Abstract
Obesity in the United States and Western countries represents a major health challenge associated with an increased risk of metabolic diseases, including cardiovascular disease, hypertension, diabetes, and certain cancers. Our past work revealed a more pronounced obesity–cancer link in certain ethnic groups, motivating us to develop a tailored dietary intervention called the Healthy Diet and Lifestyle 2 (HDLS2). The study protocol is described herein for this randomized six-month trial examining the effects of intermittent energy restriction (5:2 Diet) plus the Mediterranean dietary pattern (IER + MED) on visceral adipose tissue (VAT), liver fat, and metabolic biomarkers, compared to a standard MED with daily energy restriction (DER + MED), in a diverse participant group. Using MRI and DXA scans for body composition analysis, as well as metabolic profiling, this research aims to contribute to nutritional guidelines and strategies for visceral obesity reduction. The potential benefits of IER + MED, particularly regarding VAT reduction and metabolic health improvement, could be pivotal in mitigating the obesity epidemic and its metabolic sequelae. The ongoing study will provide essential insights into the efficacy of these energy restriction approaches across varied racial/ethnic backgrounds, addressing an urgent need in nutrition and metabolic health research. Registered Trial, National Institutes of Health, ClinicalTrials.gov (NCT05132686).
Keywords:
obesity; intermittent energy restriction (IER); daily energy restriction; Mediterranean diet (MED); visceral adipose tissue (VAT); metabolic health; randomized control trial (RCT); body composition; dietary behavioral intervention; metabolic dysfunction-associated fatty liver disease (MAFLD) 1. Introduction
In the United States and many Western countries, obesity has grown into a significant health challenge. Ten years ago, no U.S. state had an adult obesity prevalence rate higher than 34%. Obesity rates have progressively increased, and in 2022, as many as 22 states were reporting rates ≥ 35% [1]. Established associations of obesity with chronic diseases, such as cardiovascular disease, hypertension, diabetes, and 13 types of cancer [2,3,4,5,6], underscore the urgency to confront obesity’s widespread impact on metabolic health.
Our previous work in the Multiethnic Cohort Study showed significant differences in abdominal fat amount among racial/ethnic groups that correlated with disparities in the prevalence of metabolic syndrome, type-2 diabetes, and other obesity-related diseases. Notably, compared to African Americans, whites, Latinos, Native Hawaiians, and Japanese Americans exhibited higher amounts of visceral and liver fat, in that order, for a given level of total adiposity [7].
The role of visceral adipose tissue (VAT) in cancer disparities is especially intriguing, given its heightened metabolic activity, the differential propensity of racial/ethnic groups to cumulate VAT [7], and evidence of its association with breast cancer [8]. Visceral fat nests around internal abdominal organs, while liver fat localizes within the liver’s cellular structure. When fat builds up in the liver, it can directly influence portal circulation by releasing free fatty acids, leading to the elevation of triglycerides and setting the stage for non-alcoholic fatty liver disease (NAFLD) and the more comprehensive syndrome, Metabolic Dysfunction-associated Fatty Liver Disease (MAFLD) [9,10,11]. It has been suggested that VAT’s proposed link to cancer might relate to its promotion of insulin resistance, inflammation, and the release of associated bioactive compounds [12,13]. These bioactive molecules, such as the adipokines leptin and adiponectin, coupled with inflammatory cytokines like TNF-α and IL-6, have critical functions in cell communication and metabolic activities [14,15]. When secreted from VAT, these compounds may intensify chronic inflammation and metabolic imbalances, which might play a part in the initiation and promotion of several health conditions, including tumor development.
Continuous daily energy restriction (DER) and reduction of adiposity have potential long-term health benefits, including reducing the risk of chronic diseases [16]. Intermittent energy restriction (IER) has shown advantages over a standard DER, including the observation that it may be an easier diet to follow and maintain [17,18]. IER has been shown to be effective at reducing weight in normal weight and overweight/obese men, women, adolescents, and children. Moreover, IER has been found to improve insulin sensitivity, vital for diabetes prevention [19], and positively affect cardiovascular health by reducing blood pressure and improving lipid profiles [20]. It also demonstrates potential cognitive benefits and might influence lifespan [21]. However, adequate evidence of the efficacy of IER, especially among diverse populations, is lacking, as are specific data on its impact on VAT. Additional metabolic measurements are also desirable to provide detailed evidence of underlying mechanisms.
Intermittent Energy Restriction (IER) includes various fasting strategies, each with specific characteristics. Time Restrictive Eating (TER) confines food intake to a definitive window each day, often 8–10 h, promoting fasting for the remaining 14–16 h. However, to date, TRE has not demonstrated consistently strong results in terms of adherence and metabolic health outcomes compared to other IER methods [22]. The 5:2 diet involves usual eating five days per week while significantly reducing calorie intake on two consecutive days. Alternate Day Fasting (ADF) alternates between days of regular eating and days of minimal calorie intake (about 25% of normal). We selected the 5:2 diet due to its promising ability to boost adherence and metabolic health [23]. Its structured yet adaptable method of energy restriction offers a practical substitute for everyday calorie cuts, harmonizing well with the nutritional advantages of the Mediterranean diet [24].
