Effect on Body Composition of a Meal-Replacement Progression Diet in Patients 1 Month after Bariatric Surgery
Abstract
:1. Introduction
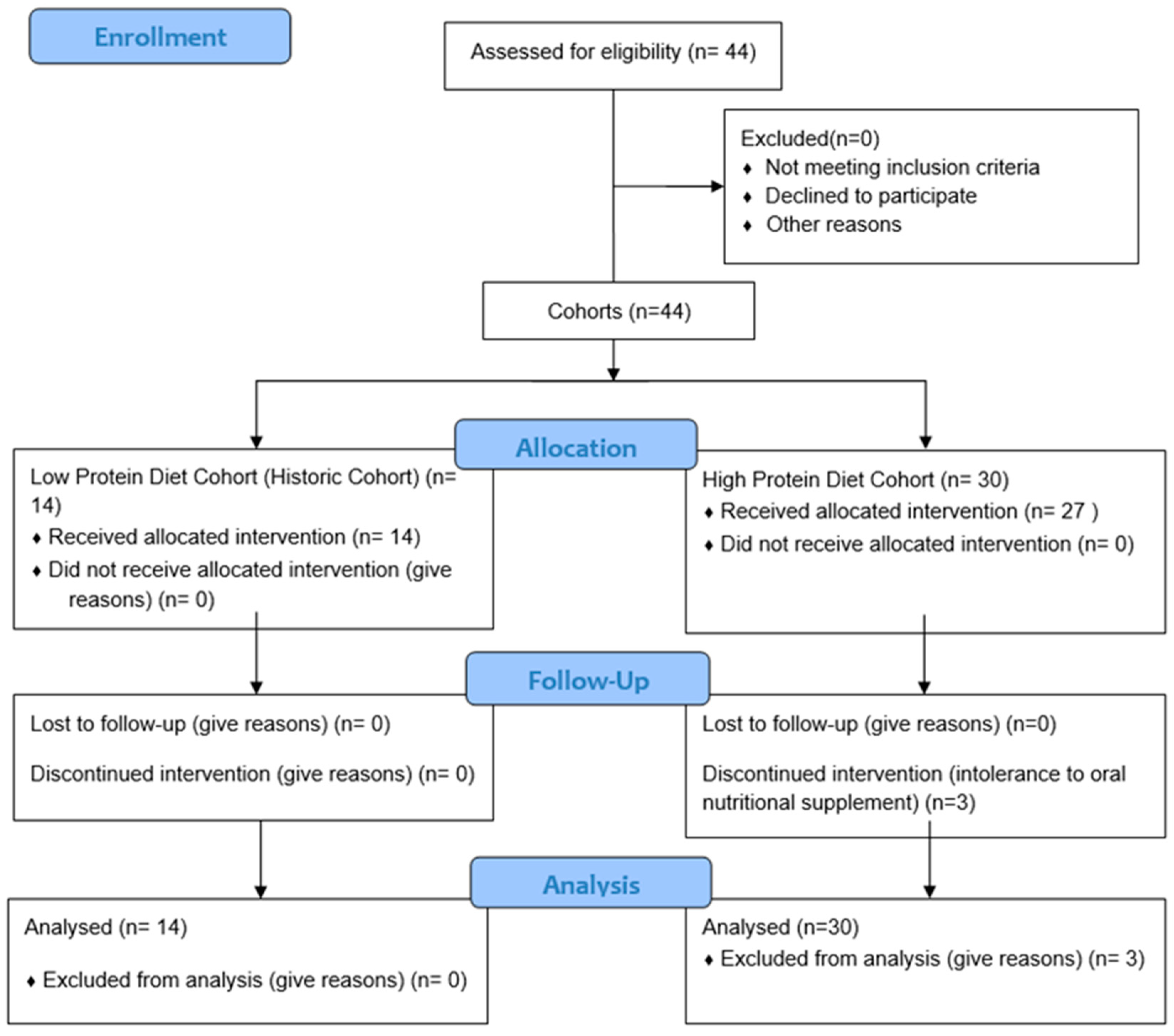
2. Materials and Methods
2.1. Study Design
2.2. Study Subject
2.3. Nutritional Intervention
2.4. Variables
2.4.1. Anthropometry
2.4.2. Body Composition
2.5. Statistical Analysis
3. Results
3.1. Sample Description
3.2. Differences in Anthropometric Parameters
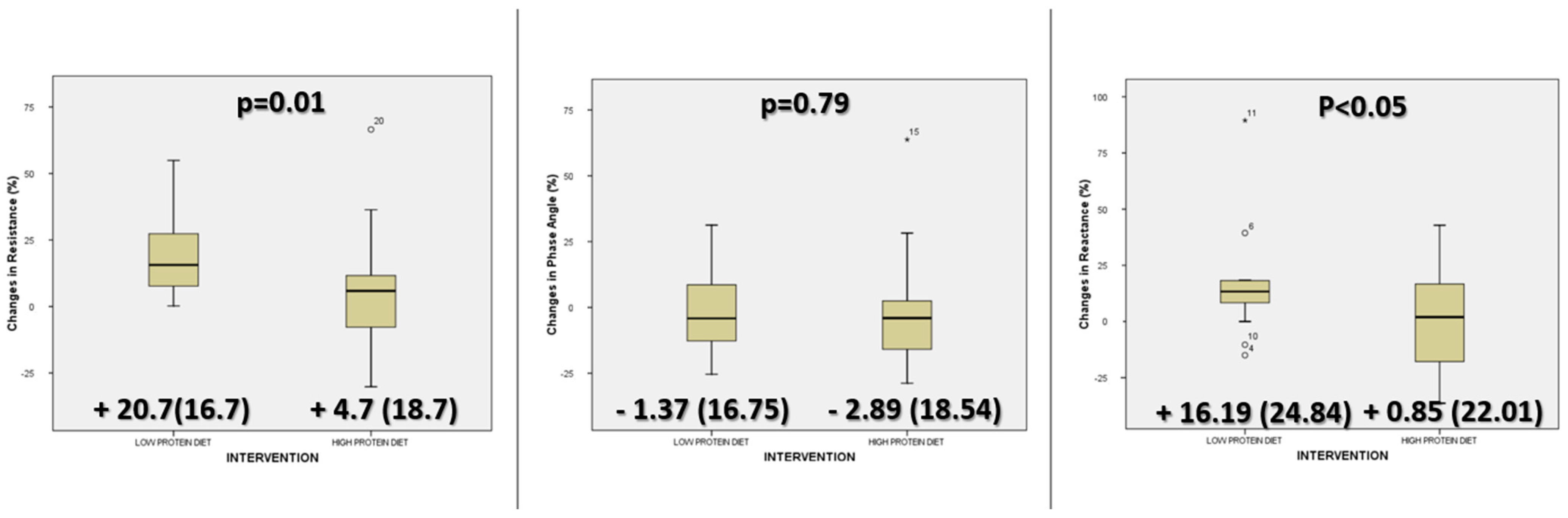
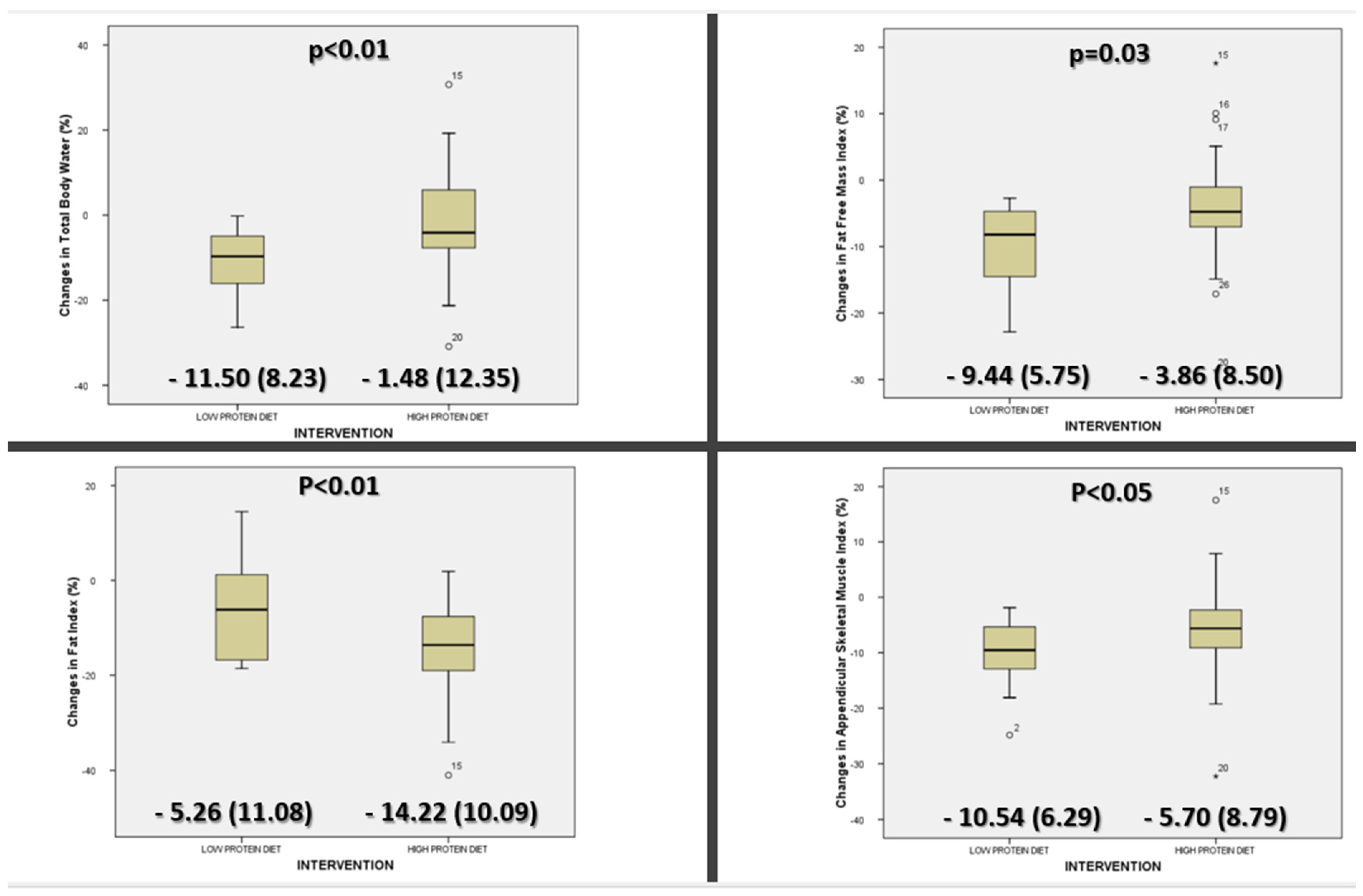
3.3. Differences in Bioimpedance Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Wang, H.; Wang, L.; Cheng, Y.; Xia, Z.; Liao, Y.; Cao, J. Efficacy of orlistat in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed. Rep. 2018, 9, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am. J. Clin. Nutr. 1992, 55, 615S–619S. [CrossRef] [PubMed]
- Pampillón, N.; Reynoso, C.; Solimano, M.; Sánchez, M.; Aguirre, M.; De Rosa, P.; Iturralde, C.; Coqueugniot, M.; Gómez, J.; Pagano, C.; et al. Update of Argentine Nutrition consensus on bariatric surgery. Actual. Nutr. 2016, 17, 19–32. [Google Scholar]
- Bavaresco, M.; Paganini, S.; Lima, T.P.; Salgado, W.; Ceneviva, R.; Dos Santos, J.E.; Nonino-Borges, C.B. Nutritional course of patients submitted to bariatric surgery. Obes. Surg. 2010, 20, 716–721. [Google Scholar] [CrossRef] [PubMed]
- McGrice, M.A.; Porter, J.A. What are gastric banding patients eating one year post-surgery? Obes. Surg. 2012, 22, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Brolin, R.E.; Robertson, L.B.; Kenler, H.A.; Cody, R.P. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann. Surg. 1994, 220, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Kruseman, M.; Leimgruber, A.; Zumbach, F.; Golay, A. Dietary, weight, and psychological changes among patients with obesity, 8 years after gastric bypass. J. Am. Diet. Assoc. 2010, 110, 527–534. [Google Scholar] [CrossRef]
