Serum Folate, Red Blood Cell Folate, and Zinc Serum Levels Are Related with Gestational Weight Gain and Offspring’s Birth-Weight of Adolescent Mothers
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Serum Folate, Red Blood Cell Folate, and Serum Zinc Determination
2.3. Anthropometric Evaluation
2.4. Gestational Weight Gain
2.5. Neonatal Outcomes
2.6. Dietary and Nutrient Intake
2.7. Other Variables
2.8. Ethical Aspects
2.9. Statistical Analysis
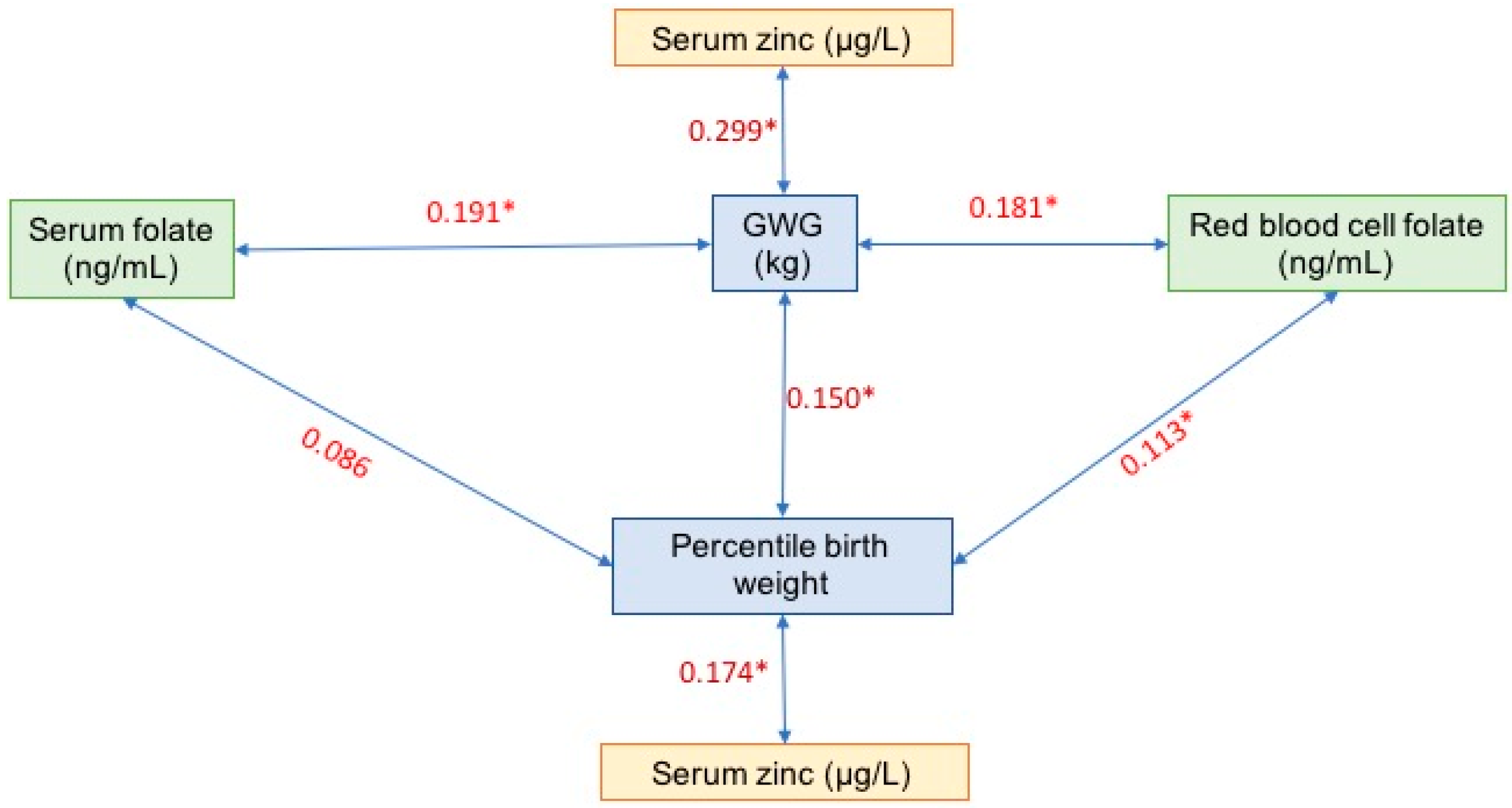
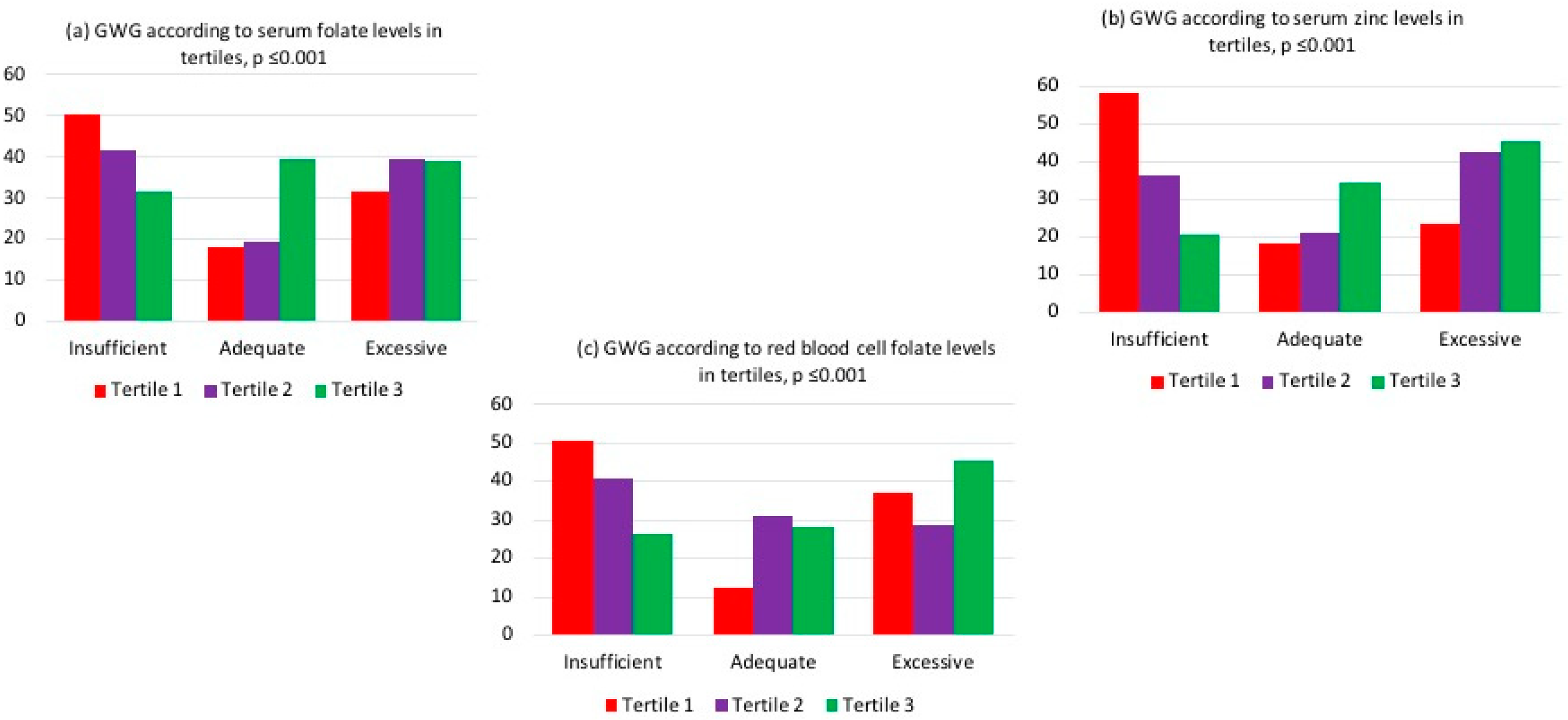
3. Results
4. Discussion
4.1. Gestational Weight Gain
4.2. Folate and Gestational Weight Gain
4.3. Folate, Small Newborns for Gestational Age, and Duration of Pregnancy
4.4. Zinc, Gestational Weight Gain, and Small Newborns for Gestational Age
4.5. Other Findings
4.6. Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- SDG Target 3.7 Sexual and Reproductive Health. Available online: https://www.who.int/data/gho/data/themes/topics/sdg-target-3_7-sexual-and-reproductive-health (accessed on 29 January 2024).
- Investing in Our Future: A Comprehensive Agenda for the Health and Well-Being of Children and Adolescents; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240037793.