Endorsement for the Mediterranean (MED) diet as a health-promoting diet is growing, with evidence pointing to its effectiveness in diminishing VAT and liver fat accumulation [25,26]. Drawing inspiration from the culinary traditions of coastal Mediterranean regions, such as those in parts of Greece, Spain, and Southern Italy, this dietary approach stresses the importance of plant-based foods [27,28,29]. This includes a wide variety of fruits, vegetables, grains, and legumes. Olive oil is typically the staple for cooking and dressings, with a preference for fish and poultry over red meats. Yogurt and cheese are the dairy mainstays, consumed in moderation. Many have heralded the health advantages of MED, particularly for heart health and certain malignancies. In parallel to the MED diet, (IER) is gaining interest as a viable method to counteract visceral and aberrant fat, particularly when related to liver conditions [30,31,32]. Even though preliminary data suggest benefits from blending the MED diet and IER to combat ectopic fat, initial studies often had relatively small or homogenous participant groups [33].
Our group conducted a randomized active-comparator pilot study (the Healthy Diet and Lifestyle Study or HDLS) from 2016–2017 to show the feasibility and effect that an IER + MED diet, compared to the often-used DASH diet, could have on VAT when followed for 12 weeks [33]. Participants in the IER + MED group successfully completed 90.6% of their IER days. No significant adverse effects were reported. On a scale of 1 (low) to 10 (high), diet compliance scores for IER + MED averaged 7.7, while DASH scores were slightly lower at 6.63. The IER + MED group experienced a significantly greater decrease in daily energy intake and greater adherence to prescribed macronutrient percentages than the DASH group. Most notably, the IER + MED group demonstrated a more substantial decrease in several anthropometric and DXA fat measures as compared to the DASH group, including VAT. This was observed even after accounting for the concurrent change in overall adiposity, where the reduction in VAT, adjusted for total fat mass change, was larger in the IER + MED group. Additionally, the IER + MED group exhibited almost double the reduction in other anthropometric measures compared to the DASH group. Roughly 73% of IER + MED participants lost at least 5% of their weight compared to 32% for DASH. Post-intervention, the IER + MED group maintained their body weight at the 6-month visit, while the DASH group saw an increase. The majority of participants in both groups continued with their prescribed diets, with the IER + MED group showing a longer adherence post-study.
Our primary focus in the Healthy Diet and Lifestyle Study 2 (HDLS2) is to compare the efficacy of IER vs. DER in reducing visceral adiposity and improving metabolic biomarkers, particularly those related to obesity-related diseases. The HDLS2 study compares the IER + MED with DER + MED diets in a 24-week randomized trial among an expected 312 participants aged 35–69. The study incorporates the same behavioral component in both study arms, involving personalized nutrition counseling as well as a moderate-level exercise program. The protocol is described herein.
2. Specific Aims/Outcomes
2.1. Aim 1: Effect of Intervention on Visceral and Ectopic Fat
To investigate the comparative effects of two dietary interventions, IER + MED and DER + MED, encompassing equivalent overall energy restrictions and analogous dietary compositions, on visceral and liver fat reduction.
Primary Outcomes:
- Intra-Abdominal VAT Volume: Assessed via abdominal MRI to detect changes attributable to the dietary interventions. Three-dimensional (3D) volumetric assessment of VAT over the abdominal region is used to improve the robustness of the VAT endpoint [34]. The approach is based on 3D volumetric Dixon fat images and automated software that performs localization and segmentation to produce VAT, subcutaneous adipose tissue (SAT), and Abdominal Adipose Tissue (AAT) volume outputs over the abdominal region of interest [35]. VAT refers to the fat deep within the abdominal cavity that surrounds organs, whereas SAT accumulates under the skin. AAT encompasses both VAT and SAT within the abdominal region.
- Liver Fat Percentage: This is quantified using MRI proton density fat fraction (PDFF) and magnetic resonance spectroscopy (MRS) to measure alterations in liver fat content. MRI-PDFF and MRS are MR-based noninvasive quantitative imaging modalities enabling liver fat assessment with high accuracy, repeatability, and reproducibility. They have emerged as a surrogate for liver biopsy in clinical trials [36].
- Relative VAT: Adjustments in VAT measures made in relation to total fat mass as determined by whole-body DXA.
- Total Fat Mass (FM): Comprehensive assessment via whole-body DXA to gauge overall changes in total adiposity.
2.2. Aim 2: Effect of Behavioral Factors on Adherence to Intervention
Examine how behavioral factors influence participant adherence to dietary interventions, which can provide insights for designing more effective dietary guidelines and interventions.
In addition to these two aims, we also include an in-person visit at Week 48 to evaluate the post-intervention maintenance of the intervention and its effects on DXA estimation of VAT [37], overall DXA-total fat mass (FM), and body weight, which will be compared among participants and to the corresponding measures taken at the end of the intervention at Week 24. The precision and accuracy of DXA for estimating VAT are considered reliable [37,38].