- Freeman, R.A.; Overs, S.E.; Zarshenas, N.; Walton, K.L.; Jorgensen, J.O. Food tolerance and diet quality following adjustable gastric banding, sleeve gastrectomy and Roux-en-Y gastric bypass. Obes. Res. Clin. Pract. 2014, 8, e115–e200. [Google Scholar] [CrossRef]
- Gesquiere, I.; Foulon, V.; Augustijns, P.; Gils, A.; Lannoo, M.; Van der Schueren, B.; Matthys, C. Micronutrient intake, from diet and supplements, and association with status markers in pre- and post-RYGB patients. Clin. Nutr. Edinb. Scotl. 2017, 36, 1175–1181. [Google Scholar] [CrossRef]
- Zarshenas, N.; Tapsell, L.C.; Neale, E.P.; Batterham, M.; Talbot, M.L. The Relationship Between Bariatric Surgery and Diet Quality: A Systematic Review. Obes. Surg. 2020, 30, 1768–1792. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2020, 16, 175–247. [Google Scholar] [CrossRef]
- Vilallonga, R.; Pereira-Cunill, J.L.; Morales-Conde, S.; Alarcón, I.; Breton, I.; Domínguez-Adame, E.; Ferrer, J.; Ruiz-De-Gordejuela, A.G.; Goday, A.; Lecube, A.; et al. A Spanish Society joint SECO and SEEDO approach to the Post-operative management of the patients undergoing surgery for obesity. Obes. Surg. 2019, 29, 3842–3853. [Google Scholar] [CrossRef] [PubMed]
- Allied Health Sciences Section Ad Hoc Nutrition Committee; Aills, L.; Blankenship, J.; Buffington, C.; Furtado, M.; Parrott, J. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2008, 4, S73–S108. [Google Scholar] [CrossRef] [PubMed]
- Moizé, V.; Andreu, A.; Rodríguez, L.; Flores, L.; Ibarzabal, A.; Lacy, A.; Jiménez, A.; Vidal, J. Protein intake and lean tissue mass retention following bariatric surgery. Clin. Nutr. Edinb. Scotl. 2013, 32, 550–555. [Google Scholar] [CrossRef]
