Extended Treatment with Micron-Size Oral Palmitoylethanolamide (PEA) in Chronic Pain: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Endpoints
2.4. Risk of Bias
2.5. Statistical Analysis
3. Results
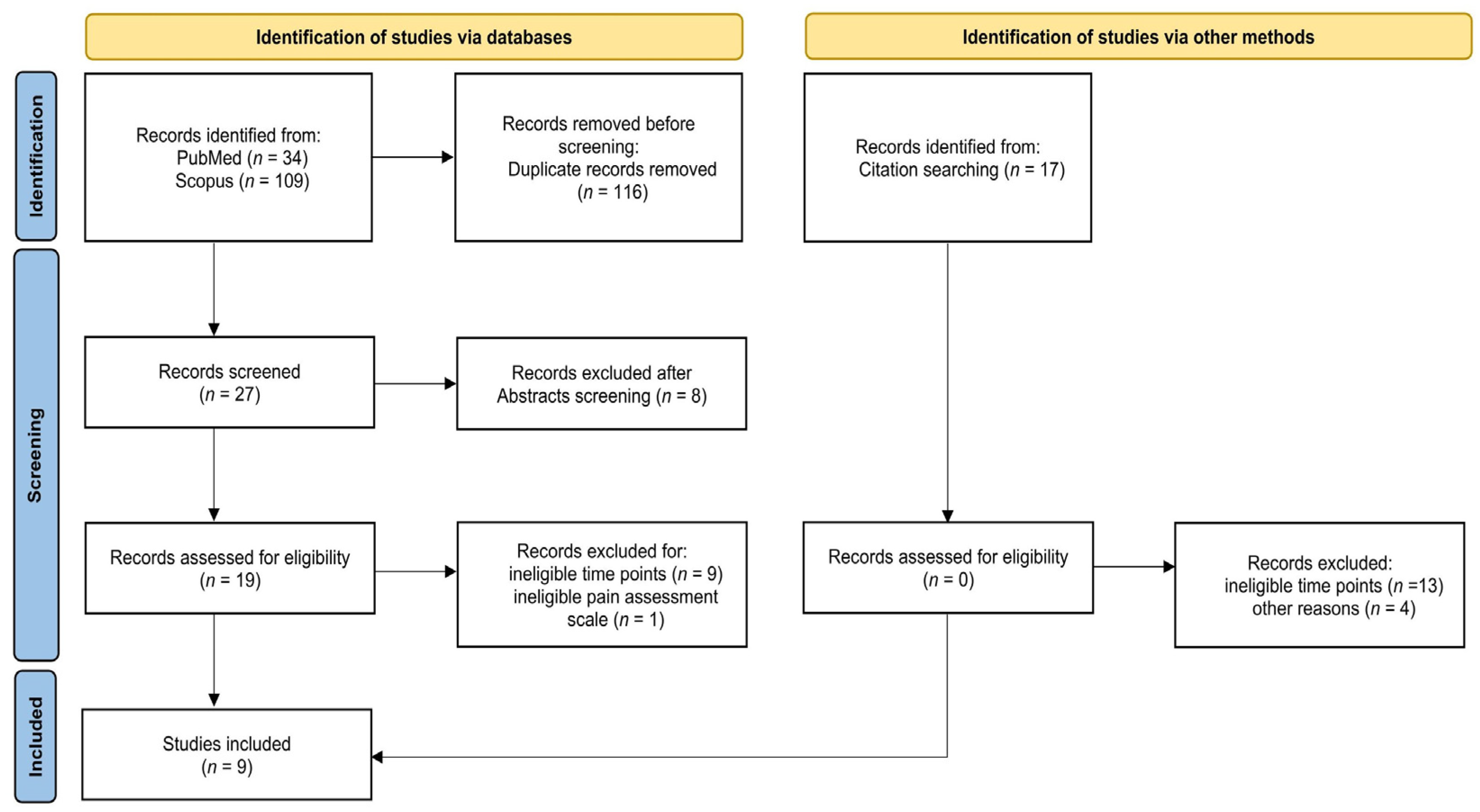
3.1. Search Results
3.2. Description of the Included Studies
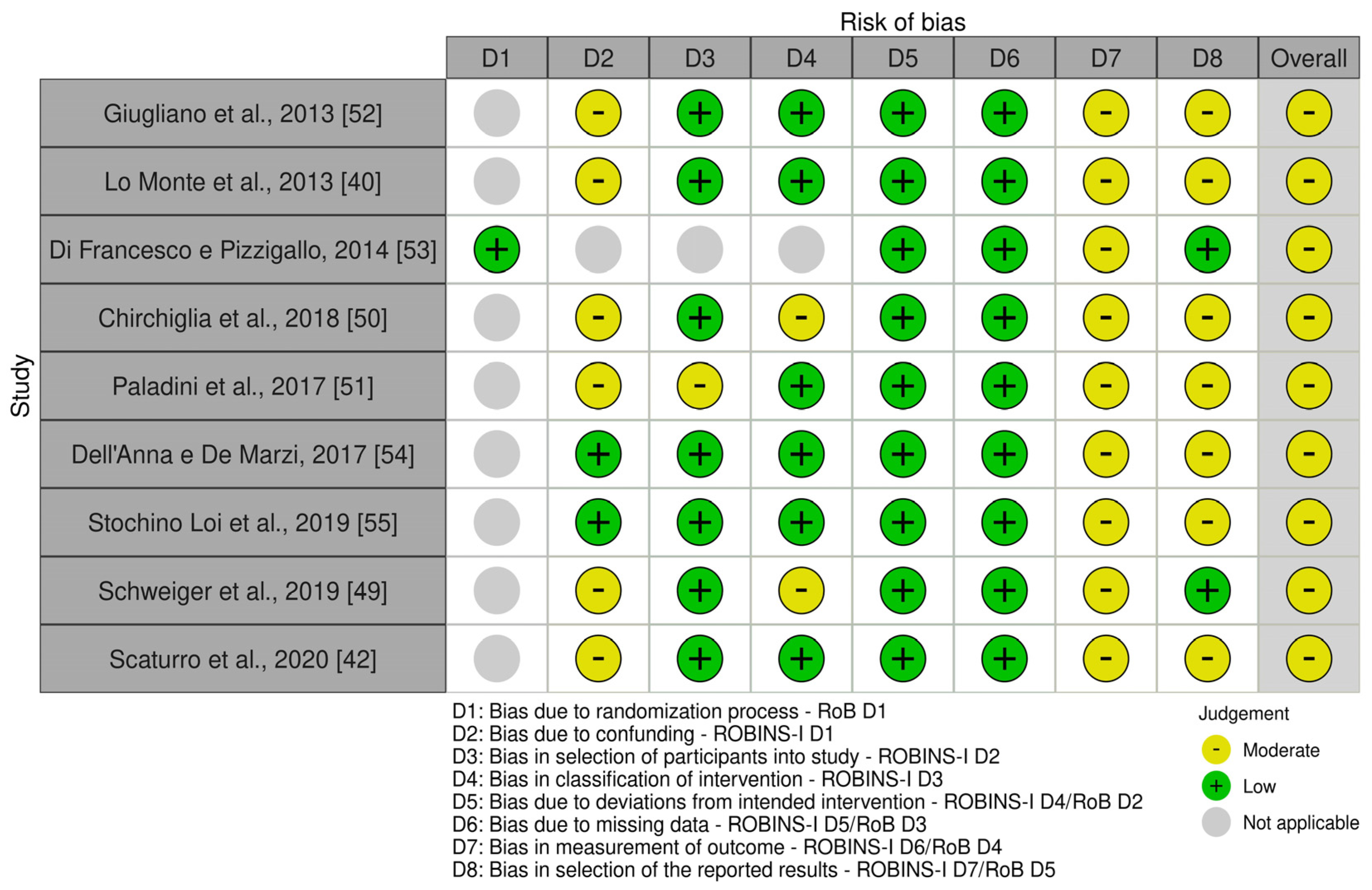
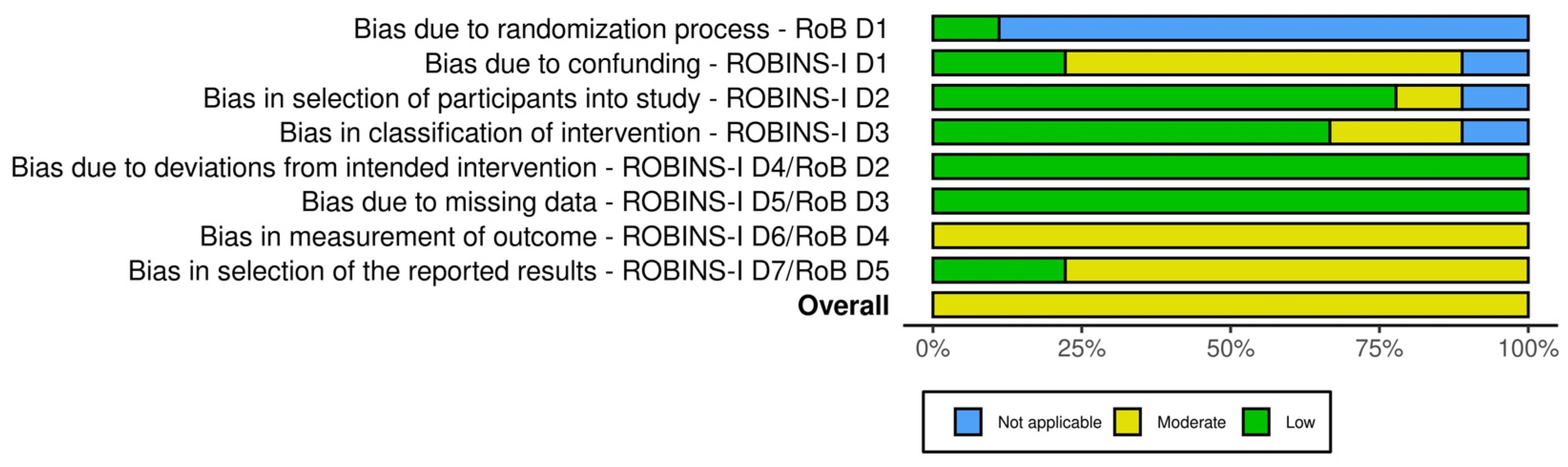
3.3. Risk of Bias
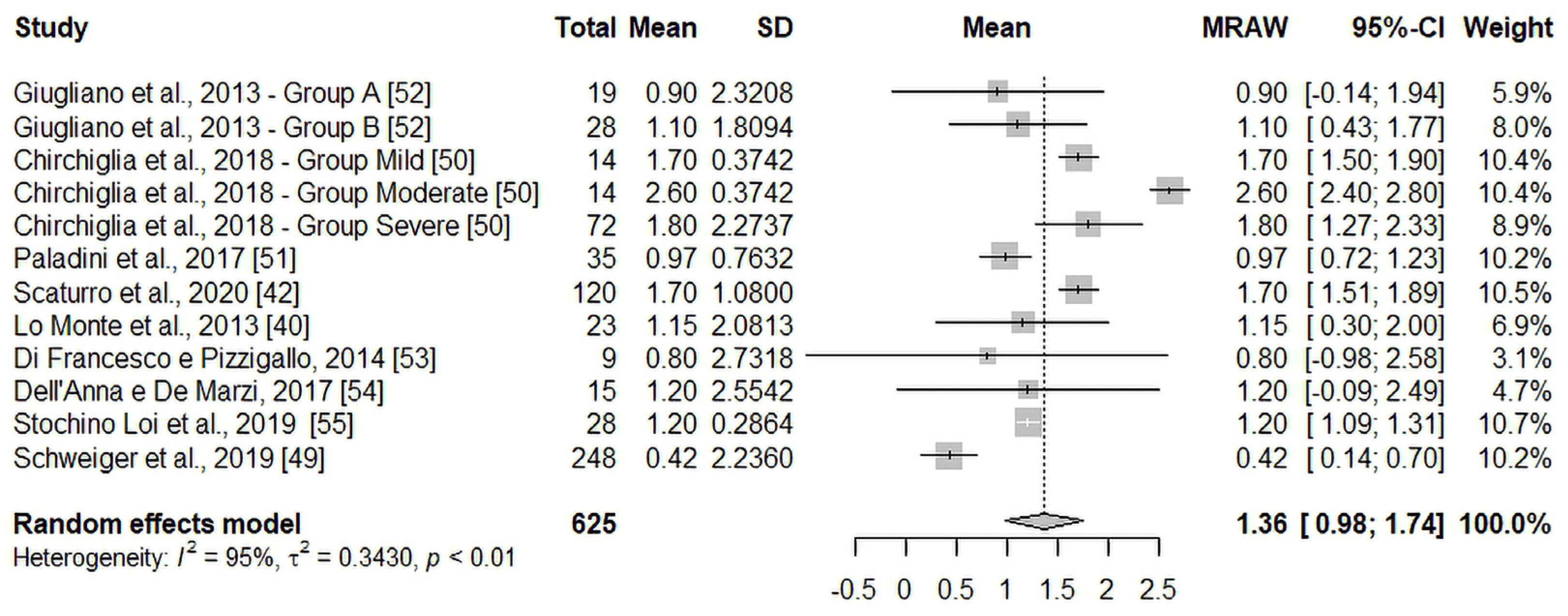
3.4. Primary Endpoint: T60 vs. T30 Pain Intensity Change
3.5. Secondary Endpoints: T30 vs. T0 Pain Intensity Change, and Percentage Variation over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kang, Y.; Trewern, L.; Jackman, J.; McCartney, D.; Soni, A. Chronic Pain: Definitions and Diagnosis. BMJ 2023, 381, e076036. [Google Scholar] [CrossRef]
- Latina, R.; De Marinis, M.G.; Giordano, F.; Osborn, J.F.; Giannarelli, D.; Di Biagio, E.; Varrassi, G.; Sansoni, J.; Bertini, L.; Baglio, G.; et al. Epidemiology of Chronic Pain in the Latium Region, Italy: A Cross-Sectional Study on the Clinical Characteristics of Patients Attending Pain Clinics. Pain. Manag. Nurs. 2019, 20, 373–381. [Google Scholar] [CrossRef]
- Hanna, M.; Perrot, S.; Varrassi, G. Critical Appraisal of Current Acute LBP Management and the Role of a Multimodal Analgesia: A Narrative Review. Pain. Ther. 2023, 12, 377–398. [Google Scholar] [CrossRef]
- Collins, A. Pain Management in Primary Care: A Review of the Updated CDC Guideline. Nurse Pract. 2024, 49, 13–19. [Google Scholar] [CrossRef]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Howard Bloch, M. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain. Physician 2017, 20, 353–362. [Google Scholar]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-Analysis. Pain. Physician 2016, 19, 11–24. [Google Scholar] [PubMed]
- Bettoni, I.; Comelli, F.; Colombo, A.; Bonfanti, P.; Costa, B. Non-Neuronal Cell Modulation Relieves Neuropathic Pain: Efficacy of the Endogenous Lipid Palmitoylethanolamide. CNS Neurol. Disord. Drug Targets 2013, 12, 34–44. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Glia and Mast Cells as Targets for Palmitoylethanolamide, an Anti-Inflammatory and Neuroprotective Lipid Mediator. Mol. Neurobiol. 2013, 48, 340–352. [Google Scholar] [CrossRef]
- Skaper, S.D.; Giusti, P.; Facci, L. Microglia and Mast Cells: Two Tracks on the Road to Neuroinflammation. FASEB J. 2012, 26, 3103–3117. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Fusco, M.; Della Valle, M.F.; Zusso, M.; Costa, B.; Giusti, P. Palmitoylethanolamide, a naturally occurring disease-modifying agent in neuropathic pain. Inflammopharmacology 2014, 22, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Keppel Hesselink, J.M. New Targets in Pain, Non-Neuronal Cells, and the Role of Palmitoylethanolamide. Open Pain. J. 2012, 5, 12–23. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Wasner, G. Central Pain Syndromes. Curr. Pain. Headache Rep. 2010, 14, 489–496. [Google Scholar] [CrossRef]