3. Materials and Methods
3.1. Study Design and Participants
HDLS2 is being conducted at the University of Hawai’i Cancer Center (UHCC) and the University of Hawai’i (UH) Magnetic Resonance Imaging (MRI) Research Center. This is a randomized trial comparing two diets over 24 weeks: the IER + MED vs. the DER + MED. The trial inclusion and exclusion criteria are presented in Table 1.

Table 1.
Inclusion and Exclusion Criteria for Study Participation.
Recruitment from the general population began in April 2022 through advertising that includes Facebook/email/flyers, media exposure, community presentations, workplace presentations, and referrals from participants and clinical partners. Specifically, we are seeking Oʻahu residents of East Asian, European White, Native Hawaiian, Pacific Islander, or Filipino ancestry, aged 35 to 69, who are slightly or more than slightly overweight and have not smoked in the last 2 years. Participants from other racial or ethnic groups are not eligible for the study. Participants should be willing to improve their eating and exercise habits and committed to following a 24-week diet and physical activity regimen. Ultimately, 312 study participants will be recruited and randomized. Joining the study comes at no cost, with benefits including support from a dietitian, access to the latest science to improve health, and compensation for time and travel.
Participants are screened for eligibility through a preliminary phone interview, covering aspects like age, ethnicity, BMI, smoking and alcohol intake status, and compatibility for MRI scans. Approximately 436 potential participants are expected to be scheduled for an eligibility visit at the UHCC Clinic, with around 30% projected to be ineligible due to factors like insufficient visceral fat level or abnormal blood biochemistry. The informed consent process explains potential risks associated with the study, such as discomfort from venipuncture or exposure to a small amount of radiation from DXA scans. Premenopausal women must agree to a pregnancy test prior to DXA and MRI scans, which needs to be negative. Once consent is obtained, DXA and MRI compatibility is also checked through questionnaires during recruitment (and before each MRI scan) to ensure participant safety. A clinic visit is then scheduled to further assess eligibility. A more in-depth medical history and a blood chemistry panel are performed to assess general health, an anthropometric examination, and a DXA and abdominal MRI scan to further assess the study body composition requirements (Table 1) and provide baseline measures.
This trial is monitored by the Data and Safety Monitoring Committee of the University of Hawaii Cancer Center (UHCC). The behavioral intervention used in this trial has been evaluated and determined to only have minimal risk for participants by our Institutional Review Board.
3.2. Randomization and Masking
At the end of the first study visit to the clinic (Week 0), participants are randomized to either the IER or DER group. Randomization is stratified by gender, age (35–54 years and 55–69 years for men and pre- and post-menopausal for women), and VAT level (≥100 cm2 and <100 cm2). The randomization procedure uses sequentially numbered opaque envelopes, each containing an assignment to one of the two dietary interventions. Recruitment and clinic staff remain blinded to the randomization assignment. The intervention staff (dietitians) are blinded to assessment measures of the study participants, apart from their baseline demographic information.
3.3. Timing of Outcome Measurements
As shown in Table 2, exposure and outcome measurements assess the impact of dietary interventions on participants throughout the study. Primary outcomes that include changes in VAT volume, liver fat percentage, and total fat mass are assessed using MRI and DXA scans at baseline, Week 12, and Week 24 to capture mid-intervention and post-intervention effects. Secondary outcomes, such as dietary adherence and metabolic biomarkers, are evaluated through self-reported questionnaires and blood tests at the same time points. Metabolic parameters such as ALT, AST, glucose, insulin, leptin, total cholesterol, HDL, LDL, and triglycerides will be measured to assess changes in metabolic biomarkers.

Table 2.
Timeline of intervention activities for HDLS2.
3.4. Diet and Physical Activity Interventions
Dietary plans are being individualized for each participant based on their sex, baseline weight, height, and age. Participants in the DER + MED group follow the MED diet continuously, incorporating a 20% energy restriction each day. This restriction is measured against their individual baseline energy requirements or a standard daily caloric intake level that will sustain the baseline weight. The Mediterranean (MED) diet intervention provides low carbohydrate intake (45% energy), with 25% energy from protein and 30% energy from fat.
The IER + MED group is prescribed a 70% energy restriction over two consecutive days each week while following the same macronutrient distribution outlined in the MED diet plan. On the remaining five days, they follow the euenergetic MED diet at the daily caloric intake level that will sustain the baseline weight. This pattern of 70% restriction on 2 of 7 days per week results in an overall energy restriction of 20%. Both dietary plans emphasize foods rich in Monounsaturated Fatty Acids (MUFAs). Intended fat intake targets include 15% energy from MUFA, 8% from Polyunsaturated Fatty Acids (PUFA), and 7% from saturated fat. The protocol allows up to 4% of carbohydrate energy to be allocated to alcohol intake to accommodate light, non-habitual drinkers. Moderate alcohol consumption, defined as up to 10 g per day, does not markedly influence hepatic fat accumulation, suggesting that such levels of intake can be compatible with the successful application of IER and DER strategies. [39].
