Fasting and Glucose Metabolism Differentially Impact Peripheral Inflammation in Human Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Cell Manipulation and Cytokine Measurements
2.3. Metabolic Flux Analysis
2.4. Statistical Analysis
2.5. Bioinformatics
3. Results
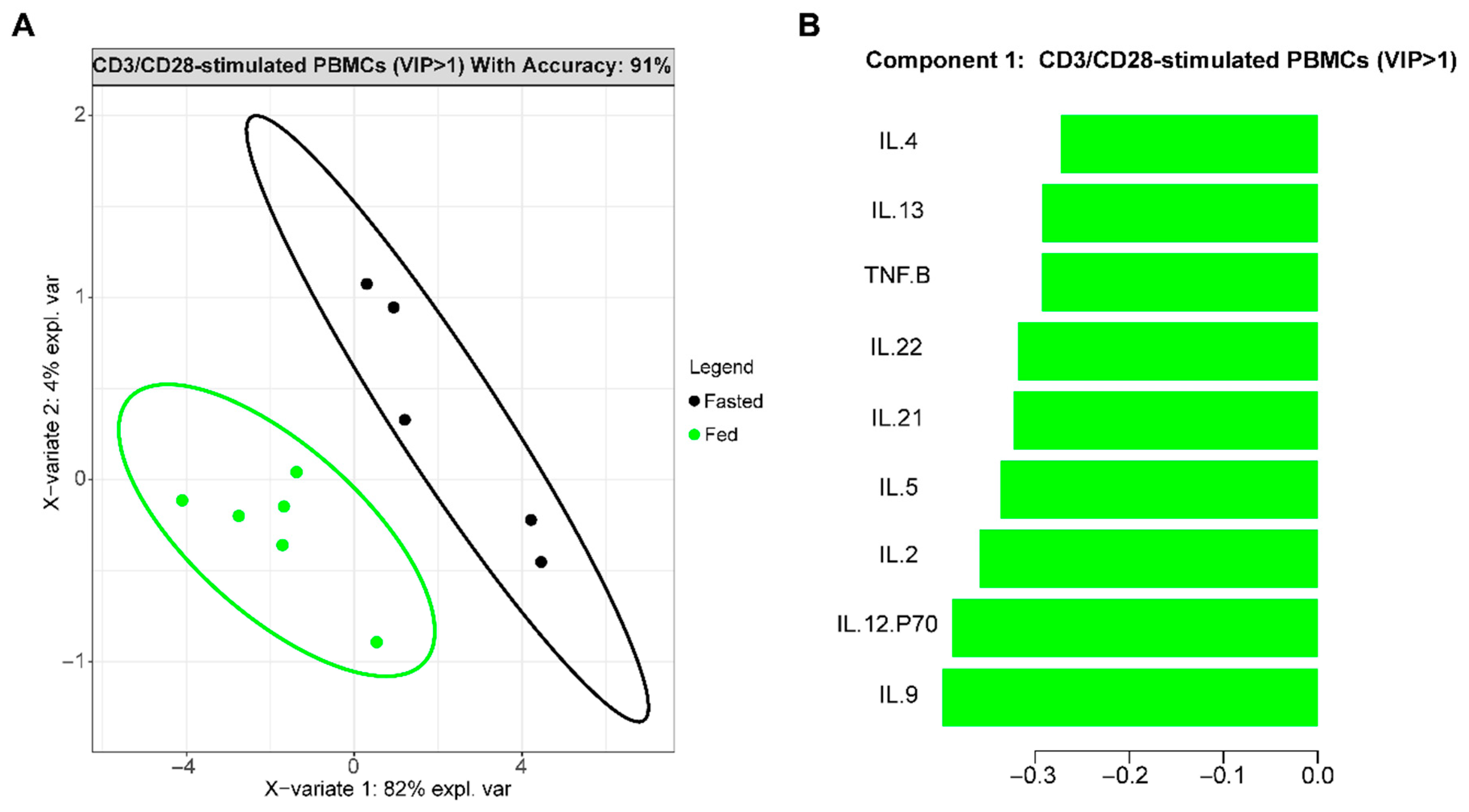
3.1. Fasting Changes Immune Cell Cytokine Profiles in T2D
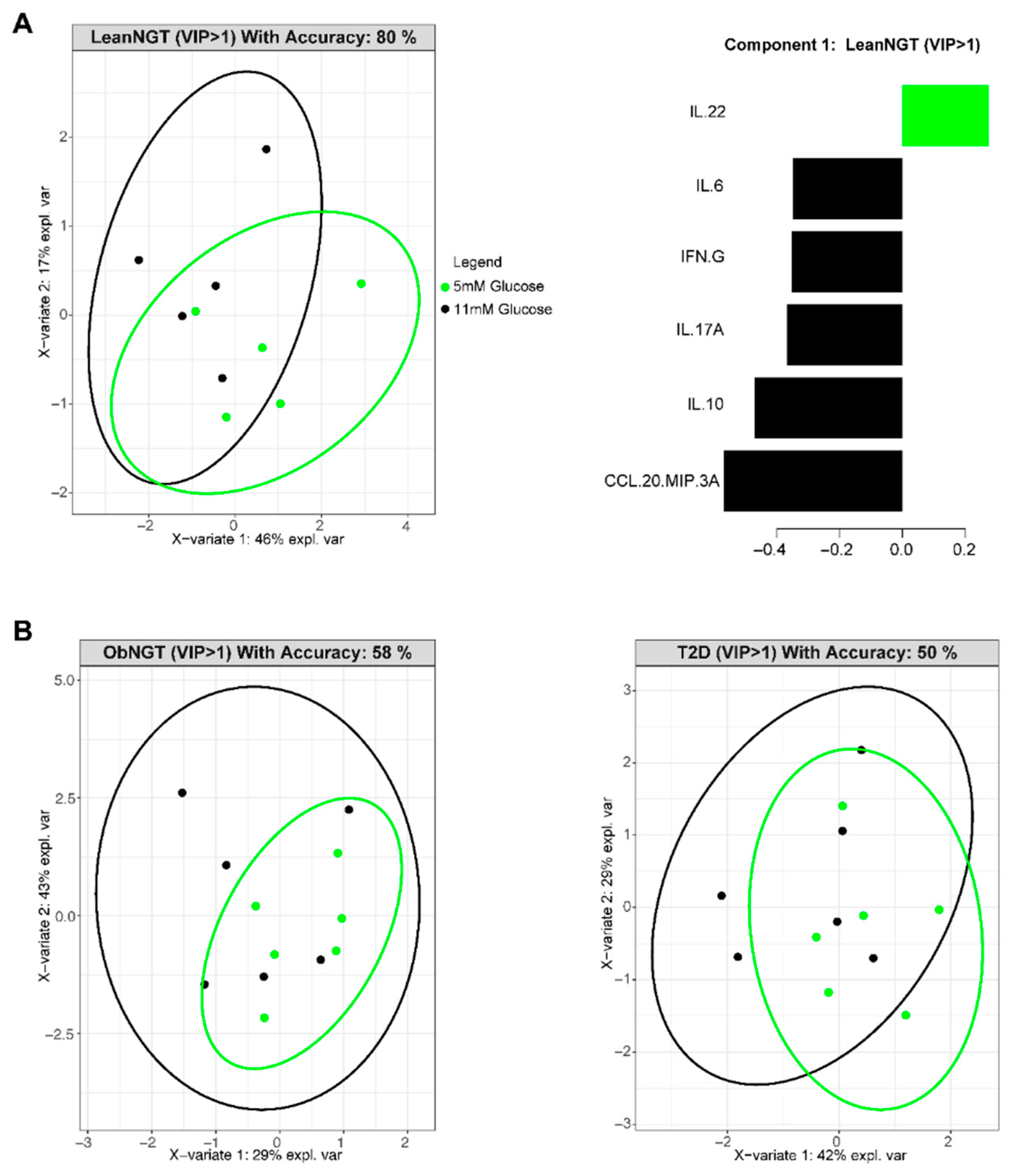
3.2. In Vitro Glucose Concentrations Do Not Alter Cytokine Production by Cells from Fasted Donors
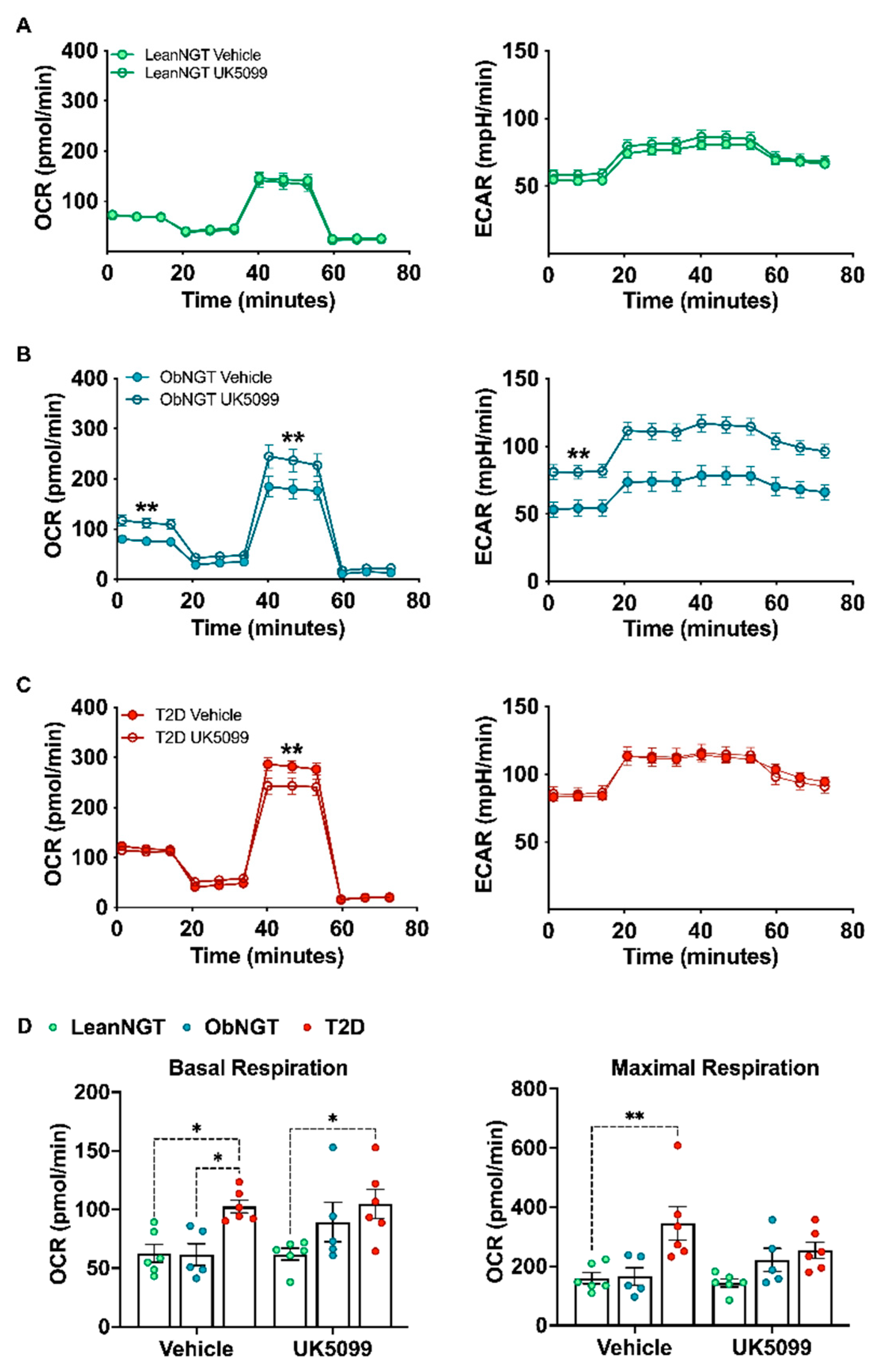
3.3. Mitochondrial Glucose Oxidation Regulates Cytokine Production while Modestly Raising OXPHOS in T-Cells from Donors with T2D
4. Discussion
5. Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yen, F.S.; Wei, J.C.; Chiu, L.T.; Hsu, C.C.; Hwu, C.M. Cardiovascular outcomes of metformin use in patients with type 2 diabetes and chronic obstructive pulmonary disease. Front. Pharmacol. 2022, 13, 919881. [Google Scholar] [CrossRef]
- Yen, F.S.; Hou, M.C.; Wei, J.C.; Shih, Y.H.; Hsu, C.Y.; Hsu, C.C.; Hwu, C.M. Liver-related long-term outcomes of alpha-glucosidase inhibitors in patients with diabetes and liver cirrhosis. Front. Pharmacol. 2022, 13, 1049094. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.P.; Wu, C.T.; Lin, J.L.; Hsiung, C.A.; Liu, H.Y.; Lai, J.N.; Yang, C.C. Metformin Treatment Is Associated with a Decreased Risk of Nonproliferative Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus: A Population-Based Cohort Study. J. Diabetes Res. 2020, 2020, 9161039. [Google Scholar] [CrossRef]
- Amin, S.V.; Khanna, S.; Parvar, S.P.; Shaw, L.T.; Dao, D.; Hariprasad, S.M.; Skondra, D. Metformin and retinal diseases in preclinical and clinical studies: Insights and review of literature. Exp. Biol. Med. 2022, 247, 317–329. [Google Scholar] [CrossRef]
- Everett, B.M.; Donath, M.Y.; Pradhan, A.D.; Thuren, T.; Pais, P.; Nicolau, J.C.; Glynn, R.J.; Libby, P.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J. Am. Coll. Cardiol. 2018, 71, 2392–2401. [Google Scholar] [CrossRef]