- Vergne-Salle, P.; Bertin, P. Chronic pain and neuroinflammation. Jt. Bone Spine 2021, 88, 105222. [Google Scholar] [CrossRef]
- Bettini, L.; Moore, K. Central Sensitization in Functional Chronic Pain Syndromes: Overview and Clinical Application. Pain. Manag. Nurs. 2016, 17, 333–338. [Google Scholar] [CrossRef]
- Gatti, A.; Lazzari, M.; Gianfelice, V.; Di Paolo, A.; Sabato, E.; Sabato, A.F. Palmitoylethanolamide in the Treatment of Chronic Pain Caused by Different Etiopathogenesis. Pain. Med. 2012, 13, 1121–1130. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Martín, A.D.; Ferrer, F.G.; Puertas, M.I.; Muro, A.L.; González, J.M.; Prieto, J.P.; Taberna, I.R. N-Palmitoylethanolamide in the Treatment of Neuropathic Pain Associated with Lumbosciatica. Pain. Manag. 2012, 2, 119–124. [Google Scholar] [CrossRef]
- Cruccu, G.; Di Stefano, G.; Marchettini, P.; Truini, A. Micronized Palmitoylethanolamide: A Post Hoc Analysis of a Controlled Study in Patients with Low Back Pain—Sciatica. CNS Neurol. Disord. Drug Targets 2019, 18, 491–495. [Google Scholar] [CrossRef]
- Capra, A.P.; Ardizzone, A.; Crupi, L.; Calapai, F.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Efficacy of Palmitoylethanolamide and Luteolin Association on Post-Covid Olfactory Dysfunction: A Systematic Review and Meta-Analysis of Clinical Studies. Biomedicines 2023, 11, 2189. [Google Scholar] [CrossRef]
- Clayton, P.; Hill, M.; Bogoda, N.; Subah, S.; Venkatesh, R. Palmitoylethanolamide: A Natural Compound for Health Management. Int. J. Mol. Sci. 2021, 22, 5305. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 2019, 11, 2175. [Google Scholar] [CrossRef] [PubMed]
- Casale, R.; Symeonidou, Z.; Ferfeli, S.; Micheli, F.; Scarsella, P.; Paladini, A. Food for Special Medical Purposes and Nutraceuticals for Pain: A Narrative Review. Pain. Ther. 2021, 10, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Moriello, A.S. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries. A Systematic Review. Int. J. Mol. Sci. 2020, 21, 9526. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/Ultramicronized Palmitoylethanolamide Displays Superior Oral Efficacy Compared to Non micronized Palmitoylethanolamide in a Rat Model of Inflammatory Pain. J. Neuroinflammation 2014, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cordaro, M.; Verde, R.; Schiano Moriello, A.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-Hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Indraccolo, U.; Favilli, A.; Dell’anna, A.; Di Francesco, A.; Dionisi, B.; Giugliano, E.; Murina, F.; Stocco, E. Looking for Responders among Women with Chronic Pelvic Pain Treated with a Comicronized Formulation of Micronized Palmitoylethanolamide and Polydatin. Biomed. Res. Int. 2022, 2022, 8620077. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Stanghellini, V.; Barbaro, M.R.; Cogliandro, R.F.; Bellacosa, L.; Santos, J.; Vicario, M.; Pigrau, M.; Alonso Cotoner, C.; Lobo, B.; et al. Randomized Clinical Trial: The Analgesic Properties of Dietary Supplementation with Palmitoylethanolamide and Polydatin in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2017, 45, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, E.; Armentano, M.; Giugliano, B.; Sena, T.; Giuliano, P.; Loffredo, C.; Mastrantonio, P. Effectiveness of the Association N-Palmitoylethanolamine and Transpolydatin in the Treatment of Primary Dysmenorrhea. J. Pediatr. Adolesc. Gynecol. 2015, 28, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Biasiotta, A.; Di Stefano, G.; La Cesa, S.; Leone, C.; Cartoni, C.; Federico, V.; Petrucci, M.; Cruccu, G. Palmitoylethanolamide Restores Myelinated-Fibre Function in Patients with Chemotherapy-Induced Painful Neuropathy. CNS Neurol. Disord. Drug Targets 2011, 10, 916–920. [Google Scholar] [CrossRef]
- Canteri, L.; Petrosino, S.; Guida, G. Reducción Del Consumo de Antiinflamatorios y Analgésicos En El Tratamiento Del Dolor Neuropático Crónico En Pacientes Afectados Por Lumbociatalgia de Tipo Compresivo y En Tratamiento Con Normast® 300 Mg. Dolor 2010, 25, 227–234. [Google Scholar]
- Guida, G.; De Martino, M.; De Fabiani, A.; Cantieri, L.; Alexandre, A.; Vassallo, G.M.; Rogai, M.; Lanaia, F.; Petrosino, S. La Palmitoiletanolamida (Normast ®) En El Dolor Neuropático Crónico Por Lumbociatalgia de Tipo Compresivo: Estudio Clínico Multicéntrico. Dolor 2010, 25, 35–42. [Google Scholar]
- Murina, F.; Graziottin, A.; Felice, R.; Radici, G.; Tognocchi, C. Vestibulodynia: Synergy Between Palmitoylethanolamide + Transpolydatin and Transcutaneous Electrical Nerve Stimulation. J. Low. Genit. Tract. Dis. 2013, 17, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Dalla Volta, G.; Zavarize, P.; Ngonga, G.; Carli, D. Ultramicronized Palmitoylethanolamide Reduces Frequency and Pain Intensity in Migraine. A Pilot Study. Int. J. Neurol. Brain Disord. 2016, 3, 1–5. [Google Scholar] [CrossRef]
- Conigliaro, R.; Drago, V.; Foster, P.; Schievano, C.; Di Marzo, V. Use of Palmitoylethanolamide in the Entrapment Neuropathy of the Median in the Wrist. Minerva Med. 2011, 102, 141–147. [Google Scholar] [PubMed]
- Del Giorno, R.; Skaper, S.; Paladini, A.; Varrassi, G.; Coaccioli, S. Palmitoylethanolamide in Fibromyalgia: Results from Prospective and Retrospective Observational Studies. Pain. Ther. 2015, 4, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Cocito, D.; Peci, E.; Ciaramitaro, P.; Merola, A.; Lopiano, L. Short-Term Efficacy of Ultramicronized Palmitoylethanolamide in Peripheral Neuropathic Pain. Pain. Res. Treat. 2014, 2014, 854560. [Google Scholar] [CrossRef] [PubMed]
- Putzu, G.A. Efficacy of Ultramicronized Palmitoylethanolamide on the Clinical Symptoms of Charcot-Marie-Tooth Neuropathy. Archiv Neurol. Neurosurgery 2016, 1, 12–14. [Google Scholar]