The implementation of the intervention aligns with the recommendation of conducting at least 14 in-person or web-based sessions led by trained experts for effective obesity management [40]. The counseling sessions focus on both diet and physical activity. No food is provided in HDLS2. Participants choose their foods in both diet groups. Clear instructions are provided during face-to-face consultations with research dietitians on following the assigned diet. Detailed information on portion sizes, recipes, and meal plans, is provided for at-home adherence. Participants are also provided with a local restaurant eating guide, Mediterranean recipes for all meals, and Hawaiian Island-Asian-inspired Mediterranean recipes to better align with local diets. A research dietitian meets with each participant through phone calls or video meetings weekly for the first four weeks following randomization and then every other week until Week 24. A detailed schema illustrating the study design and timeline is provided as Supplementary Materials (Figure S1).
Dietitians are using behavioral techniques to support diet adherence in both groups. Assigned dietitians contact participants one week after randomization to confirm diet initiation, assess understanding, and offer troubleshooting advice. Research dietitians were trained on the motivational interviewing process, originally developed to treat drug addiction, adapted from the Body and Soul Trial (Figure 1) [41,42,43]. Using theoretical constructs from Social Cognitive and Self-Determination theory to enhance self-efficacy, the research dietitian acts as a problem-solving partner by listening reflectively and offering self-motivational statements in a participant-centered, goal-oriented process to overcome obstinacy and create lasting solutions [44,45]. Building participant skills through motivational interviewing can help them address other situations influencing long-term commitment to diet-related behavior change [17]. Enhancing problem-solving skills through motivational interviewing can also help build social support and aid in addressing toxic food environments [17,46,47].
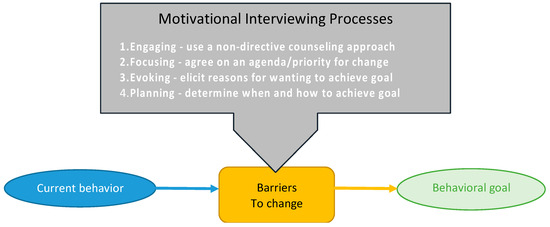
Figure 1.
Motivational Interviewing Process.
All participants are asked to increase physical activity, with a program of walking for at least one hour, five days a week. The IER + MED group is encouraged to walk only on non-IER days. Both groups receive resistance bands, providing medium resistance, at the first visit when randomization occurs. A descriptive handout is provided with the resistance bands that contain specific exercises, such as bicep curls and seated leg press exercises. Resistance training involving 3 sets of 8–10 repetitions is prescribed for two nonconsecutive days per week.
3.5. Adherence to the Intervention
Adherence to the assigned dietary intervention is being monitored through dietary records for 4 consecutive days using a mobile app called Technology Assisted Dietary Assessment (TADA) that works with most smartphones to capture before and after photos of what participants eat and drink [48]. In addition to photos before and after meals, the TADA app also records time, location, and context [49]. The mobile food record (mFR™) assesses energy, fats (MUFA, PUFA, and saturated fats), carbohydrates, protein, dietary fiber, and alcohol. For the IER + MED group, participants are asked to record their two consecutive IER days on Days 2 and 3 of the four-day mFR™. The DER + MED participants are asked to include one weekend day in their four-day mFR™. The mFR™ data are collected over a 4-day period at baseline, then at Weeks 5, 9, 11, 17, 21, 23, and 47 (Table 2). Maintenance of lifestyle changes is monitored through follow-up surveys and mFRs™. Seven 4-day mFRs™ are collected after baseline, allowing for a thorough assessment of the sustainability of diet-induced alterations. We also assess changes in physical activity by using a questionnaire assessing the average minutes of walking or alternate activity during each counseling session. To ensure participants are supported in maintaining their dietary changes, a research dietitian provides ongoing counseling, initially weekly and then biweekly, through various forms of communication, including phone and video meetings. A final session occurs in Week 48.
To objectively monitor participants’ adherence to exercise recommendations, we evaluate physical activity in Weeks 0, 11, and 23. All participants will follow a standardized physical activity regimen, including walking and resistance exercises. Physical activity will be assessed using questionnaires and ActiGraph™ monitors (ActiGraph™ GT3X+, Pensacola, FL, USA) worn for consecutive days during these weeks, with data processed using ActiLife™ Software version 6.13.6. Physical activity data will be compared between the two study groups and considered as potential confounders in the analysis. This assessment involves wearing an activity monitor (ActiGraph™) on the wrist for one week, complemented by a sleep questionnaire reflecting the time spent in bed and noting any time up during the night. During their first clinic visit, participants are taught how to use the accelerometer. Participants are instructed to wear the monitor continuously, with exceptions being not to wear it in salt water and to avoid wearing it in fresh water for durations exceeding 30 min or at depths greater than 3 feet. Before the designated wear period, they are mailed the accelerometer, instructions, and a return mailer. Standard manufacturer protocols provide activity measurements via ActiLife™ Software version 6.13.6. Our physical activity measurements are based on ActiGraph’s™ validated and transparent activity counting algorithms [50]. Data must align with self-reported wear and sleep times to be valid, requiring at least four days/nights of sufficient wear time. Activity intensities are categorized based on count rates, and the daily average of each level (e.g., sedentary, moderate) is used in the analysis.
3.6. Data Analysis
A data management system was created for the HDLS2 study based on strategies from the pilot HDLS. The system manages participant recruitment, milestone tracking, specimen handling and storage, and data entry. A detailed review and cleaning of data is performed soon after collection. Aggregation of all collected data, including DXA and MRI measures, biomarkers, and dietary intake data, will be performed prior to data analysis.