- Andreu, A.; Moizé, V.; Rodríguez, L.; Flores, L.; Vidal, J. Protein intake, body composition, and protein status following bariatric surgery. Obes. Surg. 2010, 20, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- de Paris, F.G.C.; Padoin, A.V.; Mottin, C.C.; de Paris, M.F. Assessment of Changes in Body Composition During the First Postoperative Year After Bariatric Surgery. Obes. Surg. 2019, 29, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.K.; Gonçalves, V.S.S.; Faria, S.L.C.M.; Moizé, V.; Porporatti, A.L.; Guerra, E.N.S.; De Luca Canto, G.; de Carvalho, K.M.B. Effect of Protein Intake on the Protein Status and Lean Mass of Post-Bariatric Surgery Patients: A Systematic Review. Obes. Surg. 2017, 27, 502–512. [Google Scholar] [CrossRef]
- Romeijn, M.M.; Holthuijsen, D.D.B.; Kolen, A.M.; Janssen, L.; Schep, G.; van Dielen, F.M.H.; Leclercq, W.K.G. The effect of additional protein on lean body mass preservation in post-bariatric surgery patients: A systematic review. Nutr. J. 2021, 20, 27. [Google Scholar] [CrossRef]
- Bellido, D.; García-García, C.; Talluri, A.; Lukaski, H.C.; García-Almeida, J.M. Future lines of research on phase angle: Strengths and limitations. Rev. Endocr. Metab. Disord. 2023, 24, 563–583. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef]
- Molero, J.; Olbeyra, R.; Flores, L.; Jiménez, A.; De Hollanda, A.; Andreu, A.; Ibarzabal, A.; Moizé, V.; Cañizares, S.; Balibrea, J.M.; et al. Prevalence of low skeletal muscle mass following bariatric surgery. Clin. Nutr. ESPEN 2022, 49, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Tangjittrong, S.; Udomsawaengsup, S.; Boonchaya-anant, P. Comparison of Body Composition Variables between Post-Bariatric Surgery Patients and Non-Operative Controls. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231206731. [Google Scholar] [CrossRef] [PubMed]
- Maïmoun, L.; Lefebvre, P.; Aouinti, S.; Picot, M.-C.; Mariano-Goulart, D.; Nocca, D. Acute and longer-term body composition changes after bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Carrasco Navarro, G.N.; Rojas Moncada, P.; Papapietro, K.; Salazar, G. Body composition assessment before and after weight loss following a Roux-en-Y gastric bypass. Are bioimpedanciometry estimations reliable? Nutr. Hosp. 2020, 37, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: A randomized controlled trial. Int. J. Obes. 2019, 43, 1863–1868. [Google Scholar] [CrossRef]