- Marini, I.; Cavallaro, M.; Bartolucci, M.; Alessandri-Bonetti, A.; Gatto, M.; Cordaro, M.; Checchi, L. Can Celecoxib Enhance Palmitoylethanolamide’s Effect in the Treatment of Temporo-Mandibular Arthralgia in Osteoarthritis Patients? J. Transl. Sci. 2018, 5, 1–4. [Google Scholar]
- Chirchiglia, D.; Cione, E.; Caroleo, M.C.; Wang, M.; Di Mizio, G.; Faedda, N.; Giacolini, T.; Siviglia, S.; Guidetti, V.; Gallelli, L. Effects of Add-on Ultramicronized N-Palmitol Ethanol Amide in Patients Suffering of Migraine with Aura: A Pilot Study. Front. Neurol. 2018, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Lo Monte, G.; Soave, I.; Marci, R. Utilizzo Della Palmitoiletanolamide Micronizzata (PEA)-Transpolidatina Nel Trattamento Del Dolore Perlvico Cronico in Donne Affette Da Endometriosi. Risultati Preliminari. Minerva Ginecol. 2013, 65, 453–463. [Google Scholar]
- Marini, I.; Bartolucci, M.L.; Bortolotti, F.; Gatto, M.R.; Bonetti, G.A. Palmitoylethanolamide Versus a Nonsteroidal Anti-Inflammatory Drug in the Treatment of Temporomandibular Joint Inflammatory Pain. J. Orofac. Pain. 2012, 26, 99–104. [Google Scholar] [PubMed]
- Scaturro, D.; Asaro, C.; Lauricella, L.; Tomasello, S.; Varrassi, G.; Mauro, G.L. Combination of Rehabilitative Therapy with Ultramicronized Palmitoylethanolamide for Chronic Low Back Pain: An Observational Study. Pain. Ther. 2020, 9, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Chirchiglia, D.; Paventi, S.; Seminara, P.; Cione, E.; Gallelli, L. N-Palmitoyl Ethanol Amide Pharmacological Treatment in Patients With Nonsurgical Lumbar Radiculopathy. J. Clin. Pharmacol. 2018, 58, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Passavanti, M.B.; Fiore, M.; Sansone, P.; Aurilio, C.; Pota, V.; Barbarisi, M.; Fierro, D.; Pace, M.C. The Beneficial Use of Ultramicronized Palmitoylethanolamide as Add-on Therapy to Tapentadol in the Treatment of Low Back Pain: A Pilot Study Comparing Prospective and Retrospective Observational Arms. BMC Anesthesiol. 2017, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, M.; Schievano, C.; Saleh, O. Micronized Palmitoylethanolamide Reduces Bladder Chronic Pelvic Pain Due to Different Etiologies and Improves Bladder Functions. Pelviperineology 2017, 36, 92–96. [Google Scholar]
- Cervigni, M.; Nasta, L.; Schievano, C.; Lampropoulou, N.; Ostardo, E. Micronized Palmitoylethanolamide-Polydatin Reduces the Painful Symptomatology in Patients with Interstitial Cystitis/Bladder Pain Syndrome. Biomed. Res. Int. 2019, 2019, 9828397. [Google Scholar] [CrossRef] [PubMed]