The study will assess the effects of dietary interventions on the main endpoints, such as visceral and liver fat, and explore the factors driving dietary adherence. Under Specific Aim 1, an intent-to-treat and a difference-in-differences approach to compare changes in adiposity, at Weeks 12 and 24 between treatment arms, using linear mixed models and Wald tests [51]. We will compare the effectiveness of the intervention by subgroup (sex, age, and VAT level, defined as in the randomization) to gain insight into its generalizability. Similar analyses will be conducted with the metabolic parameters endpoints, including ALT, AST, glucose, insulin, leptin, total cholesterol, HDL, LDL, and triglycerides.
In Specific Aim 2, we will identify, for each treatment arm, the predictors of dietary adherence among participant characteristics and psychosocial and behavioral factors using machine-learning approaches, such as LASSO regression, that allow for consideration of many independent variables. This analysis will provide insights into the factors influencing adherence to each dietary intervention.
Our sample size of 312 randomized participants (assuming a ~16% drop-out rate to yield 260 participants completing the 6-month intervention) with an equal split across the two study arms will provide 80% power to detect small to medium effect sizes (d = 0.35) [52] in primary and secondary outcomes, including visceral fat and body weight, with an alpha (two-sided) of 0.05 (d = 0.49) for analysis of men and women separately. Power is modestly attenuated after multiple comparison control, with (d = 0.54) with alpha = 0.005 (Bonferroni-corrected for 100 tests).
4. Results
Participant enrollment commenced in April 2022 and will end in September 2025. No results are yet available.
5. Discussion
Obesity, specifically concerning visceral adipose tissue (VAT), is a public health challenge, often addressed through DER strategies. The long-term efficacy of these methods is limited due to issues like weight regain. IER is emerging as an alternative strategy and is showing effectiveness in weight loss [53]. Studies such as Harvie et al. (2013) [54], Ash et al. (2003) [55], and Varady et al. (2011) [20] showed that IER can lead to significant weight loss, averaging 0.2–0.8 kg/week, comparable to DER [56]. In addition, studies like Arguin et al. (2012) [57] and Williams et al. (1998) [58] have reported outcomes on body composition, showing reductions in fat mass and waist circumference.
In comparative studies against more traditional dieting approaches, various forms of IER, including alternate-day fasting and time-restricted feeding, have been analyzed by Hutchison et al. (2019) [59], Liu et al. (2019) [60], and Bowen et al. (2018) [61]. These studies demonstrated their effectiveness in generating weight loss and metabolic improvements over periods ranging from 8 to 52 weeks [53]. The present research contributes to the understanding of IER’s role in obesity management, particularly in relation to VAT reduction. However, considering its implications for public health, the long-term efficacy and specific impacts on VAT will warrant more extensive research into IER’s potential in obesity and VAT management [53].
Our methodology stands out due to its comprehensive and innovative approach due to: (1) the precise quantification of fat using MRI and abdominal DXA, providing precise assessments of visceral and liver fat changes; (2) the incorporation of behavioral support techniques, such as motivational interviewing with a dietitian, to enhance practical application and adherence to dietary protocols; and (3) the continuous and detailed monitoring of participants through mobile technology, capturing dietary and lifestyle changes over time for a robust longitudinal analysis. Our inclusive recruitment strategy, designed to cover a diverse range of racial/ethnic backgrounds, aims to ensure that the study’s findings are widely applicable and representative of different population groups.
However, the study is not without limitations. Self-reported data on diet and exercise, even with the aid of technology, could introduce an element of reporting bias. However, there is no expectation that this bias would differ between study arms. The extensive nature of the intervention may also lead to a greater-than-expected participant dropout, impacting the study’s statistical robustness. Additionally, the relatively strict exclusion criteria, although necessary, might limit the generalizability of the findings. These considerations will be important in interpreting the potential impact and scope of the study’s findings.
We believe the HDLS2 study will provide valuable insights into effective diet patterns for reducing visceral and liver fat, as well as for improving metabolic biomarkers. It incorporates a comprehensive approach, considering not just the dietary and physical activity components but also the behavioral aspects of lifestyle change, which are crucial for the success of any dietary intervention. However, attention needs to be given to the integrity of the data analysis and interpretation of results, ensuring the limitations are adequately considered.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16101478/s1, Figure S1: Schema of the Healthy Diet & Lifestyle 2 Protocol (HDLS2).
Author Contributions
Conceptualization, L.L.M. and C.B.; methodology, L.L.M. and C.B.; software, L.R.W.; formal analysis, L.R.W.; data curation, L.R.W.; writing—original draft preparation, M.Y.L. and L.L.M.; writing—review and editing, K.Y., A.R., L.R.W., J.S., K.C., A.S., C.R., U.L., C.B. and L.L.M.; supervision, L.L.M. and U.L.; project administration, L.L.M.; funding acquisition, L.L.M. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the US National Cancer Institute R01 CA25879. The Nutrition Support and Biostatistics Shared Resources were funded in part by NCI grant P30 CA071789. Christoph Rettenmeier was supported in part by the Center of Biomedical Research Excellence (COBRE) under grant number P20GM139753.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the WIRB-Copernicus Group (WCG® IRB) (Protocol LEMARCHAND-2021-2 and approved 23 December 2021).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be made available upon reasonable request.
