 (0.32–1.33) | 0.49 (0.29–0.82) | 0.030 |
| Model 3 | 1 (referent) | 0.65 (0.33–1.26) | 0.50 (0.30–0.81) | 0.024 |
| Model 4 | 1 (referent) | 0.59 (0.29–1.24) | 0.44 (0.25–0.77) | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah, D.; Ingabire-Gasana, E.; Appiah, L.; Yang, J. The Relation of Serum Vitamin C Concentrations with Alzheimer’s Disease Mortality in a National Cohort of Community-Dwelling Elderly Adults. Nutrients 2024, 16, 1672. https://doi.org/10.3390/nu16111672
Appiah D, Ingabire-Gasana E, Appiah L, Yang J. The Relation of Serum Vitamin C Concentrations with Alzheimer’s Disease Mortality in a National Cohort of Community-Dwelling Elderly Adults. Nutrients. 2024; 16(11):1672. https://doi.org/10.3390/nu16111672
Chicago/Turabian StyleAppiah, Duke, Elyvine Ingabire-Gasana, Linda Appiah, and Jeanne Yang. 2024. "The Relation of Serum Vitamin C Concentrations with Alzheimer’s Disease Mortality in a National Cohort of Community-Dwelling Elderly Adults" Nutrients 16, no. 11: 1672. https://doi.org/10.3390/nu16111672
APA StyleAppiah, D., Ingabire-Gasana, E., Appiah, L., & Yang, J. (2024). The Relation of Serum Vitamin C Concentrations with Alzheimer’s Disease Mortality in a National Cohort of Community-Dwelling Elderly Adults. Nutrients, 16(11), 1672. https://doi.org/10.3390/nu16111672