- Orefice, N.S.; Alhouayek, M.; Carotenuto, A.; Montella, S.; Barbato, F.; Comelli, A.; Calignano, A.; Muccioli, G.G.; Orefice, G. Oral Palmitoylethanolamide Treatment Is Associated with Reduced Cutaneous Adverse Effects of Interferon-Β1a and Circulating Proinflammatory Cytokines in Relapsing–Remitting Multiple Sclerosis. Neurotherapeutics 2016, 13, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, M.; Cilli, V.; De Vitis, R.; Militerno, A.; Fanfani, F. Ultra-Micronized Palmitoylethanolamide Effects on Sleep-Wake Rhythm and Neuropathic Pain Phenotypes in Patients with Carpal Tunnel Syndrome: An Open-Label, Randomized Controlled Study. CNS Neurol. Disord. Drug Targets 2018, 17, 291–298. [Google Scholar] [CrossRef]
- Schweiger, V.; Martini, A.; Bellamoli, P.; Donadello, K.; Schievano, C.; Balzo, G.D.; Sarzi-Puttini, P.; Parolini, M.; Polati, E. Ultramicronized Palmitoylethanolamide (Um-PEA) as Add-on Treatment in Fibromyalgia Syndrome (FMS): Retrospective Observational Study on 407 Patients. CNS Neurol. Disord. Drug Targets 2019, 18, 326–333. [Google Scholar] [CrossRef]
- Chirchiglia, D.; Chirchiglia, P.; Signorelli, F. Nonsurgical Lumbar Radiculopathies Treated with Ultramicronized Palmitoylethanolamide (UmPEA): A Series of 100 Cases. Neurol. Neurochir. Pol. 2018, 52, 44–47. [Google Scholar] [CrossRef]
- Paladini, A.; Varrassi, G.; Bentivegna, G.; Carletti, S.; Piroli, A.; Coaccioli, S. Palmitoylethanolamide in the Treatment of Failed Back Surgery Syndrome. Pain. Res. Treat. 2017, 2017, 1486010. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, E.; Cagnazzo, E.; Soave, I.; Lo Monte, G.; Wenger, J.M.; Marci, R. The Adjuvant Use of N-Palmitoylethanolamine and Transpolydatin in the Treatment of Endometriotic Pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Pizzigallo, D. Use of Micronized Palmitoylethanolamide and Trans-Polydatin in Chronic Pelvic Pain Associated with Endometriosis. An Open-Label Study. Giorn. It Ost Gin 2014, 36, 353–357. [Google Scholar] [CrossRef]
- Dell’Anna, A.; De Marzi, C.A. Mast Cells and Microglia: New Therapeutic Targets for the Treatment of Endometriosis-Associated Symptomatology. Giorn. It Ost Gin 2017, 39, 193–201. [Google Scholar]
- Stochino Loi, E.; Pontis, A.; Cofelice, V.; Pirarba, S.; Fais, M.F.; Daniilidis, A.; Melis, I.; Paoletti, A.M.; Angioni, S. Effect of Ultramicronized-Palmitoylethanolamide and Co-Micronizedpalmitoylethanolamide/Polydatin on Chronic Pelvic Pain and Quality of Life in Endometriosis Patients: An Open-Label Pilot Study. Int. J. Womens Health 2019, 11, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; E McKenzie, J.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; A Akl, E.; E Brennan, S.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A Basic Introduction to Fixed-Effect and Random-Effects Models for Meta-Analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Gu, S.; Shi, J.; Tang, Z.; Sawhney, M.; Hu, H.; Shi, L.; Fonseca, V.; Dong, H. Comparison of Glucose Lowering Effect of Metformin and Acarbose in Type 2 Diabetes Mellitus: A Meta-Analysis. PLoS ONE 2015, 10, e0126704. [Google Scholar]
- PRISMA—Tansparent Reporting of Systematic Reviews and Meta-Analyses. Available online: http://prisma-statement.org (accessed on 29 September 2023).
