Cutaneous Disorders Masking Celiac Disease: Case Report and Mini Review with Proposal for a Practical Clinical Approach
Abstract
1. Introduction
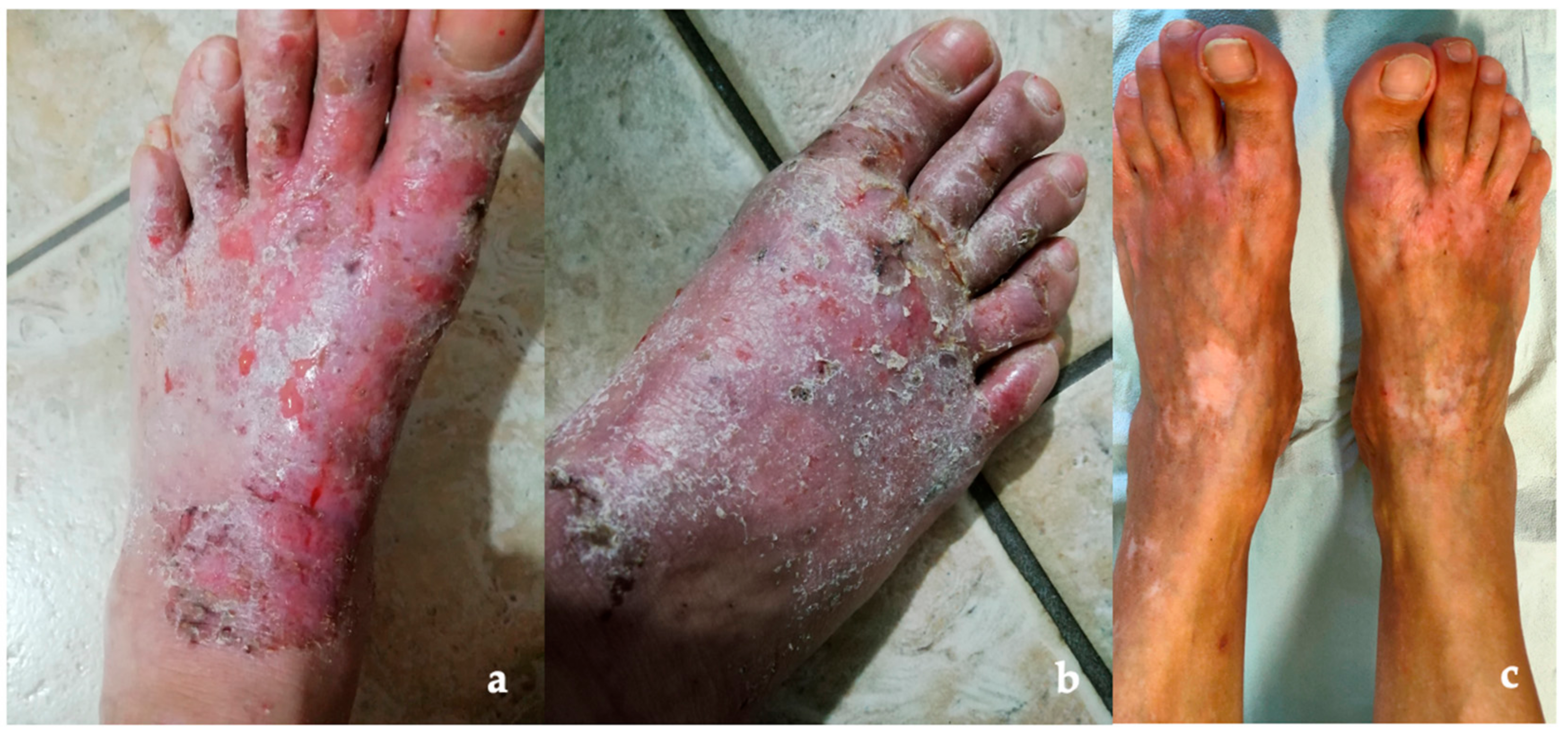
2. Case Report
3. Review: CD Association with Connective Tissue Diseases and Cutaneous Vasculitis
3.1. CD Skin Manifestations Overlapping with CTD
3.2. CD Skin Manifestation Overlapping with Cutaneous Vasculitis
3.3. Acrodermatitis Entheropatica Secondary to CD
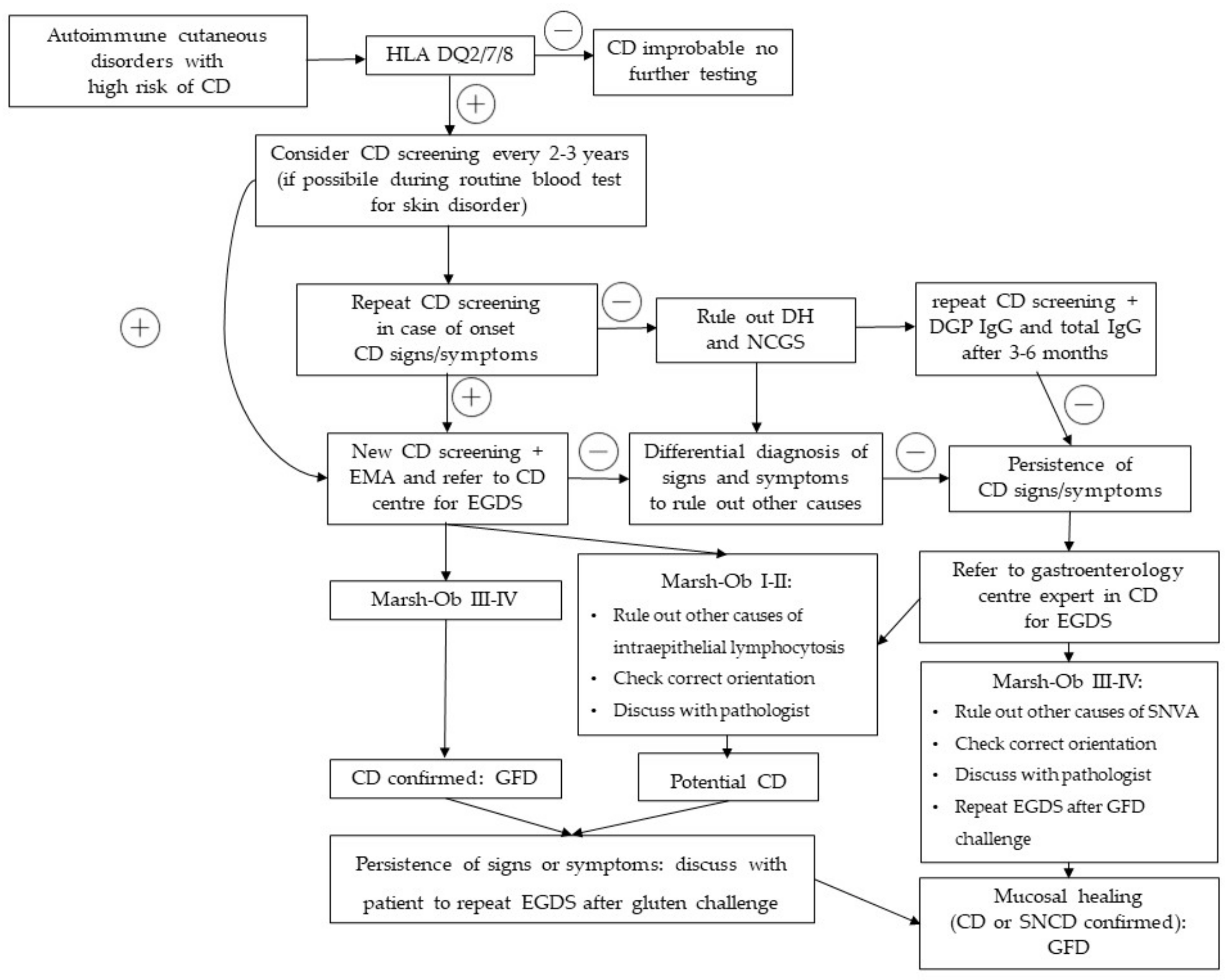
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Sabatino, A.; Corazza, G.R. Coeliac Disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Greer, K.B.; Limketkai, B.N.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-Related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef] [PubMed]
- Bonamico, M.; Mariani, P.; Thanasi, E.; Ferri, M.; Nenna, R.; Tiberti, C.; Mora, B.; Mazzilli, M.C.; Magliocca, F.M. Patchy Villous Atrophy of the Duodenum in Childhood Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Behl, S.; Khan, M.R.; Ismail, Y.; Swantek, C.; Chen, Z.-M.E.; Murray, J.A.; Absah, I. The Characteristics of Isolated Bulb Celiac Disease in Children. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 79–85. [Google Scholar] [CrossRef]
- Deb, A.; Moond, V.; Thongtan, T.; Deliwala, S.; Chandan, S.; Mohan, B.P.; Adler, D.G. Role of Duodenal Bulb Biopsy in Diagnosing Suspected Celiac Disease in Adult Patients: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2023, 10, 1097. [Google Scholar] [CrossRef]
- Schiepatti, A.; Biagi, F.; Fraternale, G.; Vattiato, C.; Balduzzi, D.; Agazzi, S.; Alpini, C.; Klersy, C.; Corazza, G.R. Short Article: Mortality and Differential Diagnoses of Villous Atrophy without Coeliac Antibodies. Eur. J. Gastroenterol. Hepatol. 2017, 29, 572–576. [Google Scholar] [CrossRef]
- Graziano, M.; Rossi, M. An Update on the Cutaneous Manifestations of Coeliac Disease and Non-Coeliac Gluten Sensitivity. Int Rev. Immunol. 2018, 37, 291–300. [Google Scholar] [CrossRef]
- Wu, J.J.; Nguyen, T.U.; Poon, K.Y.T.; Herrinton, L.J. The Association of Psoriasis with Autoimmune Diseases. J. Am. Acad. Dermatol. 2012, 67, 924–930. [Google Scholar] [CrossRef]
- De Bastiani, R.; Gabrielli, M.; Lora, L.; Napoli, L.; Tosetti, C.; Pirrotta, E.; Ubaldi, E.; Bertolusso, L.; Zamparella, M.; de Polo, M.; et al. Association between Coeliac Disease and Psoriasis: Italian Primary Care Multicentre Study. Dermatology 2015, 230, 156–160. [Google Scholar] [CrossRef]
- Birkenfeld, S.; Dreiher, J.; Weitzman, D.; Cohen, A.D. Coeliac Disease Associated with Psoriasis. Br. J. Dermatol. 2009, 161, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L. Celiac Disease and Skin: Psoriasis Association. World J. Gastroenterol. 2007, 13, 2138–2139. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.K.; Millsop, J.W.; Debbaneh, M.; Koo, J.; Linos, E.; Liao, W. Diet and Psoriasis, Part II: Celiac Disease and Role of a Gluten-Free Diet. J. Am. Acad. Dermatol. 2014, 71, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; Parente, A.; de Lorenzi, G.; di Paola, M.E.D.A.; Abenavoli, L.; Leggio, L.; Capristo, E.; de Simone, C.; Rotoli, M.; Rapaccini, G.L.; et al. Rapid Regression of Psoriasis in a Coeliac Patient after Gluten-Free Diet: A Case Report and Review of the Literature. Digestion 2003, 68, 9–12. [Google Scholar] [CrossRef]
- Caminiti, L.; Passalacqua, G.; Magazzù, G.; Comisi, F.; Vita, D.; Barberio, G.; Sferlazzas, C.; Pajno, G.B. Chronic Urticaria and Associated Coeliac Disease in Children: A Case-Control Study. Pediatr. Allergy Immunol. 2005, 16, 428–432. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Lindelöf, B.; Rashtak, S.; Rubio-Tapia, A.; Murray, J.A. Does Urticaria Risk Increase in Patients with Celiac Disease? A Large Population-Based Cohort Study. Eur. J. Dermatol. 2013, 23, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, Z.; Najafian, J.; Fatemi Naeini, F.; Fahimipour, F. Vitiligo and Autoantibodies of Celiac Disease. Int. J. Prev. Med. 2013, 4, 200. [Google Scholar]
- Zhang, J.Z.; Abudoureyimu, D.; Wang, M.; Yu, S.R.; Kang, X.J. Association between Celiac Disease and Vitiligo: A Review of the Literature. World J. Clin. Cases 2021, 9, 10430–10437. [Google Scholar] [CrossRef]
- Abenavoli, L.; Dastoli, S.; Bennardo, L.; Boccuto, L.; Passante, M.; Silvestri, M.; Proietti, I.; Potenza, C.; Luzza, F.; Nisticò, S.P. The Skin in Celiac Disease Patients: The Other Side of the Coin. Medicina 2019, 55, 578. [Google Scholar] [CrossRef]
- Ma, Y.; Zhuang, D.; Qiao, Z. Dual Threat of Comorbidity of Celiac Disease and Systemic Lupus Erythematosus. J. Int. Med. Res. 2021, 49, 03000605211012258. [Google Scholar] [CrossRef]