- Cavelti-Weder, C.; Timper, K.; Seelig, E.; Keller, C.; Osranek, M.; Lassing, U.; Spohn, G.; Maurer, P.; Muller, P.; Jennings, G.T.; et al. Development of an Interleukin-1beta Vaccine in Patients with Type 2 Diabetes. Mol. Ther. 2016, 24, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.E.; Berry, J.; Kim, S.; Canavan, B.; Grinspoon, S.K. Effects of etanercept in patients with the metabolic syndrome. Arch. Intern. Med. 2006, 166, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Ganguli, M.; Cox, K.; Means, B.; Gerling, I.; Solomon, S.S. Does therapy with anti-TNF-alpha improve glucose tolerance and control in patients with type 2 diabetes? Diabetes Care 2011, 34, e121. [Google Scholar] [CrossRef]
- Stafeev, I.S.; Michurina, S.S.; Podkuychenko, N.V.; Vorotnikov, A.V.; Menshikov, M.Y.; Parfyonova, Y.V. Interleukin-4 Restores Insulin Sensitivity in Lipid-Induced Insulin-Resistant Adipocytes. Biochemistry 2018, 83, 498–506. [Google Scholar] [CrossRef]
- Stanya, K.J.; Jacobi, D.; Liu, S.; Bhargava, P.; Dai, L.; Gangl, M.R.; Inouye, K.; Barlow, J.L.; Ji, Y.; Mizgerd, J.P.; et al. Direct control of hepatic glucose production by interleukin-13 in mice. J. Clin. Investig. 2013, 123, 261–271. [Google Scholar] [CrossRef]
- Ricardo-Gonzalez, R.R.; Red Eagle, A.; Odegaard, J.I.; Jouihan, H.; Morel, C.R.; Heredia, J.E.; Mukundan, L.; Wu, D.; Locksley, R.M.; Chawla, A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 22617–22622. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Tsai, J.N.; Chen, T.L.; Ho, K.T.; Cheng, H.Y.; Hsiao, C.W.; Shiau, M.Y. Interleukin-4 Promotes Myogenesis and Boosts Myocyte Insulin Efficacy. Mediat. Inflamm. 2019, 2019, 4182015. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Franck, N.; Egan, B.; Sjogren, R.J.; Katayama, M.; Duque-Guimaraes, D.; Arner, P.; Zierath, J.R.; Krook, A. Autocrine role of interleukin-13 on skeletal muscle glucose metabolism in type 2 diabetic patients involves microRNA let-7. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1359–E1366. [Google Scholar] [CrossRef] [PubMed]