- Lang-Illievich, K.; Klivinyi, C.; Lasser, C.; Brenna, C.T.A.; Szilagyi, I.S.; Bornemann-Cimenti, H. Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients 2023, 15, 1350. [Google Scholar] [CrossRef] [PubMed]
- Echeverria-Villalobos, M.; Tortorici, V.; Brito, B.E.; Ryskamp, D.; Uribe, A.; Weaver, T. The role of neuroinflammation in the transition of acute to chronic pain and the opioid-induced hyperalgesia and tolerance. Front. Pharmacol. 2023, 14, 1297931. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M. Microglia in the CNS and Neuropathic Pain. Adv. Exp. Med. Biol. 2018, 1099, 77–91. [Google Scholar] [PubMed]
- Mai, L.; Liu, Q.; Huang, F.; He, H.; Fan, W. Involvement of Mast Cells in the Pathophysiology of Pain. Front. Cell Neurosci. 2021, 15, 665066. [Google Scholar] [PubMed]
- Polati, E.; Martini, A.; Schweiger, V. Ultramicronized palmitoylethanolamide treatment in central neuropathic pain following longstanding spinal cord injury: Try to extinguish the fire after everything was burned. Pain 2017, 158, 763–764. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, G.; Gamba, D. Chronic Pain in Dogs and Cats: Is There Place for Dietary Intervention with Micro-Palmitoylethanolamide? Animals 2021, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.T.; Young, J.P.B.; Lamoreaux, L.; Werth, J.L.; Poole, R.M. Clinical Importance of Changes in Chronic Pain Intensity Measured on an 11-Point Numerical Pain Rating Scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Bennett, R.M.; Bushmakin, A.G.; Cappelleri, J.C.; Zlateva, G.; Sadosky, A.B. Minimal clinically important difference in the fibromyalgia impact questionnaire. J. Rheumatol. 2009, 36, 1304–1311. [Google Scholar] [CrossRef]
| Reference | Study Design | Number of Patients | Chronic Pain Condition | Pain Tool | Treatment | Concomitant Therapies | Main Results |
|---|---|---|---|---|---|---|---|
| Chirchiglia et al., 2018 [50] | Retrospective, case-series | 100 (73 males, 27 females) | Low back pain (nonsurgical lumbar radiculopathies with or without sciatica) | VAS | 1st cycle: 1200 mg/day sublingual umPEA for 10 days; 1200 mg/day umPEA tablets for 20 days 2nd cycle: 600 mg/day umPEA tablets for 1 month | 1st cycle: Paracetamol 500 mg + codeine 30 mg/day for 4 days, then for 1 month as needed 2nd cycle: Paracetamol 500 mg + codeine 30 mg/day as needed | Significant decrease in pain intensity after one month of treatment with a further improvement after the second cycle. Greater effect on patients with mild and moderate pain |
| Paladini et al., 2017 [51] | Observational, open-label | 35 (15 males, 20 females) | Low back pain (failed back surgery syndrome) | VAS | 1200 mg/day umPEA for 1 month; subsequently 600 mg/day for the second month | Tapentadol 150 mg/day Pregabalin 300 mg/day | Further and significant decrease in pain intensity after umPEA supplementation |