- Uzunov, A.V.; Cîrstoiu, M.M.; Secară, D.C.; Crîngu-Ionescu, A.; Matei, A.; Mehedințu, C.; Varlas, V.N. Mode of Delivery and Neonatal Outcome in Adolescent Pregnancy (13–16 Years Old) Associated with Anemia. Medicina 2022, 58, 1796. [Google Scholar] [CrossRef] [PubMed]
- Chavira-Suárez, E.; Ramírez-Mendieta, A.J.; Martínez-Gutiérrez, S.; Zárate-Segura, P.; Beltrán-Montoya, J.; Espinosa-Maldonado, N.C.; de la Cerda-Ángeles, J.C.; Vadillo-Ortega, F. Influence of pre-pregnancy body mass index (p-BMI) and gestational weight gain (GWG) on DNA methylation and protein expression of obesogenic genes in umbilical vein. PLoS ONE 2019, 14, e0226010. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Godínez-Martínez, E.; Sánchez-Jiménez, B.; Montiel-Ojeda, D.; Tolentino, M. Serum Concentration of Leptin in Pregnant Adolescents Correlated with Gestational Weight Gain, Postpartum Weight Retention and Newborn Weight/Length. Nutrients 2017, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Chico-Barba, G.; Flores-Quijano, M.E.; Godínez-Martínez, E.; Martínez-Rojano, H.; Ortiz-Hernandez, L.; Nájera-Medina, O.; Hernández-Trejo, M.; Hurtado-Solache, C. Association of Pregestational BMI and Gestational Weight Gain with Maternal and Neonatal Outcomes in Adolescents and Adults from Mexico City. Int. J. Environ. Res. Public Health 2021, 19, 280. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Martínez-Rojano, H.; Ortiz-Hernández, L.; Nájera-Medina, O.; Chico-Barba, G.; Godínez-Martínez, E.; Gamboa, R.; Aguirre-Minutti, E. Dietary and Nutrient Intake, Eating Habits, and Its Association with Maternal Gestational Weight Gain and Offspring’s Birth Weight in Pregnant Adolescents. Nutrients 2022, 14, 4545. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Lara-Cervantes, C.; Martínez-Rojano, H.; Chico-Barba, G.; Sánchez-Jiménez, B.; Lokier, O.; Hernández-Trejo, M.; Grosso, J.M.; Heller, S. Dietary Knowledge and Myths Vary by Age and Years of Schooling in Pregnant Mexico City Residents. Nutrients 2020, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Martínez-Rojano, H.; Ortiz-Hernández, L.; Nájera-Medina, O.; Chico-Barba, G.; Gamboa, R.; Mendoza-Flores, M.E. Individual, Family, and Social Factors Associated with Gestational Weight Gain in Adolescents: A Scoping Review. Nutrients 2023, 15, 1530. [Google Scholar] [CrossRef] [PubMed]
- Marvin-Dowle, K.; Burley, V.J.; Soltani, H. Nutrient intakes and nutritional biomarkers in pregnant adolescents: A systematic review of studies in developed countries. BMC Pregnancy Childbirth 2016, 16, 268. [Google Scholar] [CrossRef] [PubMed]
- Appiah, P.K.; Korklu, A.R.N.; Bonchel, D.A.; Fenu, G.A.; Yankey, F.W.-M. Nutritional Knowledge and Dietary Intake Habits among Pregnant Adolescents Attending Antenatal Care Clinics in Urban Community in Ghana. J. Nutr. Metab. 2021, 2021, 8835704. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Huybregts, L.; Sanghvi, T.G.; Tran, L.M.; Frongillo, E.A.; Menon, P.; Ruel, M.T. Dietary Diversity Predicts the Adequacy of Micronutrient Intake in Pregnant Adolescent Girls and Women in Bangladesh, but Use of the 5-Group Cutoff Poorly Identifies Individuals with Inadequate Intake. J. Nutr. 2018, 148, 790–797. [Google Scholar] [CrossRef]
- Singh, A.; Trumpff, C.; Genkinger, J.; Davis, A.; Spann, M.; Werner, E.; Monk, C. Micronutrient Dietary Intake in Latina Pregnant Adolescents and Its Association with Level of Depression, Stress, and Social Support. Nutrients 2017, 9, 1212. [Google Scholar] [CrossRef] [PubMed]
- Keats, E.C.; Akseer, N.; Thurairajah, P.; Cousens, S.; Bhutta, Z.A. Multiple-micronutrient supplementation in pregnant adolescents in low- and middle-income countries: A systematic review and a meta-analysis of individual participant data. Nutr. Rev. 2021, 80, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.; Luther, J.; Milne, J.; Aitken, R.; Redmer, D.; Reynolds, L.; Hay, W. Nutritional Modulation of Adolescent Pregnancy Outcome—A Review. Placenta 2006, 27, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Wang, D.; Darling, A.M.; Perumal, N.; Wang, M.; Ahmed, T.; Christian, P.; Dewey, K.G.; Kac, G.; Kennedy, S.; et al. Effects of prenatal nutritional supplements on gestational weight gain in low- and middle-income countries: A meta-analysis of individual participant data. Am. J. Clin. Nutr. 2022, 116, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Shirima, C.P.; Kinabo, J.L. Nutritional status and birth outcomes of adolescent pregnant girls in Morogoro, Coast, and Dar es Salaam regions, Tanzania. Nutrition 2005, 21, 32–38. [Google Scholar] [CrossRef]