- Varkell, N.; Braester, A.; Suprun, H.; Nusem, D.; Horn, Y. Simultaneous Occurrence of Systemic Lupus Erythematosus and Coeliac Disease-like Features. Postgrad. Med. J. 1989, 65, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Boccuti, V.; Perrone, A.; D’Introno, A.; Campobasso, A.; Sangineto, M.; Sabbà, C. An Unusual Association of Three Autoimmune Disorders: Celiac Disease, Systemic Lupus Erythematosus and Hashimoto’s Thyroiditis. Autoimmun. Highlights 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Soo Song, M.; Farber, D.; Bitton, A.; Jass FRCPA DSc, J.; Singer, M.B.; Karpati, G.; Song, M.; Farber, D.; Bitton, A.; Jass, J.; et al. Dermatomyositis Associated with Celiac Disease: Response to a Gluten-Free Diet. Can. J. Gastroenterol. Hepatol. 2006, 20, 574074. [Google Scholar] [CrossRef] [PubMed]
- Marie, I.; Lecomte, F.; Hachulla, E.; Antonietti, M.; Francois, A.; Levesque, H.; Courtois, H. An Uncommon Association: Celiac Disease and Dermatomyositis in Adults. Clin. Exp. Rheumatol. 2001, 19, 201–203. [Google Scholar] [PubMed]
- Iannone, F.; Lapadula, G. Dermatomyositis and Celiac Disease Association: A Further Case. Clin. Exp. Rheumatol. 2001, 19, 757–758. [Google Scholar] [PubMed]
- Buderus, S.; Wagner, N.; Lentze, M. Concurrence of Celiac Disease and Juvenile Dermatomyositis: Result of a Specific Immunogenetic Susceptibility? J. Pediatr. Gastroenterol. Nutr. 1997, 25, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Muddasani, S.; Rusk, A.M.; Baquerizo Nole, K.L. Gluten and Skin Disease beyond Dermatitis Herpetiformis: A Review. Int. J. Dermatol. 2021, 60, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Rubio-Tapia, A.; Chowdhary, V.; Murray, J.A.; Simard, J.F. Increased Risk of Systemic Lupus Erythematosus in 29,000 Patients with Biopsy-Verified Celiac Disease. J. Rheumatol. 2012, 39, 1964–1970. [Google Scholar] [CrossRef]
- Soltani, Z.; Baghdadi, A.; Nejadhosseinian, M.; Faezi, S.T.; Shahbazkhani, B.; Mousavi, S.A.; Kazemi, K. Celiac Disease in Patients with Systemic Lupus Erythematosus. Reumatologia 2021, 59, 85–89. [Google Scholar] [CrossRef]
- Shamseya, A.M.; Elsayed, E.H.; Donia, H.M. Study of Serology and Genetics of Celiac Disease in Patients with Juvenile Systemic Lupus Erythematosus “Celiac in Juvenile Systemic Lupus”. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1322–1327. [Google Scholar] [CrossRef]
- Mašić, M.; Močić Pavić, A.; Gagro, A.; Balažin Vučetić, A.; Ožanić Bulić, S.; Mišak, Z. From Chilblains (Pernio) to Coeliac Disease—Should We Still Consider It Random? Children 2022, 9, 1972. [Google Scholar] [CrossRef] [PubMed]
- Clair, N.E.S.; Choi Kim, C.; Semrin, G.; Woodward, A.L.; Liang, M.G.; Glickman, J.N.; Leichtner, A.M.; Binstadt, B.A. Celiac Disease Presenting with Chilblains in an Adolescent Girl. Pediatr. Dermatol. 2006, 23, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, A.; Sanchez Vivas, N.E.; Powell, J.; Jantchou, P.; Morin, M.-P. Pernio as the Clinical Presentation of Celiac Disease: A Case Report. SAGE Open Med. Case Rep. 2020, 8, 2050313X2094044. [Google Scholar] [CrossRef] [PubMed]
- D’onofrio, F.; Miele, L.; Diaco, M.; Santoro, L.; de Socio, G.; Montalto, M.; Grieco, A.; Gasbarrini, G.; Manna, R. Sjogren’s Syndrome in a Celiac Patient: Searching for Environmental Triggers. Int. J. Immunopathol. Pharmacol. 2006, 19, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Balaban, D.V.; Mihai, A.; Dima, A.; Popp, A.; Jinga, M.; Jurcut, C. Celiac Disease and Sjogren’s Syndrome: A Case Report and Review of Literature. World J. Clin. Cases 2020, 8, 4151–4161. [Google Scholar] [CrossRef]
- Harpreet, S.; Deepak, J.; Kiran, B. Multiple Autoimmune Syndrome with Celiac Disease. Reumatologia 2016, 54, 326–329. [Google Scholar] [CrossRef]
- Iltanen, S.; Collin, P.; Korpela, M.; Holm, K.; Partanen, J.; Polvi, A.; Mäki, M. Celiac Disease and Markers of Celiac Disease Latency in Patients with Primary Sjögren’s Syndrome. Am. J. Gastroenterol. 1999, 94, 1042–1046. [Google Scholar] [CrossRef]
- Bizzaro, N.; Villalta, D.; Tonutti, E.; Doria, A.; Tampoia, M.; Bassetti, D.; Tozzoli, R. IgA and IgG Tissue Transglutaminase Antibody Prevalence and Clinical Significance in Connective Tissue Diseases, Inflammatory Bowel Disease, and Primary Biliary Cirrhosis. Dig. Dis. Sci. 2003, 48, 2360–2365. [Google Scholar] [CrossRef]
- Bartoloni, E.; Bistoni, O.; Alunno, A.; Cavagna, L.; Nalotto, L.; Baldini, C.; Priori, R.; Fischetti, C.; Fredi, M.; Quartuccio, L.; et al. Celiac Disease Prevalence Is Increased in Primary Sjögren’s Syndrome and Diffuse Systemic Sclerosis: Lessons from a Large Multi-Center Study. J. Clin. Med. 2019, 8, 540. [Google Scholar] [CrossRef]
- Caio, G.; de Giorgio, R.; Ursini, F.; Fanaro, S.; Volta, U. Gastroenterology and Hepatology from Bed to Bench. Prevalence of Celiac Disease Serological Markers in a Cohort of Italian Rheumatological Patients. Gastroenterol. Hepatol. Bed Bench 2018, 11, 244. [Google Scholar]