Acknowledgments
Our team extends heartfelt appreciation to the Nutrition Support Shared Resource at the University of Hawaii Cancer Center, particularly to Jean Ishida and Keala Swafford, for their invaluable assistance with the development of dietary materials, intervention initiatives, and data entry efforts. We are grateful for the expertise provided by the Biostatistics Shared Resource, which aided in data management and analysis and the creation of the randomization protocol. Special thanks to Jenna Tsuzaki, Victoria Mak, Amaya Cheng, and Kylie Uyeda for their diligent work in clinic measurement coordination and collection. Our gratitude also extends to LaVonne Takaezu, Kellie Fujimoto, and Naomi Hee for managing eligibility screenings and organizing study visits efficiently. The efforts of Wileen Mau, Thad Park, and Shelle Santoki in phlebotomy, biospecimen collection, and processing have been instrumental. We are thankful for the support provided by Shirleen Saiki and Jamie Ishii in various study activities. Our work has greatly benefited from the contributions of En Liu, Valerie Saiki, and the team at John Shepherd’s Lab in conducting study measurements. The Video and Image Processing Laboratory (VIPER) at Purdue University, led by Edward Delp, deserves our acknowledgment for allowing the use of the mobile food record (mFR™) and for their technical support. Lastly, we recognize Michelle Harvie (Manchester Biomedical Research Centre, UK) for her support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Centers for Disease Control and Prevention. Adult Obesity Prevalence Remains High; Support for Prevention and Treatment Needed. Available online: https://www.cdc.gov/media/releases/2023/p0922-adult-obesity.html (accessed on 12 October 2023).
- Calle, E.E.; Thun, M.J. Obesity and cancer: Cancer Epidemiology. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Dubey, P.; Cistola, D.P.; Reddy, S.Y. Association Between Obesity and Cardiovascular Outcomes: Updated Evidence from Meta-analysis Studies. Curr. Cardiol. Rep. 2020, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Rapp, K.; Schroeder, J.; Klenk, J.; Stoehr, S.; Ulmer, H.; Concin, H.; Diem, G.; Oberaigner, W.; Weiland, S.K. Obesity and incidence of cancer: A large cohort study of over 145 000 adults in Austria. Br. J. Cancer 2005, 93, 1062–1067. [Google Scholar] [CrossRef]
- Riaz, H.; Khan, M.S.; Siddiqi, T.J.; Usman, M.S.; Shah, N.; Goyal, A.; Khan, S.S.; Mookadam, F.; Krasuski, R.A.; Ahmed, H. Association Between Obesity and Cardiovascular Outcomes A Systematic Review and Meta-analysis of Mendelian Randomization Studies. JAMA Netw. Open 2018, 1, e183788. [Google Scholar] [CrossRef]
- Lim, U.; Monroe, K.R.; Buchthal, S.; Fan, B.; Cheng, I.; Kristal, B.S.; Lampe, J.W.; Hullar, M.A.; Franke, A.A.; Stram, D.O.; et al. Propensity for Intra-abdominal and Hepatic Adiposity Varies Among Ethnic Groups. Gastroenterology 2019, 156, 966–975.e910. [Google Scholar] [CrossRef]
- Le Marchand, L.; Wilkens, L.R.; Castelfranco, A.M.; Monroe, K.R.; Kristal, B.S.; Cheng, I.; Maskarinec, G.; Hullar, M.A.; Lampe, J.W.; Shepherd, J.A.; et al. Circulating Biomarker Score for Visceral Fat and Risks of Incident Colorectal and Postmenopausal Breast Cancer: The Multiethnic Cohort Adiposity Phenotype Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 966–973. [Google Scholar] [CrossRef]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef] [PubMed]
- Carceller, V.; Tell, M.; Diaz, N. European association for the study of the liver (EASL)-51st international liver congress Barcelona, Spain—April 13–17, 2016. Drugs Future 2016, 41, 321–324. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999. [Google Scholar] [CrossRef]
- Mirza, M.S. Obesity, Visceral Fat, and NAFLD: Querying the Role of Adipokines in the Progression of Nonalcoholic Fatty Liver Disease. ISRN Gastroenterol. 2011, 2011, 592404–592411. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Hairston, K.G.; Hanley, A.J.G.; Tooze, J.A.; Norris, J.M.; Palmer, N.D.; Bowden, D.W.; Lorenzo, C.; Chen, Y.D.I.; Wagenknecht, L.E. Association of Visceral Adipose Tissue and Insulin Resistance with Incident Metabolic Syndrome Independent of Obesity Status: The IRAS Family Study. Obesity 2021, 29, 1195–1202. [Google Scholar] [CrossRef]