- Kanety, H.; Feinstein, R.; Papa, M.Z.; Hemi, R.; Karasik, A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J. Biol. Chem. 1995, 270, 23780–23784. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; Fam, B.C.; Cempako, G.R.; Steinberg, G.R.; Walder, K.; Kay, T.W.; Proietto, J.; Andrikopoulos, S. Deficiency in interferon-gamma results in reduced body weight and better glucose tolerance in mice. Endocrinology 2011, 152, 3690–3699. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, Q.; Ma, S.; Liu, S.; Chen, Z.; Mo, Z.; You, Z. Interleukin-17A Differentially Induces Inflammatory and Metabolic Gene Expression in the Adipose Tissues of Lean and Obese Mice. Int. J. Mol. Sci. 2016, 17, 522. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Liu, L.F.; Lamendola, C.; Shen, L.; Morton, J.; Rivas, H.; Winer, D.; Tolentino, L.; Choi, O.; Zhang, H.; et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Ip, B.; Cilfone, N.A.; Belkina, A.C.; DeFuria, J.; Jagannathan-Bogdan, M.; Zhu, M.; Kuchibhatla, R.; McDonnell, M.E.; Xiao, Q.; Kepler, T.B.; et al. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFalpha production. Obesity 2016, 24, 102–112. [Google Scholar] [CrossRef]
- Pugh, G.H.; Fouladvand, S.; SantaCruz-Calvo, S.; Agrawal, M.; Zhang, X.D.; Chen, J.; Kern, P.A.; Nikolajczyk, B.S. T cells dominate peripheral inflammation in a cross-sectional analysis of obesity-associated diabetes. Obesity 2022, 30, 1983–1994. [Google Scholar] [CrossRef]
- Martinez, N.; Vallerskog, T.; West, K.; Nunes-Alves, C.; Lee, J.; Martens, G.W.; Behar, S.M.; Kornfeld, H. Chromatin decondensation and T cell hyperresponsiveness in diabetes-associated hyperglycemia. J. Immunol. 2014, 193, 4457–4468. [Google Scholar] [CrossRef] [PubMed]
- Gessl, A.; Waldhausl, W. Increased CD69 and human leukocyte antigen-DR expression on T lymphocytes in insulin-dependent diabetes mellitus of long standing. J. Clin. Endocrinol. Metab. 1998, 83, 2204–2209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, T.; Xiang, Y.; Deng, C.; Cao, C.; Ren, Z.; Huang, G.; Zhou, Z. Variable frequencies of peripheral T-lymphocyte subsets in the diabetes spectrum from type 1 diabetes through latent autoimmune diabetes in adults (LADA) to type 2 diabetes. Front. Immunol. 