- Batar, N.; Pulat Demir, H.; Bayram, H.M. Assessment of nutritional status, body composition and blood biochemical parameters of patients following sleeve gastrectomy: 6 months follow up. Clin. Nutr. ESPEN 2021, 43, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.; Oliveira, B.M.P.M.; Correia, F. Evolution of body composition of obese patients undergoing bariatric surgery. Clin. Nutr. ESPEN 2019, 31, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, R.; Smith, J.; Avgenackis, E.; Jones, D.; Nau, P. A Comparison of the Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Body Mass Composition as Measured by Air Displacement Plethysmography. Obes. Surg. 2020, 30, 451–455. [Google Scholar] [CrossRef]
- Pakzad, M.; Miratashi Yazdi, S.A.; Talebpour, M.; Elyasinia, F.; Abolhasani, M.; Zabihi-Mahmoudabadi, H.; Najjari, K.; Geranpayeh, L. Short-Term Changes on Body Composition After Sleeve Gastrectomy and One Anastomosis Gastric Bypass. J. Laparoendosc. Adv. Surg. Tech. 2022, 32, 884–889. [Google Scholar] [CrossRef]
- Abdulsalam, F.; Ali, H.I.; Altinoz, A.; Nimeri, A. The Effect of Protein Consumption on Fat-Free Mass, Fat Mass, and Weight Loss 1 Year After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass. Obes. Surg. 2021, 31, 4741–4748. [Google Scholar] [CrossRef]
- Alshamari, S.; Elsherif, M.A.; Hanna, F.; El Akhal, L.; Abid, H.; Elhag, W. The effect of protein supplements on weight loss, body composition, protein status, and micronutrients post laparoscopic sleeve gastrectomy (LSG): A Randomised Controlled Trial (RCT). Ann. Med. Surg. 2022, 74, 103220. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.R.; Blue, M.N.M.; Trexler, E.T.; Ahuja, S.; Smith-Ryan, A.E. Provision of ready-to-drink protein following bariatric surgery: An evaluation of tolerability, body composition, and metabolic rate. Clin. Nutr. 2021, 40, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Barati-Boldaji, R.; Esmaeilinezhad, Z.; Babajafari, S.; Kazemi, A.; Clark, C.C.T.; Mazidi, M.; Ofori-Asenso, R.; Haghighat, N.; Shafiee, M.; Mazloomi, S.M. Bariatric surgery reduces branched-chain amino acids’ levels: A systematic review and meta-analysis. Nutr. Res. 2021, 87, 80–90. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.J.; Izaola-Jauregui, O.; Primo-Martín, D.; Gómez-Hoyos, E.; Torres-Torres, B.; Jiménez-Sahagún, R.; Pérez-López, P.; De Luis-Román, D.A. The morphofunctional evaluation of patients at risk of malnutrition receiving a leucine-enriched nutritional supplement. J. Funct. Foods 2023, 111, 105896. [Google Scholar] [CrossRef]
| Low-Protein Diet | High-Protein Diet | |||
|---|---|---|---|---|
| First 14 Days | Last 14 Days | First 14 Days | Last 14 Days | |
| Energy (kcal) | 217 | 1155.5 | 817 | 1155.5 |
| Protein (g) | 11.70 | 65.95 | 65.70 | 65.95 |
| Carbohydrate (g) | 37.40 | 164.45 | 97.40 | 164.45 |
| Fat (g) | 1.73 | 22.12 | 14.33 | 22.12 |
| Fibre | 2.32 | 17.39 | 17.32 | 17.39 |
| 100 mL | 600 mL | |
|---|---|---|
| Energy (kcal) | 100 | 600 |
| Proteins: g/TE% | 9.0 g/36% | 54.0 g/36% |
| Carbohydrates: g/TE% Sugars: g | 10 g/40% 1.1 g | 60 g/40% 6.6 g |
| Fat g/TE% | 2.1 g/19% | 12.6 g/19% |
| 4% | 4% |
| 10% | 10% |
| 4.7% | 4.7% |
| • EPA & DHA (mg) | 170 mg | 1220 mg |
| Fibre g/soluble-insoluble | 2.5 g/70–30% | 15 g/70–30% |
| BPL-1 | 1.6710 CFU | 1010 CFU |
| High-Protein Diet | Low-Protein Diet | p-Value | |
|---|---|---|---|
| Sex (Men/Women)% | 63.6/36.4 | 69.7/30.3 | 0.71 |
| Age (years) | 47.63 (9.96) | 46.43 (11.09) | 0.72 |
| BMI (kg/m2) | 46.46 (5.22) | 44.95 (7.87) | 0.45 |
| Resistance (ohm) | 436.20 (69.58) | 403.57 (57.87) | 0.13 |