- Baker, P.N.; Wheeler, S.J.; Sanders, T.A.; Thomas, J.E.; Hutchinson, C.J.; Clarke, K.; Berry, J.L.; Jones, R.L.; Seed, P.T.; Poston, L. A prospective study of micronutrient status in adolescent pregnancy. Am. J. Clin. Nutr. 2009, 89, 1114–1124. [Google Scholar] [CrossRef]
- Chen, X.; Lou, H.; Chen, L.; Muhuza, M.P.U.; Chen, D.; Zhang, X. Epidemiology of birth defects in teenage pregnancies: Based on provincial surveillance system in eastern China. Front. Public Health 2022, 10, 1008028. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Lillycrop, K.A.; Burdge, G.C.; Gluckman, P.D.; Hanson, M.A. Epigenetic Mechanisms and the Mismatch Concept of the Developmental Origins of Health and Disease. Pediatr. Res. 2007, 61, 5R–10R. [Google Scholar] [CrossRef]
- Atta, C.A.M.; Fiest, K.M.; Frolkis, A.D.; Jette, N.; Pringsheim, T.; Germaine-Smith, C.S.; Rajapakse, T.; Kaplan, G.G.; Metcalfe, A. Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. Am. J. Public Health 2016, 106, e24–e34. [Google Scholar] [CrossRef]
- Ballestín, S.S.; Campos, M.I.G.; Ballestín, J.B.; Bartolomé, M.J.L. Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review. Nutrients 2021, 13, 3134. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; De Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Ota, E.; Mori, R.; Middleton, P.; Tobe-Gai, R.; Mahomed, K.; Miyazaki, C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2015, 2015, CD000230. [Google Scholar] [CrossRef] [PubMed]
- Mridha, M.K.; Matias, S.L.; Arnold, C.D.; Dewey, K.G. Factors associated with nutritional status and dietary practices of Bangladeshi adolescents in early pregnancy. Ann. New York Acad. Sci. 2018, 1416, 66–76. [Google Scholar] [CrossRef]
- Relton, C.L.; Pearce, M.S.; Parker, L. The influence of erythrocyte folate and serum vitamin B12 status on birth weight. Br. J. Nutr. 2005, 93, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Fekete, K.; Berti, C.; Trovato, M.; Lohner, S.; Dullemeijer, C.; Souverein, O.W.; Cetin, I.; Decsi, T. Effect of folate intake on health outcomes in pregnancy: A systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr. J. 2012, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Gobernación. Norma Oficial Mexicana NOM-007-SSA2-2016, Para la Atención de la Mujer Durante el Embarazo, Parto y Puerperio, y de la Persona Recién Nacida. Diario Oficial de la Federación. (Secretariat of the Interior. Official Mexican Standard NOM-007-SSA2-2016, For the Care of Women during Pregnancy, Childbirth, and the Postpartum Period, and of the Newborn Person. Official Gazette of the Federation). Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5432289&fecha=07/04/2016#gsc.tab=0 (accessed on 23 February 2024).
- Zhang, Y.; Mustieles, V.; Wang, Y.-X.; Sun, Y.; Agudelo, J.; Bibi, Z.; Torres, N.; Oulhote, Y.; Slitt, A.; Messerlian, C. Folate concentrations and serum perfluoroalkyl and polyfluoroalkyl substance concentrations in adolescents and adults in the USA (National Health and Nutrition Examination Study 2003-16): An observational study. Lancet Planet. Health 2023, 7, e449–e458. [Google Scholar] [CrossRef] [PubMed]
- WHO. Serum and red blood cell folate concentrations for assessing folate status in populations. In Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2015; Available online: http://apps.who.int/iris/bitstream/10665/162114/1/WHO_NMH_NHD_ EPG_15.01.pdf?ua=1 (accessed on 20 March 2024).
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and Laboratory Studies: A Reference Table for Clinicians. Obstetrics & Gynecology 2009, 114, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. In Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011; Available online: https://iris.who.int/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?sequence=22 (accessed on 27 April 2024).