2022, 13, 974864. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.Y.M.; Carroll, E.C.; Callender, L.A.; Hood, G.A.; Berryman, V.; Pattrick, M.; Finer, S.; Hitman, G.A.; Ackland, G.L.; Henson, S.M. Type 2 diabetes is associated with the accumulation of senescent T cells. Clin. Exp. Immunol. 2019, 197, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Miya, A.; Nakamura, A.; Miyoshi, H.; Takano, Y.; Sunagoya, K.; Hayasaka, K.; Shimizu, C.; Terauchi, Y.; Atsumi, T. Impact of Glucose Loading on Variations in CD4(+) and CD8(+) T Cells in Japanese Participants with or without Type 2 Diabetes. Front. Endocrinol. 2018, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Wadowski, M.; Goruk, S.; Cameron, L.; Sharma, A.M.; Field, C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care 2017, 5, e000379. [Google Scholar] [CrossRef]
- Nicholas, D.A.; Proctor, E.A.; Agrawal, M.; Belkina, A.C.; Van Nostrand, S.C.; Panneerseelan-Bharath, L.; Jones, A.R.; Raval, F.; Ip, B.C.; Zhu, M.; et al. Fatty Acid Metabolites Combine with Reduced beta Oxidation to Activate Th17 Inflammation in Human Type 2 Diabetes. Cell Metab. 2019, 30, 447–461 e445. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv. Nutr. 2017, 8, 213–225. [Google Scholar] [CrossRef]
- Dror, E.; Dalmas, E.; Meier, D.T.; Wueest, S.; Thevenet, J.; Thienel, C.; Timper, K.; Nordmann, T.M.; Traub, S.; Schulze, F.; et al. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 2017, 18, 283–292. [Google Scholar] [CrossRef]
- Aziz, N. Measurement of Circulating Cytokines and Immune-Activation Markers by Multiplex Technology in the Clinical Setting: What Are We Really Measuring? Forum Immunopathol. Dis. Ther. 2015, 6, 19–22. [Google Scholar] [CrossRef]
- Spranger, J.; Kroke, A.; Mohlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.S.; da Silva, M.V.; da Silva, C.A.; Borges, M.F.; Palhares, H.; Rocha, L.P.; Correa, R.R.M.; Rodrigues Junior, V.; Dos Reis, M.A.; Machado, J.R. Analysis of serum inflammatory mediators in type 2 diabetic patients and their influence on renal function. PLoS ONE 2020, 15, e0229765. [Google Scholar] [CrossRef] [PubMed]