| Reactance (ohm) | 46.90 (8.90) | 43.79 (7.27) | 0.26 |
| Phase Angle (°) | 6.16 (0.82) | 6.28 (0.66) | 0.65 |
| ASMI (kg/m2) | 9.79 (0.91) | 9.96 (1.35) | 0.63 |
| FFMI (kg/m2) | 22.29 (2.21) | 22.63 (2.88) | 0.67 |
| FI (kg/m2) | 24.17 (4.44) | 22.32 (5.59) | 0.24 |
| TBW (%) | 39.33 (5.15) | 42.76 (5.33) | 0.06 |
| High-Protein Diet | Low-Protein Diet | |||
|---|---|---|---|---|
| Men (n = 7) | Women (n = 23) | Men (n = 4) | Women (n = 10) | |
| Sex (%) | 36.4 | 63.6 | 30.3 | 69.7 |
| Age (years) | 51.57 (7.41) | 46.43 (10.46) | 50.25 (5.73) | 44.9 (12.56) |
| BMI (kg/m2) | 45.71 (4.77) | 46.69 (5.43) | 40.64 (6.44) | 46.67 (8.00) |
| Resistance (ohm) | 411 (51.11) | 443.87 (73.53) | 378.5 (33.52) | 413.6 (63.81) |
| Reactance (ohm) | 43.71 (8.88) | 47.87 (8.87) | 41.25 (2.75) | 44.80 (8.35) |
| Phase Angle (°) | 6.1 (1.12) | 6.18 (0.73) | 6.5 (0,22) | 6.19 (0.77) |
| ASMI (kg/m2) | 9.55 (0.94) | 9.87 (0.91) | 9.47 (1.03) | 10.16 (1.45) |
| FFMI (kg/m2) | 20.43 (1.98) | 22.86 (1.98) * | 20.38 (1.96) | 23.53 (2.76) |
| FI (kg/m2) | 25.28 (3.90) | 23.83 (4.61) | 20.25 (4.76) | 23.14 (5.90) |
| TBW (%) | 41.06 (4.52) | 38.80 (5.31) | 47.75 (4.68) | 40.76 (4.26) * |
| High-Protein Diet | Low-Protein Diet | |||||
|---|---|---|---|---|---|---|
| Prebariatric | One Month Postbariatric | p-Value | Prebariatric | One Month Postbariatric | p-Value | |
| Weight (kg) | 123.89 (17.13) | 111.81 (16.27) | <0.01 | 122.24 (23.24) | 112.69 (19.61) | <0.01 |
| BMI (kg/m2) | 46.39 (5.29) | 41.94 (5.67) | <0.01 | 44.95 (7.87) | 41.49 (7.01) | <0.01 |
| Resistance (ohm) | 436.20 (69.58) | 451.63 (80.04) | 0.26 | 403.57 (57.87) | 481.19 (66.22) | <0.01 |
| Reactance (ohm) | 46.90 (8.90) | 46.30 (9.38) | 0.76 | 43.79 (7.27) | 50.30 (10.04) | <0.02 |
| Phase Angle (°) | 6.16 (0.82) | 5.94 (1.26) | 0.32 | 6.28 (0.66) | 6.13 (0.83) | 0.60 |
| ASMI (kg/m2) | 9.80 (0.93) | 9.23 (1.13) | <0.01 | 9.96 (1.35) | 8.89 (1.21) | <0.01 |