- Li, F.; Wilkens, L.R.; Novotny, R.; Fialkowski, M.K.; Paulino, Y.C.; Nelson, R.; Bersamin, A.; Martin, U.; Deenik, J.; Boushey, C.J. Anthropometric measurement standardization in the US-affiliated pacific: Report from the Children’s Healthy Living Program. Am. J. Hum. Biol. 2016, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.G.P.D.S.; Da Gama, S.G.N.; De Barros, D.C.; Saunders, C.; Mattos, I.E. Validity of Self-Reported Weight, Height, and BMI in Mothers of the Research Birth in Brazil. Rev. de Saude publica 2017, 51, 115. [Google Scholar] [CrossRef]
- Carrilho, T.R.B.; Rasmussen, K.M.; Farias, D.R.; Costa, N.C.F.; Batalha, M.A.; Reichenheim, M.E.; Ohuma, E.O.; Hutcheon, J.A.; Kac, G.; Brazilian Maternal and Child Nutrition Consortium. Agreement between self-reported pre-pregnancy weight and measured first-trimester weight in Brazilian women. BMC Pregnancy Childbirth 2020, 20, 734. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Standard Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- Kuczmarski, R.R.J.; Ogden, C.L.C.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and Development. Vital Health Stat. 2002, 246, 1–190. [Google Scholar]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy. Weight Guidelines Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009; ISBN 978-0-309-13113-1. [Google Scholar]
- Adu-Afarwuah, S.; Lartey, A.; Okronipa, H.; Ashorn, P.; Ashorn, U.; Zeilani, M.; Arimond, M.; Vosti, S.A.; Dewey, K.G. Maternal Supplementation with Small-Quantity Lipid-Based Nutrient Supplements Compared with Multiple Micronutrients, but Not with Iron and Folic Acid, Reduces the Prevalence of Low Gestational Weight Gain in Semi-Urban Ghana: A Randomized Controlled Trial. J. Nutr. 2017, 147, 697–705. [Google Scholar] [CrossRef]
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Suitor, C.W.; Bailey, L.B. Dietary Folate Equivalents: Interpretation and Application. J. Am. Diet. Assoc. 2000, 100, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Socioeconomic Level Index of the Mexican Association of Market Research and Public Opinion Agencies (AMAI) 2014 AMAI Regulation NSE 8 × 7. Available online: www.amai.org/NSE/NivelSocioeconomicoAMA.pdf (accessed on 20 March 2024).
- Maia, P.A.; Figueiredo, R.C.; Anastácio, A.S.; da Silveira, C.L.P.; Donangelo, C.M. Zinc and copper metabolism in pregnancy and lactation of adolescent women. Nutrition 2007, 23, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Muter, J.; Ewington, L.; Puttemans, P.; Petraglia, F.; Brosens, J.J.; Benagiano, G. Adolescent Preeclampsia: Pathological Drivers and Clinical Prevention. Reprod. Sci. 2019, 26, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, A.; Chmurzynska, A. Folate and choline absorption and uptake: Their role in fetal development. Biochimie 2018, 158, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Socha, M.W.; Flis, W.; Wartęga, M. Epigenetic Genome Modifications during Pregnancy: The Impact of Essential Nutritional Supplements on DNA Methylation. Nutrients 2024, 16, 678. [Google Scholar] [CrossRef]
- Behere, R.V.; Yajnik, C.S. Low vitamin B-12–high folate status in adolescents and pregnant women may have deleterious effects on health of the offspring. Am. J. Clin. Nutr. 2021, 113, 1057–1059. [Google Scholar] [CrossRef]