- Bibbò, S.; Pes, G.M.; Usai-Satta, P.; Salis, R.; Soro, S.; Quarta Colosso, B.M.; Dore, M.P. Chronic Autoimmune Disorders Are Increased in Coeliac Disease. Medicine 2017, 96, e8562. [Google Scholar] [CrossRef]
- Ayar, K.; Tunç, R.; Pekel, H.; Esen, H.H.; Küçük, A.; Çifçi, S.; Ataseven, H.; Özdemir, M. Prevalence of Sicca Symptoms and Sjögren’s Syndrome in Coeliac Patients and Healthy Controls. Scand. J. Rheumatol. 2020, 49, 233–238. [Google Scholar] [CrossRef]
- Erbasan, F.; Çoban, D.T.; Karasu, U.; Çekin, Y.; Yeşil, B.; Çekin, A.H.; Süren, D.; Terzioğlu, M.E. Primary Sjögren’s Syndrome in Patients with Celiac Disease. Turk. J. Med. Sci. 2017, 47, 430–434. [Google Scholar] [CrossRef]
- Caglar, E.; Ugurlu, S.; Ozenoglu, A.; Can, G.; Kadioglu, P.; Dobrucali, A. Autoantibody Frequency in Celiac Disease. Clinics 2009, 64, 1195–1200. [Google Scholar] [CrossRef]
- Patinen, P.; Aine, L.; Collin, P.; Hietanen, J.; Korpela, M.; Enckell, G.; Kautiainen, H.; Konttinen, Y.T.; Reunala, T. Oral Findings in Coeliac Disease and Sjögren’s Syndrome. Oral Dis. 2004, 10, 330–334. [Google Scholar] [CrossRef]
- Gómez-Puerta, J.A.; Gil, V.; Cervera, R.; Miquel, R.; Jiménez, S.; Ramos-Casals, M.; Font, J. Coeliac Disease Associated with Systemic Sclerosis. Ann. Rheum. Dis. 2004, 63, 104–105. [Google Scholar] [CrossRef]
- Marguerie, C.; Kaye, S.; Vyse, T.; Mackworth-Young, C.; Walport, M.J.; Black, C. Malabsorption caused by coeliac disease in patients who have scleroderma. Rheumatology 1995, 34, 858–861. [Google Scholar] [CrossRef]
- Forbess, L.J.; Gordon, J.K.; Doobay, K.; Bosworth, B.P.; Lyman, S.; Davids, M.L.; Spiera, R.F. Low Prevalence of Coeliac Disease in Patients with Systemic Sclerosis: A Cross-Sectional Study of a Registry Cohort. Rheumatology 2013, 52, 939–943. [Google Scholar] [CrossRef]
- Luft, L.M.; Barr, S.G.; Martin, L.O.; Chan, E.K.; Fritzler, M.J. Autoantibodies to Tissue Transglutaminase in Sjögren’s Syndrome and Related Rheumatic Diseases. J. Rheumatol. 2003, 30, 2613–2619. [Google Scholar] [PubMed]
- Rosato, E.; de Nitto, D.; Rossi, C.; Libanori, V.; Donato, G.; di Tola, M.; Pisarri, S.; Salsano, F.; Picarelli, A. High Incidence of Celiac Disease in Patients with Systemic Sclerosis. J. Rheumatol. 2009, 36, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Nisihara, R.; Utiyama, S.R.; Azevedo, P.M.; Skare, T.L. Celiac Disease Screening in Patients with Scleroderma. Arq. Gastroenterol. 2011, 48, 163–164. [Google Scholar] [CrossRef][Green Version]
- Conti, V.; Leone, M.C.; Casato, M.; Nicoli, M.; Granata, G.; Carlesimo, M. High Prevalence of Gluten Sensitivity in a Cohort of Patients with Undifferentiated Connective Tissue Disease. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 54–57. [Google Scholar][Green Version]
- Bondavalli, P.; Quadri, G.; Parodi, A.; Rebora, A. Failure of Gluten-Free Diet in Celiac Disease-Associated Alopecia Areata. Acta Derm. Venereol. 1998, 78, 319. [Google Scholar] [CrossRef]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; Destefano, G.M.; Rothman, L.; et al. Alopecia Areata Is Driven by Cytotoxic T Lymphocytes and Is Reversed by JAK Inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef]
- Corazza, G.R.; Andreani, M.L.; Venturo, N.; Bernardi, M.; Tosti, A.; Gasbarrini, G. Celiac Disease and Alopecia Areata: Report of a New Association. Gastroenterology 1995, 109, 1333–1337. [Google Scholar] [CrossRef]
- Volta, U.; Bardazzi, F.; DeFranceschi, L.; Tosti, A.; Molinaro, N.; Ghetti, S.; Tetta, C.; Grassi, A.; Bianchi, F. Serological Screening for Coeliac Disease in Vitiligo and Alopecia Areata. Br. J. Dermatol. 1997, 136, 801–802. [Google Scholar] [CrossRef]
- Cooper, B.; Holmes, G.; Cooke, W. Coeliac Disease and Immunological Disorders. Br. Med. J. 1978, 1, 537–539. [Google Scholar] [CrossRef]
- Zammit-Maempel, I.; Adamson’ And, A.R.; Halsey, J.P. Sclerodactyly Complicating Coeliac Disease. Rheumatology 1986, 25, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Confino-Cohen, R.; Chodick, G.; Shalev, V.; Leshno, M.; Kimhi, O.; Goldberg, A. Chronic Urticaria and Autoimmunity: Associations Found in a Large Population Study. J. Allergy Clin. Immunol. 2012, 129, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Borzova, E.; Grattan, C.; Asero, R.; Pogorelov, D.; Maurer, M. Autoimmune Comorbidity in Chronic Spontaneous Urticaria: A Systematic Review. Autoimmun. Rev. 2017, 16, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Paravati, F.; El Hachem, M.; Duse, M.; Bergamini, M.; Simeone, G.; Barbagallo, M.; Bernardini, R.; Bottau, P.; Bugliaro, F.; et al. Management of Chronic Urticaria in Children: A Clinical Guideline. Ital. J. Pediatr. 2019, 45, 101. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-R.; Carlson, J.A. Clinical Approach to Cutaneous Vasculitis. Am. J. Clin. Dermatol. 2008, 9, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, L.; Beteta-Gorriti, V.; Alvarez, N.; de Castro, C.G.; de Dios, A.; Palacios, L.; Santos-Juanes, J. Cutaneous and Mucosal Manifestations Associated with Celiac Disease. Nutrients 2018, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.; Dikman, S.; Spiera, H.; Schultz, N.; Janowitz, H.D. Cutaneous Vasculitis Complicating Coeliac Disease. Gut 1981, 22, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Holdstock, D.; Oleesky, S. Vasculitis in Coeliac Diseases. Br. Med. J. 1970, 4, 369. [Google Scholar] [CrossRef]