- Ziccardi, P.; Nappo, F.; Giugliano, G.; Esposito, K.; Marfella, R.; Cioffi, M.; D’Andrea, F.; Molinari, A.M.; Giugliano, D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002, 105, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Samaras, K.; Botelho, N.K.; Chisholm, D.J.; Lord, R.V. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity 2010, 18, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Elands, R.J.J.; Simons, C.C.J.M.; van Dongen, M.; Schouten, L.J.; Verhage, B.A.J.; van den Brandt, P.A.; Weijenberg, M.P. A Systematic Literature Review and Meta-Regression Analysis on Early-Life Energy Restriction and Cancer Risk in Humans. PLoS ONE 2016, 11, e0158003. [Google Scholar] [CrossRef] [PubMed]
- Middleton, K.R.; Anton, S.D.; Perri, M.G. Long-Term Adherence to Health Behavior Change. Am. J. Lifestyle Med. 2013, 7, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.; Howell, A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects-A Narrative Review of Human and Animal Evidence. Behav. Sci. 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl. Res. J. Lab. Clin. Med. 2014, 164, 302–311. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss. Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Chew, H.S.J.; Ang, W.H.D.; Tan, Z.Y.A.; Ang, W.W.; Chan, K.S.; Lau, Y. Umbrella review of time-restricted eating on weight loss, fasting blood glucose, and lipid profile. Nutr. Rev. 2023, 81, 1180–1199. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Przulj, D.; Pesola, F.; McRobbie, H.; Peerbux, S.; Phillips-Waller, A.; Bisal, N.; Smith, K.M. A randomised controlled trial of the 5:2 diet. PLoS ONE 2021, 16, e0258853. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Zhang, J.; Xu, J. Intermittent Versus Continuous Energy Restriction for Weight Loss and Metabolic Improvement: A Meta-Analysis and Systematic Review. Obesity 2021, 29, 108–115. [Google Scholar] [CrossRef] [PubMed]
- EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [CrossRef]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Bes-Rastrollo, M.; Itsiopoulos, C.; Thomas, C.J. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef] [PubMed]
- Dernini, S.; Berry, E.M. Mediterranean Diet: From a Healthy Diet to a Sustainable Dietary Pattern. Front. Nutr. 2015, 2, 15. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Lagiou, P. Healthy Traditional Mediterranean Diet: An Expression of Culture, History, and Lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.N.; Howell, T. Could Intermittent Energy Restriction and Intermittent Fasting Reduce Rates of Cancer in Obese, Overweight, and Normal-Weight Subjects? A Summary of Evidence. Adv. Nutr. 2016, 7, 690–705. [Google Scholar] [CrossRef]
- Hsu, C.C.; Ness, E.; Kowdley, K.V. Nutritional Approaches to Achieve Weight Loss in Nonalcoholic Fatty Liver Disease. Adv. Nutr. 2017, 8, 253–265. [Google Scholar] [CrossRef]
- Lee, H.A.; Moon, H.; Kim, Y.; Lee, H.A.; Kim, H.Y. Effect of 12-week intermittent calorie restriction compared to standard of care in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Curr. Control. Trials Cardiovasc. Med. 2023, 24, 490. [Google Scholar] [CrossRef] [PubMed]
- Panizza, C.E.; Lim, U.; Yonemori, K.M.; Cassel, K.D.; Wilkens, L.R.; Harvie, M.N.; Maskarinec, G.; Delp, E.J.; Lampe, J.W.; Shepherd, J.A.; et al. Effects of Intermittent Energy Restriction Combined with a Mediterranean Diet on Reducing Visceral Adiposity: A Randomized Active Comparator Pilot Study. Nutrients 2019, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Maislin, G.; Ahmed, M.M.; Gooneratne, N.; Thorne-Fitzgerald, M.; Kim, C.; Teff, K.; Arnardottir, E.S.; Benediktsdottir, B.; Einarsdottir, H.; Juliusson, S.; et al. Single Slice vs. Volumetric MR Assessment of Visceral Adipose Tissue: Reliability and Validity Among the Overweight and Obese. Obesity 2012, 20, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Estrada, S.; Lu, R.; Conjeti, S.; Orozco-Ruiz, X.; Panos-Willuhn, J.; Breteler, M.M.B.; Reuter, M. FatSegNet: A fully automated deep learning pipeline for adipose tissue segmentation on abdominal dixon MRI. Magn. Reson. Med. 2020, 83, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Shvetsov, Y.B.; Wong, M.C.; Garber, A.; Monroe, K.; Ernst, T.M.; Buchthal, S.D.; Lim, U.; Le Marchand, L.; Heymsfield, S.B.; et al. Subcutaneous and visceral fat assessment by DXA and MRI in older adults and children. Obesity 2022, 30, 920–930. [Google Scholar]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, S.; Zanjani, S.; Gjellan, S.; Leinhard, O.D.; Kihlberg, J.; Smedby, Ö.; Johansson, L.; Kullberg, J.; Ahlström, H.; Lindström, T.; et al. Effects of moderate red wine consumption on liver fat and blood lipids: A prospective randomized study. Ann. Med. 2011, 43, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Resnicow, K.; Jackson, A.; Blissett, D.; Wang, T.; McCarty, F.; Rahotep, S.; Periasamy, S. Results of the Healthy Body Healthy Spirit Trial. Health Psychol. 2005, 24, 339–348. [Google Scholar] [CrossRef]