- Whelan, E.; Armson, B.A.; Ashley-Martin, J.; MacSween, K.; Woolcott, C. Gestational Weight Gain and Interpregnancy Weight Change in Adolescent Mothers. J. Pediatr. Adolesc. Gynecol. 2017, 30, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Campos-Nonato, I.; Galván-Valencia, O.; Hernández-Barrera, L.; Oviedo-Solís, C.; Barquera, S. Prevalence of obesity and associated risk factors in Mexican adults: Results of the ENSANUT 2022. Salud Publica. Mex. 2023, 65 (Suppl. 1), S238–S247. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Cederberg, H.; Wheeler, S.; Poston, L.; Hutchinson, C.; Seed, P.; Oliver, R.; Baker, P. Relationship between maternal growth, infant birthweight and nutrient partitioning in teenage pregnancies. BJOG 2009, 117, 200–211. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.A.; et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. USA 2007, 104, 19351–19356. [Google Scholar] [CrossRef]
- McGee, M.; Bainbridge, S.; Fontaine-Bisson, B. A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes. Nutr. Rev. 2018, 76, 469–478. [Google Scholar] [CrossRef]
- Olapeju, B.; Saifuddin, A.; Wang, G.; Ji, Y.; Hong, X.; Raghavan, R.; Summers, A.; Keiser, A.; Ji, H.; Zuckerman, B.; et al. Maternal postpartum plasma folate status and preterm birth in a high-risk US population. Public Health Nutr 2019, 22, 1281–1291. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.; Mao, B.; Qiu, J.; Guo, H.; Yi, B.; He, X.; Lin, X.; Lv, L.; Xu, X.; et al. Maternal Folic Acid Supplementation, Dietary Folate Intake, and Low Birth Weight: A Birth Cohort Study. Front. Public Health 2022, 10, 844150. [Google Scholar] [CrossRef]
- Angeles-Agdeppa, I.; Denney, L.; Toledo, M.B.; Obligar, V.A.; Jacquier, E.F.; Carriquiry, A.L.; Capanzana, M.V. Inadequate nutrient intakes in Filipino schoolchildren and adolescents are common among those from rural areas and poor families. Food Nutr. Res. 2019, 63, 3435. [Google Scholar] [CrossRef] [PubMed]
- Darnton-Hill, I.; Mkparu, U.C. Micronutrients in Pregnancy in Low- and Middle-Income Countries. Nutrients 2015, 7, 1744–1768. [Google Scholar] [CrossRef]
- Hodgetts, V.; Morris, R.; Francis, A.; Gardosi, J.; Ismail, K. Effectiveness of folic acid supplementation in pregnancy on reducing the risk of small-for-gestational age neonates: A population study, systematic review and meta-analysis. BJOG 2014, 122, 478–490. [Google Scholar] [CrossRef]
- Adams, J.B.; Kirby, J.K.; Sorensen, J.C.; Pollard, E.L.; Audhya, T. Evidence based recommendations for an optimal prenatal supplement for women in the US: Vitamins and related nutrients. Matern. Health Neonatol. Perinatol. 2022, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A.; Hamid, A.; Kaur, J. Folate status in various pathophysiological conditions. IUBMB Life 2008, 60, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Belfort, G.P.; Santos, M.M.A.d.S.; Pessoa, L.d.S.; Dias, J.R.; Heidelmann, S.P.; Saunders, C. Determinants of low birth weight in the children of adolescent mothers: A hierarchical analysis. Cienc. Saude Coletiva 2018, 23, 2609–2620. [Google Scholar] [CrossRef] [PubMed]
- Siega-Riz, A.M.; Savitz, D.A.; Zeisel, S.H.; Thorp, J.M.; Herring, A. Second trimester folate status and preterm birth. Am. J. Obstet. Gynecol. 2004, 191, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef]
- Pretorius, R.A.; Palmer, D.J. High-Fiber Diet during Pregnancy Characterized by More Fruit and Vegetable Consumption. Nutrients 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, L.B.; Lobo, C.V.; Carmo, A.S.D.; e Souza, R.C.V.; dos Santos, L.C. Dietary Patterns During Pregnancy and Their Association with Gestational Weight Gain and Anthropometric Measurements at Birth. Matern. Child Health J. 2022, 26, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Suárez, C.C.; Vásquez-Garibay, E.M.; Romero-Velarde, E.; Romo-Huerta, H.P.; García De Alba García, J.E.; Troyo-Sanromán, R. Hábitos de alimentación y factores culturales en adolescentes embarazadas [Food habits and culture factors in pregnant adolescents]. Arch. Latinoam. Nutr. 2008, 58, 19–26. (In Spanish) [Google Scholar]