- Mishra, P.; Sirka, C.S.; Das, R.R.; Nanda, D. Secondary Acrodermatitis Enteropathica-like Lesions in a Child with Newly Diagnosed Coeliac Disease. Paediatr. Int. Child Health 2016, 36, 72–75. [Google Scholar] [CrossRef]
- Fasano, A. Celiac Disease-How to Handle a Clinical Chameleon. N. Engl. J. Med. 2003, 25, 2568–2570. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Aziz, I.; Peerally, M.F.; Barnes, J.H.; Kandasamy, V.; Whiteley, J.C.; Partridge, D.; Vergani, P.; Cross, S.S.; Green, P.H.; Sanders, D.S. The Clinical and Phenotypical Assessment of Seronegative Villous Atrophy; A Prospective UK Centre Experience Evaluating 200 Adult Cases over a 15-Year Period (2000–2015). Gut 2017, 66, 1563–1572. [Google Scholar] [CrossRef]
- Schiepatti, A.; Maimaris, S.; Raju, S.A.; Green, O.L.; Mantica, G.; Therrien, A.; Flores-Marin, D.; Linden, J.; Fernández-Bañares, F.; Esteve, M.; et al. Persistent Villous Atrophy Predicts Development of Complications and Mortality in Adult Patients with Coeliac Disease: A Multicentre Longitudinal Cohort Study and Development of a Score to Identify High-Risk Patients. Gut 2023, 72, 2095–2102. [Google Scholar] [CrossRef]
- Crocco, M.; Calvi, A.; Canzoneri, F.; Malerba, F.; Zampatti, N.; Chiaro, A.; Arrigo, S.; Gandullia, P.; Proietti, S.; Bonassi, S. The Influence of SARS-CoV-2 Pandemic on the Diagnosis of Celiac Disease and Clinical Practice in Pediatric Gastroenterology. Nutrients 2023, 15, 559. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Aronico, N.; Bianchi, P.I.; D’Agate, C.C.; Neri, M.; Volta, U.; Mumolo, M.G.; Astegiano, M.; Calabrò, A.S.; Zingone, F.; et al. Diagnostic Delay in Adult Coeliac Disease: An Italian Multicentre Study. Dig. Liver Dis. 2023, 55, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fasano, A. Celiac Disease Diagnosis: Simple Rules Are Better than Complicated Algorithms. Am. J. Med. 2010, 123, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.A.; Diamond, B.; Rotterdam, H.; Green, P.H.R. Seronegative Celiac Disease: Increased Prevalence with Lesser Degrees of Villous Atrophy. Dig. Dis. Sci. 2004, 49, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.; Gigliobianco, A.; Lombardi, D.; Gasbarrini, G. Low Prevalence of Antigliadin and Anti-Endomysium Antibodies in Subclinical/Silent Celiac Disease. Am. J. Gastroenterol. 2001, 96, 1507–1510. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.M. Prevalence of Antitissue Transglutaminase Antibodies in Different Degrees of Intestinal Damage in Celiac Disease. J. Clin. Gastroenterol. 2003, 36, 219–221. [Google Scholar] [CrossRef]
- Hoerter, N.A.; Shannahan, S.E.; Suarez, J.; Lewis, S.K.; Green, P.H.R.; Leffler, D.A.; Lebwohl, B. Diagnostic Yield of Isolated Deamidated Gliadin Peptide Antibody Elevation for Celiac Disease. Dig. Dis. Sci. 2017, 62, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Valitutti, F.; Leonard, M.M.; Kenyon, V.; Montuori, M.; Piemontese, P.; Francavilla, R.; Malamisura, B.; Norsa, L.; Calvi, A.; Lionetti, M.E.; et al. Early Antibody Dynamics in a Prospective Cohort of Children at Risk of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 574–577. [Google Scholar] [CrossRef]
- Vekić-Mužević, M.; Tomić, L.; Pondeljak, N.; Lazić Mosler, E. Positivity of Celiac Disease-Specific Antibodies and Non-Celiac Hypersensitivity in Psoriasis. Acta Dermatovenerol. Alp. Pannonica Adriat. 2023, 32, 87–92. [Google Scholar] [CrossRef]
- Malamut, G.; Meresse, B.; Kaltenbach, S.; Derrieux, C.; Verkarre, V.; Macintyre, E.; Ruskone-Fourmestraux, A.; Fabiani, B.; Radford-Weiss, I.; Brousse, N.; et al. Small Intestinal CD4+ T-Cell Lymphoma Is a Heterogenous Entity with Common Pathology Features. Clin. Gastroenterol. Hepatol. 2014, 12, 599–608.e1. [Google Scholar] [CrossRef]
- Malamut, G.; Verkarre, V.; Suarez, F.; Viallard, J.-F.; Lascaux, A.-S.; Cosnes, J.; Bouhnik, Y.; Lambotte, O.; Béchade, D.; Ziol, M.; et al. The Enteropathy Associated with Common Variable Immunodeficiency: The Delineated Frontiers with Celiac Disease. Am. J. Gastroenterol. 2010, 105, 2262–2275. [Google Scholar] [CrossRef] [PubMed]