- Resnicow, K.; Jackson, A.; Wang, T.; De, A.K.; McCarty, F.; Dudley, W.N.; Baranowski, T. A Motivational Interviewing Intervention to Increase Fruit and Vegetable Intake Through Black Churches: Results of the Eat for Life Trial. Am. J. Public Health 2001, 91, 1686–1693. [Google Scholar] [CrossRef]
- Resnicow, K.; Kramish Campbell, M.; Carr, C.; McCarty, F.; Wang, T.; Periasamy, S.; Rahotep, S.; Doyle, C.; Williams, A.; Stables, G. Body and soul: A dietary intervention conducted through African-American churches. Am. J. Prev. Med. 2004, 27, 97–105. [Google Scholar] [CrossRef]
- Miller, W.; Rollnick, S. Motivational Interviewing: Preparing People for Change, 2nd ed. J. Healthc. Qual. 2003, 25, 46. [Google Scholar] [CrossRef]
- Nouwen, A.; Ford, T.; Balan, A.T.; Twisk, J.W.; Ruggiero, L.; White, D. Longitudinal motivational predictors of dietary self-care and diabetes control in adults with newly diagnosed type 2 diabetes mellitus. Health Psychol. 2011, 30, 771–779. [Google Scholar] [CrossRef]
- Sallis, J.F.; Grossman, R.M.; Pinski, R.B.; Patterson, T.L.; Nader, P.R. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987, 16, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Rieger, E.; Sellbom, M.; Murray, K.; Caterson, I. Measuring social support for healthy eating and physical activity in obesity. Br. J. Health Psychol. 2018, 23, 1021–1039. [Google Scholar] [CrossRef] [PubMed]
- Boushey, C.J.; Spoden, M.; Zhu, F.M.; Delp, E.J.; Kerr, D.A. New mobile methods for dietary assessment: Review of image-assisted and image-based dietary assessment methods. Proc. Nutr. Soc. 2017, 76, 283–294. [Google Scholar] [CrossRef]
- Fengqing, Z.; Bosch, M.; Insoo, W.; SungYe, K.; Boushey, C.J.; Ebert, D.S.; Delp, E.J. The Use of Mobile Devices in Aiding Dietary Assessment and Evaluation. IEEE J. Sel. Top. Signal Process. 2010, 4, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Neishabouri, A.; Nguyen, J.; Samuelsson, J.; Guthrie, T.; Biggs, M.; Wyatt, J.; Cross, D.; Karas, M.; Migueles, J.H.; Khan, S.; et al. Quantification of acceleration as activity counts in ActiGraph wearable. Sci. Rep. 2022, 12, 11958. [Google Scholar] [CrossRef]
- Daw, J.R.; Hatfield, L.A. Matching and Regression to the Mean in Difference-in-Differences Analysis. Health Serv. Res. 2018, 53, 4138–4156. [Google Scholar] [CrossRef]
- Lindner, R.; Cohen, J. Statistical Power Analysis for the Behavioral Sciences, rev ed.; Erlbaum: Hillsdale, NJ, USA, 1988; Volume 67, p. 1007. [Google Scholar]
- Wei, X.; Cooper, A.; Lee, I.; Cernoch, C.A.; Huntoon, G.; Hodek, B.; Christian, H.; Chao, A.M. Intermittent Energy Restriction for Weight Loss: A Systematic Review of Cardiometabolic, Inflammatory and Appetite Outcomes. Biol. Res. Nurs. 2022, 24, 410–428. [Google Scholar] [CrossRef]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Ash, S.; Reeves, M.M.; Yeo, S.; Morrison, G.; Carey, D.; Capra, S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with Type II diabetes: A randomised trial. Int. J. Obes. 2003, 27, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.S.; Clarke, R.E.; Coulter, S.N.; Rounsefell, K.N.; Walker, R.E.; Rauch, C.E.; Huggins, C.E.; Ryan, L. Intermittent energy restriction and weight loss: A systematic review. Eur. J. Clin. Nutr. 2016, 70, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Arguin, H.; Dionne, I.J.; Sénéchal, M.; Bouchard, D.R.; Carpentier, A.C.; Ardilouze, J.-L.; Tremblay, A.; Leblanc, C.; Brochu, M. Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: A pilot study. Menopause 2012, 19, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.V.; Mullen, M.L.; Kelley, D.E.; Wing, R.R. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care 1998, 21, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Liu, B.; Wood, R.E.; Vincent, A.D.; Thompson, C.H.; O’Callaghan, N.J.; Wittert, G.A.; Heilbronn, L.K. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity 2019, 27, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hutchison, A.T.; Thompson, C.H.; Lange, K.; Heilbronn, L.K. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes. Res. Clin. Pract. 2019, 13, 408–415. [Google Scholar] [CrossRef]
- Bowen, J.; Brindal, E.; James-Martin, G.; Noakes, M. Randomized Trial of a High Protein, Partial Meal Replacement Program with or without Alternate Day Fasting: Similar Effects on Weight Loss, Retention Status, Nutritional, Metabolic, and Behavioral Outcomes. Nutrients 2018, 10, 1145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).