| Maternal Variables | Mean ± SD | Interval |
|---|---|---|
| Age (years) | 15.8 ± 1.3 | 12–19 |
| Gynecological (years) | 4.3 ± 1.6 | 1–10 |
| Beginning of antenatal care (weeks) | 27 ± 6 | 11–37 |
| Delivery (a) | Cesarean-section | 173 (42.7) |
| Natural | 233 (57.3) | |
| Height (cm) | 155.9 ± 5.5 | 139.4–176 |
| Prepregnancy BMI | 21.5 ± 3.6 | 13.5–39.1 |
| Prepregnancy BMI (percentile) | 52.5 ± 10.1 | 1–99 |
| Prepregnancy BMI (a) | Low weight | 36 (8.9) |
| Healthy | 286 (70.4) | |
| Overweight | 62 (15.3) | |
| Obesity | 22 (5.4) | |
| Prepregnancy weight (kg) | 52.5 ± 10 | 28–100 |
| Adequacy of gestational weight gain (%) | 114 ± 66 | −128–359 |
| Adequacy of gestational weight gain (kg) | 12.7 ± 6 | −7.7–35.5 |
| Gestational weight gain (a) | Inadequate | 156 (38.4) |
| Recommendable | 101 (24.9) | |
| Excessive | 149 (36.7) | |
| Hemoglobin (g/dL) | 12.7 (11.8–13.3) | 9.2–15.9 |
| Serum folate (ng/dL) | 13.2 (9.4–0.3) | 3.93–94.4 |
| Red blood cell folate (ng/dL) | 370 (260–490) | 63.5–1684 |
| Serum zinc (μg/L) | 665 (575–798) | 164–1481 |
| Perinatal outcomes | ||
| Birth weight (g) | 2929 ± 477.7 | 1030–4105 |
| Birth weight for gestational age (a) | Small | 85 (20.9) |
| Adequate | 302 (74.4) | |
| Large | 19 (4.7) | |
| Length (cm) | 48.8 ± 2.7 | 31–53 |
| Gestational age (weeks) | 39 (37.6–40.0) | 26.6–42 |
| Preterm | 57 (13.8) | |
| Term | 349 (86.2) | |
| Sex (gender) (a) | Women | 189 (46.7) |
| Men | 217 (53.3) |
| Gestational Weight Gain | Birth Weight | |||||
|---|---|---|---|---|---|---|
| Insufficient, n = 156 | Adequate n = 101 | Excessive, n = 149 | Small, n = 85 | Adequate n = 302 | Large, n = 19 | |
| Micronutrients | ||||||
| Serum folate (ng/mL) | 11 (8–16) 1–2,1–3 | 17 (11–29) | 14 (10–22) | 14 (9–19) | 13 (10–23) | 12 (8–16) |
| Red blood cell folate (ng/mL) | 327 (217–429) 1–2,1–3 | 389 (323–530) | 388 (277–548) * | 349 (278–453) 1–2 | 378 (278–501) | 290 (245–441) |
| Serum zinc (μg/L) | 602 (533–694) 1–2,1–3 | 715 (611–845) | 711 (632–870) * | 616 (514–713) 1–2,1–3 | 679 (589–801) | 732 (610–884) * |
| Hemoglobin (g/dL) | 12.1 (11.2–13.0) 1–2,1–3 | 13.3 (12.5–13.7) | 12.7 (11.9–13.1) | 12.6 (11.6–13.2) | 12.8 (11.9–13.4) | 12.4 (11.4–12.9) |
| Dietetic variables | ||||||
| Energy (kcal) | 1821 (1491–2188) | 1803 (1524–2283) | 1776 (1402–2100) | 1798 (1456–2191) | 1794 (1450–2166) | 1940 (1697–2252) |
| Fiber (g) | 13 (9–19) | 12 (8–16) | 12 (8–15) 1–3 | 13 (8–19) | 12 (9–17) | 11 (7–15) |
| Folate (µg) | 854 (771–965) | 822 (766–950) | 840 (767–921) | 977 (771–997) | 838 (767–920) | 818 (786–874) |
| Thiamine B1 (mg) | 1.1 (0.8–1.5) | 1.0 (0.8–1.5) 1–2 | 1.1 (0.8–1.4) | 1.2 (0.8–1.7) | 1.0 (0.8–1.4) | 1.1 (0.9–1.3) |
| Riboflavin B2 (mg) | 1.4 (1.0–1.9) | 1.3 (0.8–1.8) | 1.4 (0.9–1.8) | 1.4 (0.9–1.8) | 1.3 (0.9–1.8) | 1.3 (1.0–1.7) |
| Pyridoxine B6 (mg) | 1.2(0.8–1.8) | 1.1 (0.7–1.8) | 1.0 (0.7–1.4) | 1.3 (0.7–1.8) | 1.1 (0.7–1.6) | 0.9 (0.8–1.3) |
| Cyanocobalamin B12 (µg) | 2.4 (1.4–3.5) | 2.6 (1.4–3.6) | 2.2 (1.3–3.5) | 2.5 (1.5–3.7) | 2.3 (1.3–3.5) | 1.5 (1.2–2.6) |
| Pantothenic acid (mg) | 2.4 (1.8–3.4) | 2.4 (1.3–4.0) | 2.3 (1.4–3.3) | 2.7 (1.5–3.9) | 2.3 (1.6–3.2) | 2.5 (1.7–2.8 |
| Niacin (mg) | 14 (9–19) | 14 (10–18) | 12 (9–15) | 15 (10–20) | 13 (9–18) | 12 (10–18) |
| Iron (mg) | 12 (9–19) | 12 (9–17) | 12 (9–15) | 12 (9–20) | 12 (9–16) | 11 (9–14) |
| Zinc (mg) | 5 (4–8) | 6 (4–8) | 5 (4–8) | 5 (4–8) | 5 (4–8) | 4 (4–6) |