- DeGaetani, M.; Tennyson, C.A.; Lebwohl, B.; Lewis, S.K.; Abu Daya, H.; Arguelles-Grande, C.; Bhagat, G.; Green, P.H.R. Villous Atrophy and Negative Celiac Serology: A Diagnostic and Therapeutic Dilemma. Am. J. Gastroenterol. 2013, 108, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Cincotta, M.; Biagi, F.; Sanders, D.S. Enteropathies with Villous Atrophy but Negative Coeliac Serology in Adults: Current Issues. BMJ Open Gastroenterol. 2021, 8, e000630. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Sanders, D.S.; Zuffada, M.; Luinetti, O.; Iraqi, A.; Biagi, F. Overview in the Clinical Management of Patients with Seronegative Villous Atrophy. Eur. J. Gastroenterol. Hepatol. 2019, 31, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Iacono, G.; Di Prima, L.; Pirrone, G.; Cavataio, F.; Ambrosiano, G.; Sciumè, C.; Geraci, G.; Florena, A.; Teresi, S.; et al. Antiendomysium Antibodies Assay in the Culture Medium of Intestinal Mucosa: An Accurate Method for Celiac Disease Diagnosis. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Salmi, T.T.; Collin, P.; Korponay-Szabó, I.R.; Laurila, K.; Partanen, J.; Huhtala, H.; Király, R.; Lorand, L.; Reunala, T.; Mäki, M.; et al. Endomysial Antibody-Negative Coeliac Disease: Clinical Characteristics and Intestinal Autoantibody Deposits. Gut 2006, 55, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Trovato, C.M.; Oliva, S.; Pietropaoli, N.; Pignataro, M.G.; Berni, S.; Tancredi, A.; Cucchiara, S.; Giordano, C.; Montuori, M. A New Double Immunohistochemistry Method to Detect Mucosal Anti-Transglutaminase IgA Deposits in Coeliac Children. Dig. Liver Dis. 2022, 54, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, M.; Maglio, M.; Korponay-Szabó, I.R.; Vass, V.; Mearin, M.L.; Meijer, C.; Niv-Drori, H.; Ribes-Koninckx, C.; Roca, M.; Shamir, R.; et al. Intestinal Anti-Transglutaminase 2 Immunoglobulin A Deposits in Children at Risk for Coeliac Disease (CD): Data from the PreventCD Study. Clin. Exp. Immunol. 2018, 191, 311–317. [Google Scholar] [CrossRef]
- Rahmanipour, E.; Ghorbani, M.; Ganji, A.; Mirzaei, Z.; Ghavami, V.; Shahbazkhani, B.; Attarian, F.; Amiri, M. Clinical Profile of Patients with Seronegative Celiac Disease. Gastroenterol. Hepatol. Bed Bench 2023, 16, 203–209. [Google Scholar]
- Rose, C.; Armbruster, F.P.; Ruppert, J.; Igl, B.-W.; Zillikens, D.; Shimanovich, I. Autoantibodies against Epidermal Transglutaminase Are a Sensitive Diagnostic Marker in Patients with Dermatitis Herpetiformis on a Normal or Gluten-Free Diet. J. Am. Acad. Dermatol. 2009, 61, 39–43. [Google Scholar] [CrossRef]
- Chorzelski, T.P.; Sulej, J.; Tchorzewska, H.; Jablonska, S.; Beutner, E.H.; Kumar, V. IgA Class Endomysium Antibodies in Dermatitis Herpetiformis and Coeliac Disease. Ann. N. Y. Acad. Sci. 1983, 420, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Silvester, J.A.; Kurada, S.; Szwajcer, A.; Kelly, C.P.; Leffler, D.A.; Duerksen, D.R. Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients with Celiac Disease and Persistent Villous Atrophy on Gluten-Free Diets: A Meta-Analysis. Gastroenterology 2017, 153, 689–701.e1. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Merone, M.; Manfredi, M.; Grossi, V.; Landini, A.; Alessio, M.G.; Previtali, G.; Trevisan, M.T.; Porcelli, B.; Fabris, M.; et al. Positive Tissue Transglutaminase Antibodies with Negative Endomysial Antibodies: Unresolved Issues in Diagnosing Celiac Disease. J. Immunol. Methods 2021, 489, 112910. [Google Scholar] [CrossRef] [PubMed]
- Čelakovská, J.; Michaela, K.; Petra, C.; Martin, N.; Eva, Č.; Josef, B.; Ctirad, A.; Miloslav, S. Dermatitis Herpetiformis Duhring—Evaluation of Disease Severity and Tissue Transglutaminase Levels in 122 Patients on Dapsone Therapy. Food Agric. Immunol. 2021, 32, 237–252. [Google Scholar] [CrossRef]
- Reunala, T.; Chorzelski, T.P.; Viander, M.; Sulej, J.; Vainio, E.; Kumar, V.; Beutner, E.H. IgA Anti-Endomysial Antibodies in Dermatitis Herpetiformis: Correlation with Jejunal Morphology, Gluten-Free Diet and Anti-Gliadin Antibodies. Br. J. Dermatol. 1987, 117, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Mir, F.; Bechtold, M.L.; Holly, J.S.L.; Hammad, H. Celiac Disease Presenting with Acquired Acrodermatitis Enteropathica. Am. J. Gastroenterol. 2015, 110, 1396. [Google Scholar] [CrossRef]
- Lebwohl, B.; Söderling, J.; Roelstraete, B.; Lebwohl, M.G.; Green, P.H.R.; Ludvigsson, J.F. Risk of Skin Disorders in Patients with Celiac Disease: A Population-Based Cohort Study. J. Am. Acad. Dermatol. 2021, 85, 1456–1464. [Google Scholar] [CrossRef]
- Doe, W.F.; Evans, D.; Hobbs, J.R.; Booth, C.C. Coeliac disease, vasculitis, and cryoglobulinaemia. Gut 1972, 13, 112–123. [Google Scholar] [CrossRef]