| FFMI (kg/m2) | 22.27 (2.24) | 21.36 (2.45) | 0.01 | 22.63 (2.88) | 20.46 (2.69) | <0.01 |
| FI (kg/m2) | 24.12 (4.50) | 20.58 (4.29) | 0.01 | 22.32 (5.58) | 21.04 (5.03) | <0.01 |
| TBW (%) | 39.33 (5.16) | 38.41 (4.55) | 0.34 | 42.76 (5.33) | 37.74 (5.44) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gómez, J.J.; Ramos-Bachiller, B.; Primo-Martín, D.; Calleja-Fernández, A.; Izaola-Jauregui, O.; Jiménez-Sahagún, R.; González-Gutiérrez, J.; López Andrés, E.; Pinto-Fuentes, P.; Pacheco-Sánchez, D.; et al. Effect on Body Composition of a Meal-Replacement Progression Diet in Patients 1 Month after Bariatric Surgery. Nutrients 2024, 16, 106. https://doi.org/10.3390/nu16010106
López-Gómez JJ, Ramos-Bachiller B, Primo-Martín D, Calleja-Fernández A, Izaola-Jauregui O, Jiménez-Sahagún R, González-Gutiérrez J, López Andrés E, Pinto-Fuentes P, Pacheco-Sánchez D, et al. Effect on Body Composition of a Meal-Replacement Progression Diet in Patients 1 Month after Bariatric Surgery. Nutrients. 2024; 16(1):106. https://doi.org/10.3390/nu16010106
Chicago/Turabian StyleLópez-Gómez, Juan J., Beatriz Ramos-Bachiller, David Primo-Martín, Alicia Calleja-Fernández, Olatz Izaola-Jauregui, Rebeca Jiménez-Sahagún, Jaime González-Gutiérrez, Eva López Andrés, Pilar Pinto-Fuentes, David Pacheco-Sánchez, and et al. 2024. "Effect on Body Composition of a Meal-Replacement Progression Diet in Patients 1 Month after Bariatric Surgery" Nutrients 16, no. 1: 106. https://doi.org/10.3390/nu16010106
APA StyleLópez-Gómez, J. J., Ramos-Bachiller, B., Primo-Martín, D., Calleja-Fernández, A., Izaola-Jauregui, O., Jiménez-Sahagún, R., González-Gutiérrez, J., López Andrés, E., Pinto-Fuentes, P., Pacheco-Sánchez, D., & De Luis-Román, D. A. (2024). Effect on Body Composition of a Meal-Replacement Progression Diet in Patients 1 Month after Bariatric Surgery. Nutrients, 16(1), 106. https://doi.org/10.3390/nu16010106