- Yazdani-Biuki, B.; Stelzl, H.; Brezinschek, H.P.; Hermann, J.; Mueller, T.; Krippl, P.; Graninger, W.; Wascher, T.C. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-alpha antibody infliximab. Eur. J. Clin. Investig. 2004, 34, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, H.; Storgaard, H.; Rask-Madsen, C.; Steffen Hermann, T.; Ihlemann, N.; Baunbjerg Nielsen, D.; Spohr, C.; Kober, L.; Vaag, A.; Torp-Pedersen, C. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J. Vasc. Res. 2005, 42, 517–525. [Google Scholar] [CrossRef]
- Nicholas, D.; Proctor, E.A.; Raval, F.M.; Ip, B.C.; Habib, C.; Ritou, E.; Grammatopoulos, T.N.; Steenkamp, D.; Dooms, H.; Apovian, C.M.; et al. Advances in the quantification of mitochondrial function in primary human immune cells through extracellular flux analysis. PLoS ONE 2017, 12, e0170975. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Pugh, G.H.; Tevonian, E.; Thompson, K.; Lauffenburger, D.A.; Kern, P.A.; Nikolajczyk, B.S. Regulatory T Cells Control Effector T Cell Inflammation in Human Prediabetes. Diabetes 2022, 71, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Lars Ståhle, S.W. Partial least squares analysis with cross-validation for the two-class problem: A Monte Carlo study. J. Chemom. 1987, 1, 185–196. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Rocke, D.M. Tumor classification by partial least squares using microarray gene expression data. Bioinformatics 2002, 18, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Rohart, F.; Gautier, B.; Singh, A.; Le Cao, K.A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Battaglia, G.M.; Zheng, D.; Hickner, R.C.; Houmard, J.A. Effect of exercise training on metabolic flexibility in response to a high-fat diet in obese individuals. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1440–E1445. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Strassburger, K.; Herder, C.; Knebel, B.; Kotzka, J.; Szendroedi, J.; Roden, M.; GDS group. Metabolic flexibility and oxidative capacity independently associate with insulin sensitivity in individuals with newly diagnosed type 2 diabetes. Diabetologia 2016, 59, 2203–2207. [Google Scholar] [CrossRef] [PubMed]