- Rensch, M.J.; Szyjkowski, R.; Shaffer, R.T.; Fink, S.; Kopecky, C.; Grissmer, L.; Enzenhauer, R.; Kadakia, S. The prevalence of celiac disease autoantibodies in patients with systemic lupus erythematosus. Am. J. Gastroenterol. 2001, 96, 1113–1135. [Google Scholar] [CrossRef]
- Szodoray, P.; Barta, Z.; Lakos, G.; Szakáll, S.; Zeher, M. Coeliac disease in Sjögren’s syndrome—A study of 111 Hungarian patients. Rheumatol. Int. 2004, 24, 278–282. [Google Scholar] [CrossRef]
- Gabrielli, M.; Candelli, M.; Cremonini, F.; Ojetti, V.; Santarelli, L.; Nista, E.C.; Nucera, E.; Schiavino, D.; Patriarca, G.; Gasbarrini, G.; et al. Idiopathic chronic urticaria and celiac disease. Dig. Dis. Sci. 2005, 50, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, M.; Erdem, T.; Ertekin, V.; Selimoğlu, M.A. The mucocutaneous manifestations associated with celiac disease in childhood and adolescence. Pediatr. Dermatol. 2007, 24, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Adult celiac disease followed by onset of systemic lupus erythematosus. J. Clin. Gastroenterol. 2008, 42, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Jarrick, S.; Murray, J.A.; Emilsson, L. Celiac Disease and Risk of Henoch-Schonlein Purpura: Population-based Cohort Study. J. Clin. Gastroenterol. 2018, 52, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Soylu, A.; Öztürk, Y.; Doğan, Y.; Özmen, D.; Yılmaz, Ö.; Kuyum, P.; Kavukçu, S. Screening of celiac disease in children with Henoch-Schoenlein purpura. Rheumatol. Int. 2016, 36, 713–717. [Google Scholar] [CrossRef]
- Şahin, Y.; Şahin, S.; Adrovic, A.; Kutlu, T.; Çokuğras, F.Ç.; Barut, K.; Erkan, T.; Kasapçopur, Ö. Serological screening for celiac disease in children with systemic lupus erythematosus. Eur. J. Rheumatol. 2019, 6, 142–145. [Google Scholar] [CrossRef]
- Kosmeri, C.; Siomou, E.; Challa, A.; Tsabouri, S. Investigation of Autoimmune Disease in Children with Chronic Spontaneous Urticaria. Int. Arch. Allergy Immunol. 2019, 180, 250–254. [Google Scholar] [CrossRef]
- AlEnzi, F.; Yateem, M.; Shaikh, M.; AlSohaibani, F.; Alhaymouni, B.; Ahmed, A.; Al-Mayouf, S.M. The Value of Screening for Celiac Disease in Systemic Lupus Erythematosus: A Single Experience of a Tertiary Medical Center. Rheumatol. Ther. 2020, 7, 649–656. [Google Scholar] [CrossRef]
- Meijer-Boekel, C.; van den Akker, M.E.; van Bodegom, L.; Escher, J.; van Geloven, N.; van Overveld, F.; Rings, E.H.H.M.; Smit, L.; de Vries, M.C.; Mearin, M.L. Early Diagnosis of Coeliac Disease in the Preventive Youth Health Care Centres in the Netherlands: Study Protocol of a Case Finding Study (GLUTENSCREEN). BMJ Paediatr. Open 2021, 5, e001152. [Google Scholar] [CrossRef]
- Raiteri, A.; Granito, A.; Giamperoli, A.; Catenaro, T.; Negrini, G.; Tovoli, F. Current Guidelines for the Management of Celiac Disease: A Systematic Review with Comparative Analysis. World J. Gastroenterol. 2022, 28, 154–175. [Google Scholar] [CrossRef]
- Fröhlich-Reiterer, E.; Elbarbary, N.S.; Simmons, K.; Buckingham, B.; Humayun, K.N.; Johannsen, J.; Holl, R.W.; Betz, S.; Mahmud, F.H. ISPAD Clinical Practice Consensus Guidelines 2022: Other Complications and Associated Conditions in Children and Adolescents with Type 1 Diabetes. Pediatr. Diabetes 2022, 23, 1451–1467. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.B.; Losowsky, M.S. Patchiness and Duodenal-Jejunal Variation of the Mucosal Abnormality in Coeliac Disease and Dermatitis Herpetiformis. Gut 1976, 17, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Bonamico, M.; Thanasi, E.; Mariani, P.; Nenna, R.; Luparia, R.P.L.; Barbera, C.; Morra, I.; Lerro, P.; Guariso, G.; De Giacomo, C.; et al. Duodenal Bulb Biopsies in Celiac Disease: A Multicenter Study. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Brocchi, E.; Tomassetti, P.; Volta, U.; Piscitelli, L.; Bonora, M.; Campana, D.; Corinaldesi, R. Adult Coeliac Disease Diagnosed by Endoscopic Biopsies in the Duodenal Bulb. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1413–1415. [Google Scholar] [CrossRef]
- Mooney, P.D.; Kurien, M.; Evans, K.E.; Rosario, E.; Cross, S.S.; Vergani, P.; Hadjivassiliou, M.; Murray, J.A.; Sanders, D.S. Clinical and Immunologic Features of Ultra-Short Celiac Disease. Gastroenterology 2016, 150, 1125–1134. [Google Scholar] [CrossRef]
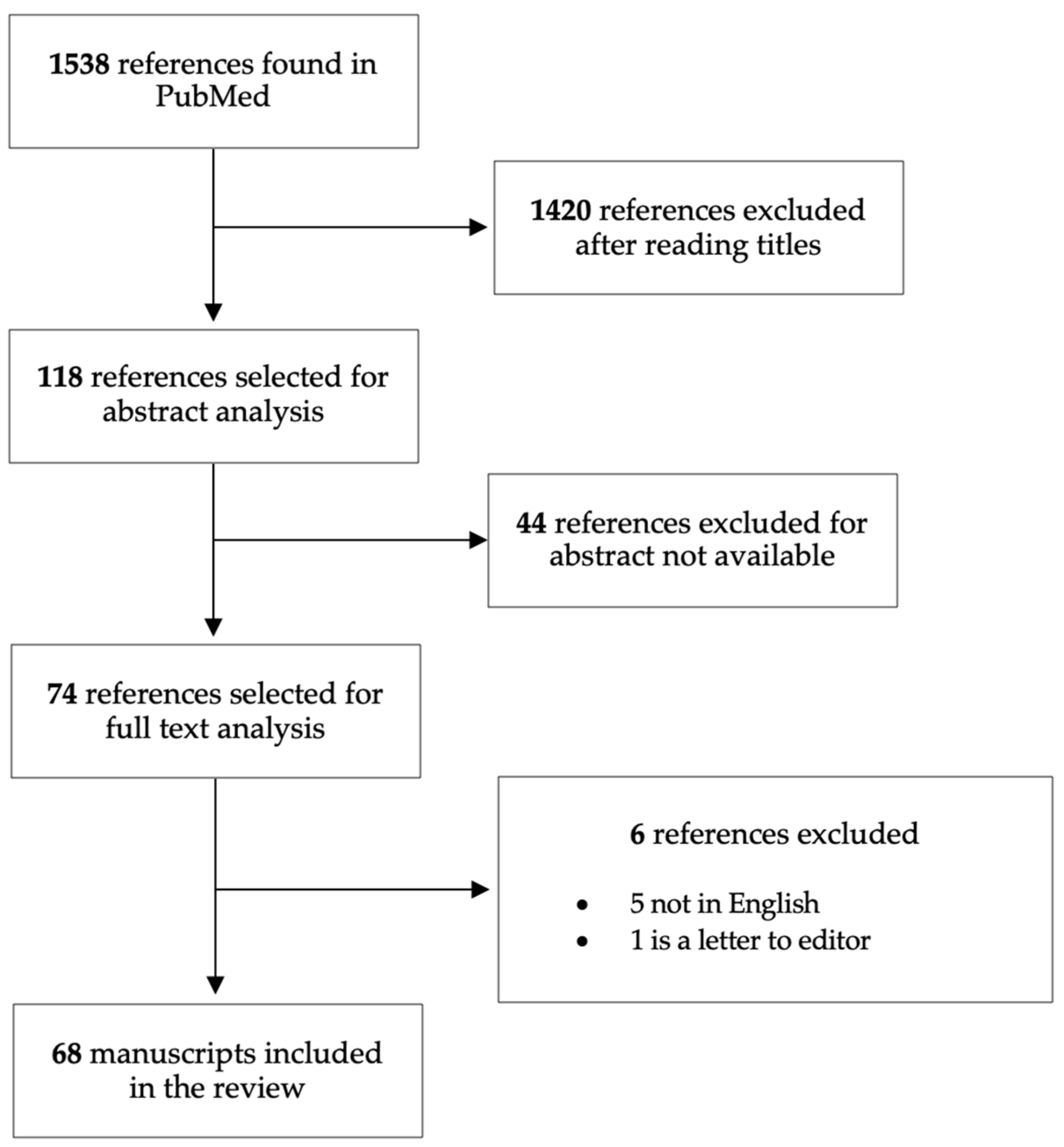
| Authors | Year | Population | Primary Objective | Results |
|---|---|---|---|---|
| Doe WF et al. [98] | 1972 | n = 4 adult patients (M 42 y, F 31 y; F 67 y; and M 50 y) | To evaluate four cases of CD, vasculitis, and mixed cryoglobulinemia | |
| Marguerie C et al. [47] | 1995 | n = 2 (a 35-year-old Caucasian woman and a 40-year-old man) | To describe two cases of concomitant scleroderma and CD | |
| Iltanen S et al. [37] | 1999 | n = 34 (pSS): median age 55 y, range 26–70; F 82% n= 28 (healthy controls): median age 53 years, range 25–81; F 43% | To evaluate the prevalence of CD in patients with pSS | Prevalence of CD was 14,7% in the pSS patients |