| Scaturro et al., 2020 [42] | Prospective, observational | 120 (37 males, 83 females) | Low back pain (lumbosciatica/ lumbocruralgia) | NRS | 1200 mg/day umPEA for 20 days; subsequently 600 mg/day for 40 days | Pregabalin 150 mg/day or oxycodone 10 mg/day, daily functional rehabilitation, relaxation massage | Significant reduction in chronic pain to a not clinically relevant intensity; improvement in QoL; decrease in pain-dependent disability |
| Lo Monte et al., 2013 [40] | Pilot, open-label | 24 females | Chronic pelvic pain (endometriosis) | VAS | 800 + 80 mg/day mPEAPol for 90 days | Analgesics and hormonal therapies | Significant reduction in pelvic pain, dysmenorrhea and dyspareunia; improvement in QoL; decrease NSAIDs consumption |
| Giugliano et al., 2013 [52] | Prospective, open-label | 47 females Group A: 19 recto-vaginal endometriosis; Group B: 28 ovarian endometriosis | Chronic pelvic pain (endometriosis) | VAS | 800 + 80 mg/day mPEAPol for 90 days | Estrogen–progestin pill (13 patients group A, 18 patients group B), anti-inflammatory drugs (6 patients group A, 10 patients group B) | Significant reduction in pelvic pain, dysmenorrhea, dyspareunia regardless of lesion site already after 30 days, reaching the maximum relief after 60 days |
| Di Francesco and Pizzigallo, 2014 [53] | Three-arm, randomized, open-label | 30 females Group A: 10 mPEAPol; Group B: 10 leuprorelin acetate; Group C: 10 ethinylestradiol + drospirenone | Chronic pelvic pain (endometriosis) | NRS | 800 + 80 mg/day mPEAPol for 6 months | NA | Significant decrease in painful symptoms in all groups regardless of the treatment; improvement in QoL; not interference with woman fertility |
| Dell’Anna and De Marzi, 2017 [54] | Observational, open-label | 17 females | Chronic pelvic pain (endometriosis) | NRS | 600 mg/day umPEA + 1200 + 120 mg/day mPEAPol for 4 months | Analgesics and anti-inflammatory drugs as needed | Significant reduction in pelvic pain, dysmenorrhea, dyschezia, dyspareunia and dysuria; improvement in QoL; not interference with fertility |
| Stochino Loi et al., 2019 [55] | Single arm, non-randomized, open-label | 30 females | Chronic pelvic pain (endometriosis) | VAS | 1200 mg/day umPEA for 10 days; subsequently 800 + 80 mg/day mPEAPol for 80 days | Ketoprofen 80 mg, maximum twice daily | Significant reduction in pelvic pain, dyspareunia, dysmenorrhea, dyschezia; improvement in QoL and psychological well-being; significant reduction in the ketoprofen consumption |
| Schweiger et al., 2019 [49] | Retrospective, observational | 359 (23 males, 336 females) | Fibromyalgia syndrome (FMS) | VAS | 1800 mg/day umPEA for 10 days; subsequently 1200 mg/day for 20 days; followed by 600 mg/day until the 15th months | Various FMS drug treatments (SSRI, SNRI, GBPs, TCA, BZD, OPI, NSAIDs, MR, ACT) | Significant improvement in pain intensity and QoL |