- Hoskova, E.; Kopecky, J., Jr.; Veleba, J.; Velebova, K.; Melenovsky, V.; Pelikanova, T. 2-OR: Metabolic inflexibility in patients with type 2 diabetes and heart failure. Diabetes 2019, 68 (Suppl. 1), 2-OR. [Google Scholar] [CrossRef]
- Bental, M.; Deutsch, C. Metabolic changes in activated T cells: An NMR study of human peripheral blood lymphocytes. Magn. Reson. Med. 1993, 29, 317–326. [Google Scholar] [CrossRef]
- Greiner, E.F.; Guppy, M.; Brand, K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J. Biol. Chem. 1994, 269, 31484–31490. [Google Scholar] [CrossRef] [PubMed]
- Frauwirth, K.A.; Thompson, C.B. Regulation of T lymphocyte metabolism. J. Immunol. 2004, 172, 4661–4665. [Google Scholar] [CrossRef]
- Jacobs, S.R.; Herman, C.E.; Maciver, N.J.; Wofford, J.A.; Wieman, H.L.; Hammen, J.J.; Rathmell, J.C. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 2008, 180, 4476–4486. [Google Scholar] [CrossRef]
- MacDonald, H.R. Energy metabolism and T-cell-mediated cytolysis. II. Selective inhibition of cytolysis by 2-deoxy-D-glucose. J. Exp. Med. 1977, 146, 710–719. [Google Scholar] [CrossRef]
- Yang, J.Q.; Kalim, K.W.; Li, Y.; Zhang, S.; Hinge, A.; Filippi, M.D.; Zheng, Y.; Guo, F. RhoA orchestrates glycolysis for TH2 cell differentiation and allergic airway inflammation. J. Allergy Clin. Immunol. 2016, 137, 231–245 e234. [Google Scholar] [CrossRef]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef]
- Ma, M.; Ma, C.; Li, P.; Ma, C.; Ping, F.; Li, W.; Xu, L.; Zhang, H.; Sun, Q.; Li, Y. Low glucose enhanced metformin’s inhibitory effect on pancreatic cancer cells by suppressing glycolysis and inducing energy stress via up-regulation of miR-210-5p. Cell Cycle 2020, 19, 2168–2181. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control Prevention; U.S. Dept of Health and Human Services. National Diabetes Statistics Report 2020: Estimates of Diabetes and Its Burden in the United States; Centers for Disease Control Prevention (CDC): Atlanta, GA, USA, 2020. [Google Scholar]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, H.; Liu, T.; Qin, C.; Sesaki, H.; Guo, S.; Radovick, S.; Hussain, M.; Maheshwari, A.; Wondisford, F.E.; et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019, 29, 1511–1523 e1515. [Google Scholar] [CrossRef]
- Kumari, S.; Bubak, M.T.; Schoenberg, H.M.; Davidyan, A.; Elliehausen, C.J.; Kuhn, K.G.; VanWagoner, T.M.; Karaman, R.; Scofield, R.H.; Miller, B.F.; et al. Antecedent Metabolic Health and Metformin (ANTHEM) Aging Study: Rationale and Study Design for a Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2373–2377. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalantar, G.H.; Saraswat, S.; SantaCruz-Calvo, S.; Gholamrezaeinejad, F.; Javidan, A.; Agrawal, M.; Liu, R.; Kern, P.A.; Zhang, X.D.; Nikolajczyk, B.S. Fasting and Glucose Metabolism Differentially Impact Peripheral Inflammation in Human Type 2 Diabetes. Nutrients 2024, 16, 1404. https://doi.org/10.3390/nu16101404
Kalantar GH, Saraswat S, SantaCruz-Calvo S, Gholamrezaeinejad F, Javidan A, Agrawal M, Liu R, Kern PA, Zhang XD, Nikolajczyk BS. Fasting and Glucose Metabolism Differentially Impact Peripheral Inflammation in Human Type 2 Diabetes. Nutrients. 2024; 16(10):1404. https://doi.org/10.3390/nu16101404
Chicago/Turabian StyleKalantar, Gabriella H., Shubh Saraswat, Sara SantaCruz-Calvo, Fatemeh Gholamrezaeinejad, Aida Javidan, Madhur Agrawal, Rui Liu, Philip A. Kern, Xiaohua Douglas Zhang, and Barbara S. Nikolajczyk. 2024. "Fasting and Glucose Metabolism Differentially Impact Peripheral Inflammation in Human Type 2 Diabetes" Nutrients 16, no. 10: 1404. https://doi.org/10.3390/nu16101404
APA StyleKalantar, G. H., Saraswat, S., SantaCruz-Calvo, S., Gholamrezaeinejad, F., Javidan, A., Agrawal, M., Liu, R., Kern, P. A., Zhang, X. D., & Nikolajczyk, B. S. (2024). Fasting and Glucose Metabolism Differentially Impact Peripheral Inflammation in Human Type 2 Diabetes. Nutrients, 16(10), 1404. https://doi.org/10.3390/nu16101404