| Rensch MJ et al. [99] | 2001 | n = 103 (SLE) | To evaluate the prevalence of CD autoantibodies in SLE patients | 23.3% of the SLE patients tested positive for AGA, whereas none tested positive for EMA nor had histological evidence of CD |
| Luft L et al. [49] | 2003 | n = 50 (SS) n = 50 (SLE) n = 30 (RA) n = 30 (SSc) n = 50 (healthy controls) | To evaluate the prevalence of anti-tTG in a cohort of SS patients and other systemic rheumatic diseases | Prevalence of anti-tTG: SS 12% vs. heathy controls 4% vs. SLE 6% vs. SSc 7% vs. RA 2%. A total of 5/6 SS patients with anti-tTG had symptoms, signs, or small bowel biopsy findings consistent with a diagnosis of CD |
| Szodoray P et al. [100] | 2004 | n = 111 (SS) | To evaluate the prevalence of CD in patients with SS | Prevalence of CD: SS patients 4.5% vs. 0.45–0.55% in non-SS population |
| Patinen P et al. [45] | 2004 | n = 20 (CD and SS): age mean 61 years, range 48–78; F 95% | To evaluate the prevalence of oral mucosal and dental abnormalities in patients with SS and CD | Prevalence of oral mucosal abnormalities: SS 80% vs. CD + SS 65% vs. CD 40%. The CD + SS patients had higher salivary flow rates and lower inflammatory focus scores in the salivary glands than the SS patients |
| Gabrielli M et al. [101] | 2005 | n = 80 (CU): age mean 48 ± 18 y; F 72.5% n = 264 (healthy controls): age mean 45 ± 16 y; F 72.7% | To evaluate the prevalence of CD in a population of adult patients with CU and healthy controls | No differences in CD prevalence in the two groups (1.25% in the CU group vs. 0.38% in the healthy controls) |
| Caminiti L et al. [15] | 2005 | n = 79 (CU): median age 7 y, range 2–18; F 51.9% | To evaluate the prevalence of CD in children with urticaria | CD prevalence: CU 5.0% vs. healthy controls 0.67%; (p = 0.0003) |
| Seyhan M et al. [102] | 2007 | n = 55 children and adolescents (mean age 10 ± 4.6 years; F 58.2%) | To evaluate the prevalence of mucocutaneous manifestations of CD in childhood and adolescence | The most prevalent dermatologic diagnosis was xerosis (69.1%) |
| Freeman HJ et al. [103] | 2008 | n = 246 (biopsy-defined CD) | To evaluate the prevalence of SLE in CD patients | SLE occurrence in the CD patients was higher than in the general population (2.4% biopsy-proven CD) |
| Rosato E et al. [50] | 2009 | n = 50 (SSc): mean age 51 ± 14.5 y; F 86% | To evaluate the incidence of CD in patients with SSc | The incidence of CD in the patients with SSc was 8% |
| Nisihara R et al. [51] | 2011 | n = 105 SSc Brazilian patients (mean age 43.2 years; F 92.3%) | To evaluate the prevalence between CD and SSc | No association between CD and EMA positivity |
| Ludvigsson JF et al. [28] | 2012 | n = 29,048 (biopsy-verified CD): median age 30 y, range 0–95; F 61.9% n = 144,352 (healthy controls) | To evaluate the prevalence of SLE in patients with CD | The CD patients were at a 3-fold increased risk of SLE compared to the general population (HR 3.49%; 2.48–4.90) |
| Forbess LJ et al. [48] | 2013 | n = 72 (SSc): mean age 51 ± 13 y; F 88% | To evaluate the prevalence of CD in SSc patients | The prevalence of CD in the SSc patients was 4% |
| Ludvigsson JF et al. [16] | 2013 | n = 28,900 (biopsy-verified CD): median age 30 years, range 0–95; F (61.8%) n = 143,397 (healthy controls): median age 30 years, range 0–95; F 61.9% | To evaluate the risk of urticaria in CD patients | The patients with CD were at an increased risk of later urticaria (HR = 1.51; 95% CI = 1.36–1.68 based on 453 observed events vs. 300 expected in CD patients without a diagnosis of earlier urticaria) The patients with CD were at an almost two-fold increased risk of chronic urticaria (HR = 1.92; 95% CI = 1.48–2.48, based on 79 observed events vs. 41 expected) |
| Conti V et al. [52] | 2015 | n = 52 (UCT): median age 44 years, range 21–69; F 98% | To evaluate the prevalence of CD in UCTD | Prevalence of CD in UCTD was 11.5% |
| Ludvigsson JF et al. [104] | 2018 | n = 29,096 (CD) n = 144,522 (healthy controls) (F 62% in both groups) | To evaluate the prevalence of HSP in CD patients | No association between CD and HSP (0.06% in the CD patients vs. 0.07% in the controls; HR 0.96) |
| Soylu A et al. [105] | 2016 | n = 42 (children with HSP): mean age 126 ± 49 months; F 40% | To evaluate CD prevalence in children with HSP | CD seropositivity prevalence: HSP 12% vs. Turkish school children 2.5% (p < 0.001) |
| Erbasan F et al. [43] | 2017 | n = 82 (CD): age mean 40.5 y, range 20–62; F 73% | To evaluate the prevalence of SS in CD patients | The prevalence of dry-eye symptoms was 29.3%, but nine patients (11%) were using systemic medications that could contribute to their dry-eye symptoms |