| Serving of food | ||||||
| Fruits | 1.6 (0.1–3.2) | 1.2 (0.3–2.5) | 1.1 (0.1–2.7) | 1.4 (0.0–3.4) | 1.4 (0.2–2.8) | 0.7 (0.0–1.2) |
| Vegetables | 1.7 (0.8–3.1) 1–3,2–3 | 1.8 (0.7–2.9) | 1.2 (0.4–2.8) | 1.2 (0.5–3.1) | 1.5 (0.6–2.9) | 1.4 (0.6–2.2) |
| Legumes | 0.0 (0–0.5) | 0.0 (0–0.5) | 0.0 (0–0.5) | 0.0 (0.0–0.7) | 0.0 (0.0–0.5) | 0.0 (0.0–0.6) |
| Sugar | 3.3 (1.2–6.1) 1–3,2–3 | 2.8 (1.2–4.4) | 4 (2.1–6.0) | 3.7 (2.1–6.2) | 3.3 (1.5–6.0) | 4.5 (1.3–7.0) |
| Animal-sourced foods | 5 (3.5–7.1) | 5.1 (3.4–6.6) | 4.9 (2.8–6.6) | 5.1 (3.8–6.6) | 4.8 (3.0–6.8) | 4.2 (3.6–7.0) |
| Cereal | 8 (6–11) | 8 (7–10) | 8 (5–11) | 8 (6–11) | 8.3 (6–11) | 10 (6–11) |
| Milk and derivate | 1.0 (0.5–1.9) | 1 (0–2) | 1 (0–2) | 1.0 (0.0–1.9) | 1.0 (0.1–1.9) | 1.0 (0.6–1.6) |
| Fat and oils | 3.9 (1.5–6.5) 1–3,2–3 | 3.4 (1.5–7.7) | 5.3 (3.0–7.0) | 4.0 (1.5–6.6) | 4.2 (2.0–7.0) 2–3 | 5.1 (4.1–9.0) |
| Sweetened beverages | 1 (0–3) 1–3,2–3 | 1 (0–3) | 2 (2–4) | 2 (1–4) | 2 (0–3) | 2 (1–3) |
| Gestational Weight Gain | Birth Weight | ||
|---|---|---|---|
| Insufficient, n = 156 | Excessive, n = 149 | SGA, n = 85 | |
| OR (CI 95%) | OR (CI 95%) | OR (CI 95%) | |
| Serum folate below the median | 2.1 (1.3–3.3) | 0.6 (0.3–0.9) | 0.9 (0.5–1.5) |
| Red blood cell folate (ng/mL) below median | 1.6 (1.0–2.6) | 0.7 (0.4–1.1) | 1.6 (0.9–2.7) |
| Serum zinc (μg/L) below median | 3.3 (2.1–5.2) | 0.5 (0.3–0.7) | 1.2 (1.2–3.4) |
| <15 years old | 0.5 (0.3–0.8) | 1.2 (0.7–1.9) | 1.2 (0.7–2.0) |
| Hemoglobin < 12.5 (g/dL) | 1.9 (1.2–3.1) | 1.0 (0.6–1.6) | 0.9 (0.5–1.6) |
| pBMI overweight/obese | 0.3 (0.2–0.7) | 4.9 (2.1–8.5) | 0.5 (0.2–0.9) |
| Dietetic variables | |||
| Fiber < 25 g | 0.3 (0.1–0.6) | 2.2 (1.0–4.9) | 0.5 (0.2–1.0) |
| Fruits inadequate intake | 0.3 (0.2–0.7) | 1.3 (0.6–2.8) | 0.8 (0.4–1.7) |
| Legumes inadequate intake | 1.4 (0.4–4.4) | 0.4 (0.1–1.4) | 2.7 (0.6–11.3) |
| Fat and oils | 0.6 (0.4–1.1) | 2.1 (1.3–3.4) | 0.8 (0.5–1.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Gamboa, R.; Tolentino, M.; Toledo-Barrera, A.X.; Ramírez-González, C.; Mendoza-Flores, M.E.; Hernández-Trejo, M.; Godínez-Martínez, E. Serum Folate, Red Blood Cell Folate, and Zinc Serum Levels Are Related with Gestational Weight Gain and Offspring’s Birth-Weight of Adolescent Mothers. Nutrients 2024, 16, 1632. https://doi.org/10.3390/nu16111632
Sámano R, Martínez-Rojano H, Chico-Barba G, Gamboa R, Tolentino M, Toledo-Barrera AX, Ramírez-González C, Mendoza-Flores ME, Hernández-Trejo M, Godínez-Martínez E. Serum Folate, Red Blood Cell Folate, and Zinc Serum Levels Are Related with Gestational Weight Gain and Offspring’s Birth-Weight of Adolescent Mothers. Nutrients. 2024; 16(11):1632. https://doi.org/10.3390/nu16111632
Chicago/Turabian StyleSámano, Reyna, Hugo Martínez-Rojano, Gabriela Chico-Barba, Ricardo Gamboa, Maricruz Tolentino, Alexa Xiomara Toledo-Barrera, Cristina Ramírez-González, María Eugenia Mendoza-Flores, María Hernández-Trejo, and Estela Godínez-Martínez. 2024. "Serum Folate, Red Blood Cell Folate, and Zinc Serum Levels Are Related with Gestational Weight Gain and Offspring’s Birth-Weight of Adolescent Mothers" Nutrients 16, no. 11: 1632. https://doi.org/10.3390/nu16111632
APA StyleSámano, R., Martínez-Rojano, H., Chico-Barba, G., Gamboa, R., Tolentino, M., Toledo-Barrera, A. X., Ramírez-González, C., Mendoza-Flores, M. E., Hernández-Trejo, M., & Godínez-Martínez, E. (2024). Serum Folate, Red Blood Cell Folate, and Zinc Serum Levels Are Related with Gestational Weight Gain and Offspring’s Birth-Weight of Adolescent Mothers. Nutrients, 16(11), 1632. https://doi.org/10.3390/nu16111632