| Reference | T0 n | T0 NRS/VAS Mean ± SD | T30 n | T30 NRS/VAS Mean ± SD | T60 n | T60 NRS/VAS Mean ± SD |
|---|---|---|---|---|---|---|
| Giugliano et al., 2013 [52] Group A | 19 | 5.8 ± 2.8 | 19 | 3.8 ± 2.4 | 19 | 2.9 ± 1.7 |
| Giugliano et al., 2013 [52] Group B | 28 | 4.6 ± 2.4 | 28 | 2.7 ± 1.7 | 28 | 1.6 ± 1.6 |
| Chirchiglia et al., 2018 [50] Group Mild | 14 | 3.5 ± 0.75 | 14 | 1.7 ± 0.37 | 14 | 0 ± 0 |
| Chirchiglia et al., 2018 [50] Group Moderate | 14 | 5.3 ± 0.37 | 14 | 2.6 ± 0.37 | 14 | 0 ± 0 |
| Chirchiglia et al., 2018 [50] Group Severe | 72 | 8.7 ± 0.85 | 72 | 6.4 ± 1.27 | 72 | 4.6 ± 2.46 |
| Paladini et al., 2017 [51] | 35 | 4.3 ± 0.65 | 35 | 2.7 ± 0.53 | 35 | 1.7 ± 0.65 |
| Scaturro et al., 2020 [42] | 120 | 6.3 ± 1.10 | 120 | 3.7 ± 0.99 | 120 | 2.0 ± 0.99 |
| Lo Monte et al., 2013 [40] | 24 | 5.1 ± 2.65 | 23 | 3.0 ± 2.09 | 23 | 1.9 ± 1.65 |
| Di Francesco and Pizzigallo, 2014 [53] | 10 | 5.3 ± 3.63 | 9 | 4.8 ± 2.58 | 9 | 4.0 ± 2.40 |
| Dell’Anna and De Marzi, 2017 [54] | 17 | 7.8 ± 1.53 | 16 | 6.1 ± 2.04 | 15 | 4.9 ± 2.56 |
| Stochino Loi et al., 2019 [55] | 30 | 7.2 ± 1.2 | 30 | 4.1 ± 0.3 | 28 | 2.9 ± 0.2 |
| Schweiger et al., 2019 [49] | 359 | 7.58 ± 1.52 | 303 | 6.3 ± 1.98 | 248 | 5.9 ± 2.09 |
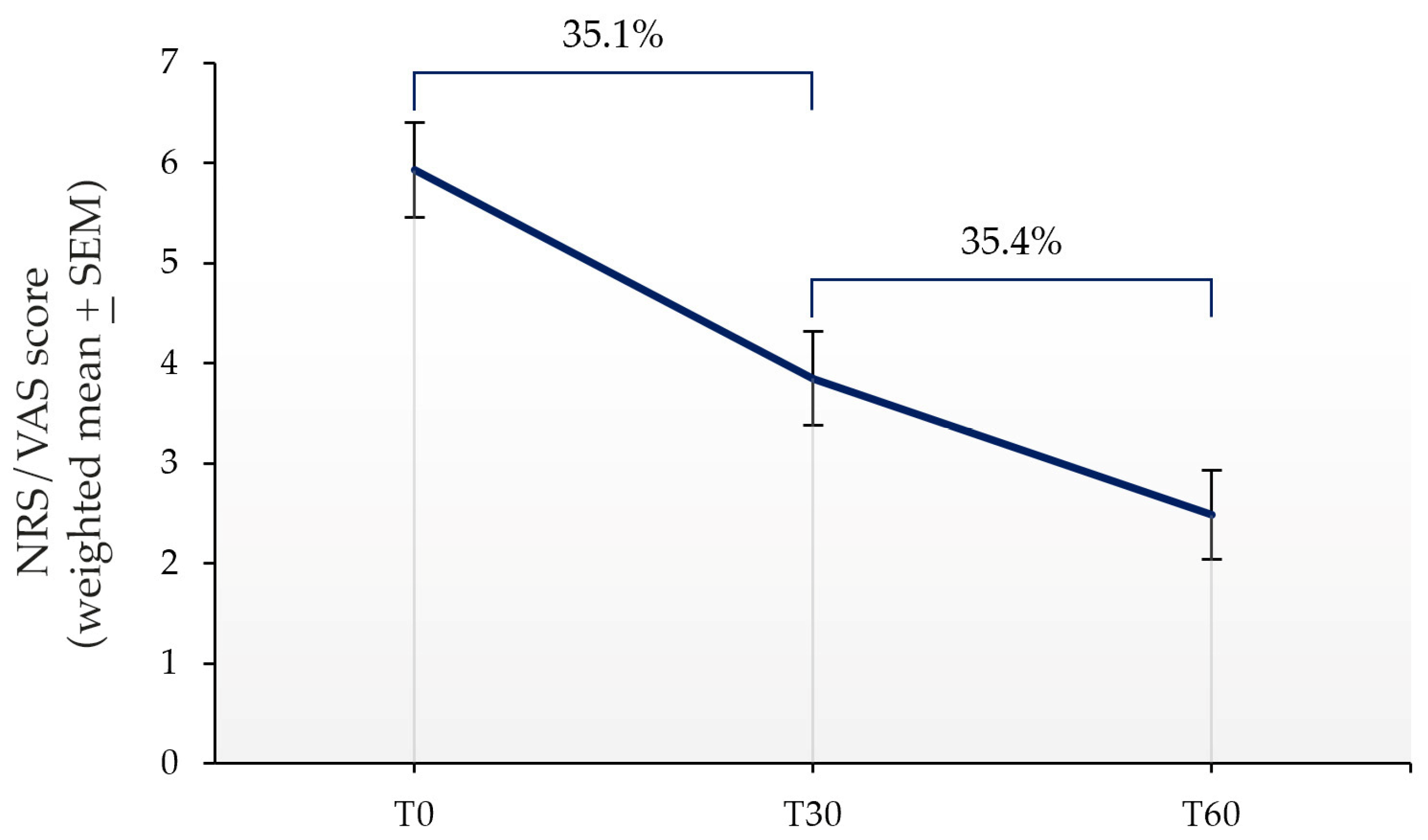
| T0 | T30 | T60 | |
|---|---|---|---|
| NRS/VAS score (Weighted mean ± SEM) | 5.93 ± 0.47 | 3.85 ± 0.47 | 2.49 ± 0.45 |
| Effect vs. T0 (score) | 2.08 | 3.44 | |
| Effect vs. T30 (score) | 1.36 | ||
| Effect vs. previous follow-up (%) | 35.1 | 35.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweiger, V.; Schievano, C.; Martini, A.; Polati, L.; Del Balzo, G.; Simari, S.; Milan, B.; Finco, G.; Varrassi, G.; Polati, E. Extended Treatment with Micron-Size Oral Palmitoylethanolamide (PEA) in Chronic Pain: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1653. https://doi.org/10.3390/nu16111653
Schweiger V, Schievano C, Martini A, Polati L, Del Balzo G, Simari S, Milan B, Finco G, Varrassi G, Polati E. Extended Treatment with Micron-Size Oral Palmitoylethanolamide (PEA) in Chronic Pain: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(11):1653. https://doi.org/10.3390/nu16111653
Chicago/Turabian StyleSchweiger, Vittorio, Carlo Schievano, Alvise Martini, Luca Polati, Giovanna Del Balzo, Salvatore Simari, Beatrice Milan, Gabriele Finco, Giustino Varrassi, and Enrico Polati. 2024. "Extended Treatment with Micron-Size Oral Palmitoylethanolamide (PEA) in Chronic Pain: A Systematic Review and Meta-Analysis" Nutrients 16, no. 11: 1653. https://doi.org/10.3390/nu16111653
APA StyleSchweiger, V., Schievano, C., Martini, A., Polati, L., Del Balzo, G., Simari, S., Milan, B., Finco, G., Varrassi, G., & Polati, E. (2024). Extended Treatment with Micron-Size Oral Palmitoylethanolamide (PEA) in Chronic Pain: A Systematic Review and Meta-Analysis. Nutrients, 16(11), 1653. https://doi.org/10.3390/nu16111653