| Caio G et al. [40] | 2018 | n = 230 (67 RA, 52 SS, 42 SSc, 35 SLE, 15 MCTD, 11 PM, and 10 DM): age range 18–84 y; F (81.7%) | To evaluate the prevalence of CD seropositivity in a cohort of patients referred to an Italian rheumatological clinic | CD (antibodies) prevalence: rheumatological patients: 3% (in SS 5.8%; in SSc 8%; in RA 1.5%; and in SLE 2.8%) |
| Bartoloni E et al. [39] | 2019 | n = 580 (SLE): age mean 46 ± 13 y; age range 19–83; F 89% n = 354 (pSS): age mean 55 ± 12 y; age range 21–90; F 97% n = 524 (SSc): age mean 61 ± 14 y; age range 15–87; F 90% n = 14,298 (healthy controls): age mean 53 ± 22 y; age range 15–90; F 91% | To evaluate CD prevalence in SLE, pSS, and SSc | CD prevalence: pSS 6.8% vs. controls 0.6%, p < 0.0001); SLE (1.4%, p = 0.058) and SSc (1.3%, p = 0.096) |
| Sahin Y et al. [106] | 2019 | n = 50 (JSLE) (age mean 15.5 ± 3.4 y; F 88%) | To evaluate the prevalence of CD in children with SLE | No CD in children with SLE |
| Kosmeri C et al. [107] | 2019 | n = 49 (children with CU): median age 8.8 years, range 1–15; F 46.9% | To evaluate the association between autoimmune diseases and CU in children | The prevalence of CD biopsy-confirmed in CU was 2%. The prevalence of high serum levels of anti-thyroid antibodies but normal thyroid function was 8.1%. No other specific autoantibodies were detected |
| Shamseya AM et al. [30] | 2020 | n = 100 (JSLE): age mean 34.6 ± 9.6 y; age at diagnosis 11.9 ± 3.4 y; F 90% n = 40 (healthy controls): age mean 35.5 ± 9.3 y; F 87.5% | To evaluate the prevalence of CD in patients with JSLE | Positive serology in 10% of the SLE patients (vs. 0% in the controls), biopsy-confirmed CD in 6% |
| AlEnzi F et al. [108] | 2020 | n = 81 (SL): age mean 34.1 ± 11.5 y; F 94% n = 34 (JSLE): age mean 10.3 ± 2.7 y; F 82% n = 62 (RA): age mean 48.8 ± 10 y; F 94% n = 73 (JIA): age mean 10.0 ± 2.6 y; F 62% | To evaluate the prevalence of CD in adults and children with SLE and compare them with RA and JIA | The prevalence of serologic CD positivity was higher (but not statistically significant, p = 0.27) among the SLE patients (13.1% vs. 10.4% among the RA and JIA patients), but nobody had biopsy-proven CD |
| Ayar K et al. [42] | 2020 | n = 80 (CD): age mean 40.5 ± 13.5 y n = 100 (healthy controls): age mean 39.7 ± 12.5 y | To evaluate the prevalence of sicca symptoms and SS in CD patients | Prevalence of ocular symptoms: CD 22% vs. healthy controls 13%, (p = 0.113); oral symptoms: CD 26% vs. healthy controls 10%, (p = 0.005); SS CD: 2.8% according to ACR criteria and 5.0% according to AECG vs. healthy controls 3.0% and 2.0% |
| Lebwohl B et al. [97] | 2021 | n = 43,300 (CD): age mean 31.3 ± 25.2 y; F 62.4% n = 198532 (healthy controls): mean age 30.6 ± 25 years; F 62.3% | To evaluate the risk of skin disorders in CD patients | The CD patients had an increased risk of multiple common skin disorders (HR 1.55). Higher risk in the men and in the patients aged 18–40 years; strongest association after DH was vitiligo (OR 2.20), followed by alopecia areata (OR 2.07), psoriasis (OR 1.86), eczema (OR 1.59), and urticaria (OR 1.53) |
| Soltani Z et al. [29] | 2021 | n = 130 (SLE): age mean 31.5 ± 8.3 y; F 81.5% | To evaluate the prevalence of CD in patients with SLE | Prevalence of 3% for biopsy-proven CD in the patients with SLE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ancona, S.; Bianchin, S.; Zampatti, N.; Nosratian, V.; Bigatti, C.; Ferro, J.; Trambaiolo Antonelli, C.; Viglizzo, G.; Gandullia, P.; Malerba, F.; et al. Cutaneous Disorders Masking Celiac Disease: Case Report and Mini Review with Proposal for a Practical Clinical Approach. Nutrients 2024, 16, 83. https://doi.org/10.3390/nu16010083
Ancona S, Bianchin S, Zampatti N, Nosratian V, Bigatti C, Ferro J, Trambaiolo Antonelli C, Viglizzo G, Gandullia P, Malerba F, et al. Cutaneous Disorders Masking Celiac Disease: Case Report and Mini Review with Proposal for a Practical Clinical Approach. Nutrients. 2024; 16(1):83. https://doi.org/10.3390/nu16010083
Chicago/Turabian StyleAncona, Silvana, Silvia Bianchin, Noemi Zampatti, Valentina Nosratian, Carolina Bigatti, Jacopo Ferro, Chiara Trambaiolo Antonelli, Gianmaria Viglizzo, Paolo Gandullia, Federica Malerba, and et al. 2024. "Cutaneous Disorders Masking Celiac Disease: Case Report and Mini Review with Proposal for a Practical Clinical Approach" Nutrients 16, no. 1: 83. https://doi.org/10.3390/nu16010083
APA StyleAncona, S., Bianchin, S., Zampatti, N., Nosratian, V., Bigatti, C., Ferro, J., Trambaiolo Antonelli, C., Viglizzo, G., Gandullia, P., Malerba, F., & Crocco, M. (2024). Cutaneous Disorders Masking Celiac Disease: Case Report and Mini Review with Proposal for a Practical Clinical Approach. Nutrients, 16(1), 83. https://doi.org/10.3390/nu16010083





