Membrane Lipids in Ultra-High-Risk Patients: Potential Predictive Biomarkers of Conversion to Psychosis
Abstract
1. Introduction
2. Methods and Materials
2.1. Clinical Population and Assessments
2.2. Blood Samples
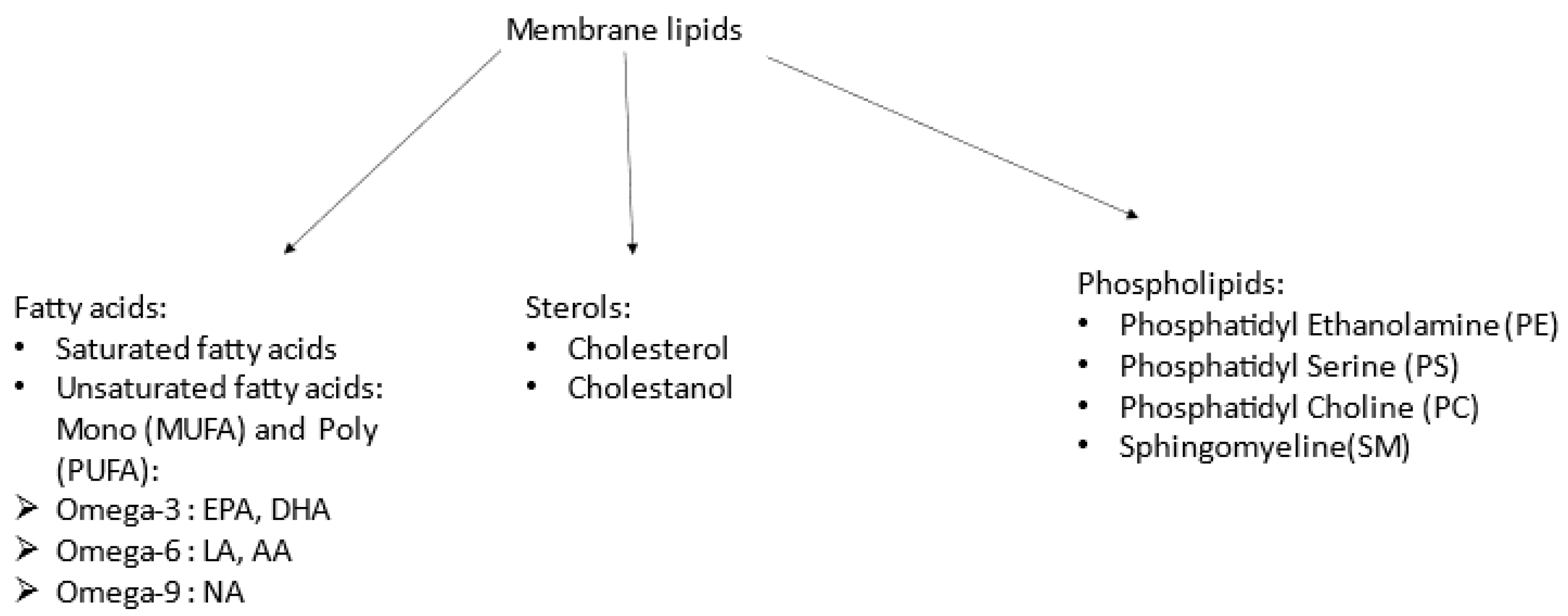
2.3. Lipid Analysis
- -
- Fatty acids: After extraction, they were then trans-methylated in acid conditions and separated by gas chromatography–mass spectrometry using a Thermo GC/MS/FID TRACE DSQ2 device.
- -
- Phospholipids: Lipid extracts were suspended in 200 µL cyclohexane/isopropanol/water/ammonium acetate, 500 mM (58/40/0/2) volume (Solvent A). For phospholipid analysis, a total of 10 µL was injected onto a 3.0 mm × 250 mm length PVA-SIL column (YMC Europe GmbH, D-46514, Schermbeck, Germany), at a flow rate of 150 µL/min, with a total run time of 70 min. A 2 mm frit cap and a short reverse-phase guard cartridge (in-line guard C18-silica, 3 µm, 4 × 20 mm2, CIL-Cluzeau, 92419, Courbevoie, France) were used to prevent the capillary clogging. A passage through the guard cartridge was used to decrease ion suppression. The mobile phase gradient used consisted of solvent A and solvent B (cyclohexane/isopropanol/water/ammonium acetate 500 mM (50/40/8/2). In each measurement, gradient elution was applied to separate each lipid class. Both the application of HPLC solvent gradient and mass spectrometer scan functions were controlled by the Analyst Software (AB Sciex) data system. The samples were analysed using an electrospray ionisation tandem mass spectrometry (ESI/MS/MS, 6500 ABSciex, TQ, Applied Biosystems-Sciex, Concord, ON, Canada) either with scan mode or multiple-reaction monitoring (MRM). The specific detection of lipid classes was based on the mass-to-charge ratio (m/z) value of their precursor ion scanning, which was related to their head group fragments. The scans were conducted in negative-ion mode. Based on the precursor ion scanning value, the PL was identified at 184 (m/z) for PC and SM, 185 (m/z) for PS, and neutral loss scanning 141 (m/z) for PE. A comprehensive description of the methodology can be found in Lamazière et al. [32].
- -
- Sterols: Sterols were extracted with a solvent mixture containing chloroform/methanol 2/1 (v/v) spiked with internal standards. Lipids were partitioned in chloroform after the addition of saline and evaporation under nitrogen, and saponified by methanol potassium hydroxide. The fatty acids released were then methylated with BF3-methanol to prevent them from interfering with the chromatography of sterols. Sterols were further re-extracted in hexane and silylated, with evaporation under nitrogen; then, we added 150 µL cyclohexane 10% BSTFA and the resultant derivatives were separated by gas chromatography (GC) (Hewlett–Packard 6890 series) in a medium-polarity capillary column RTX-65, (Restesk, Evry, France). The mass spectrometer (Agilent 5975 inert XL) in series with the GC was set up for the detection of positive ions, which were produced in the electron impact mode at 70 eV. Sterols were identified by the fragmentogram in the scanning mode and quantified by selective monitoring of the specific ions after normalisation with the internal standards and calibration with weighed standards. For more detailed descriptions, see Chevy et al. [35].
2.4. Statistical Analysis
3. Results
3.1. Comparison at Baseline between Converters and Non-Converters
3.2. Prediction of Conversion to Psychosis
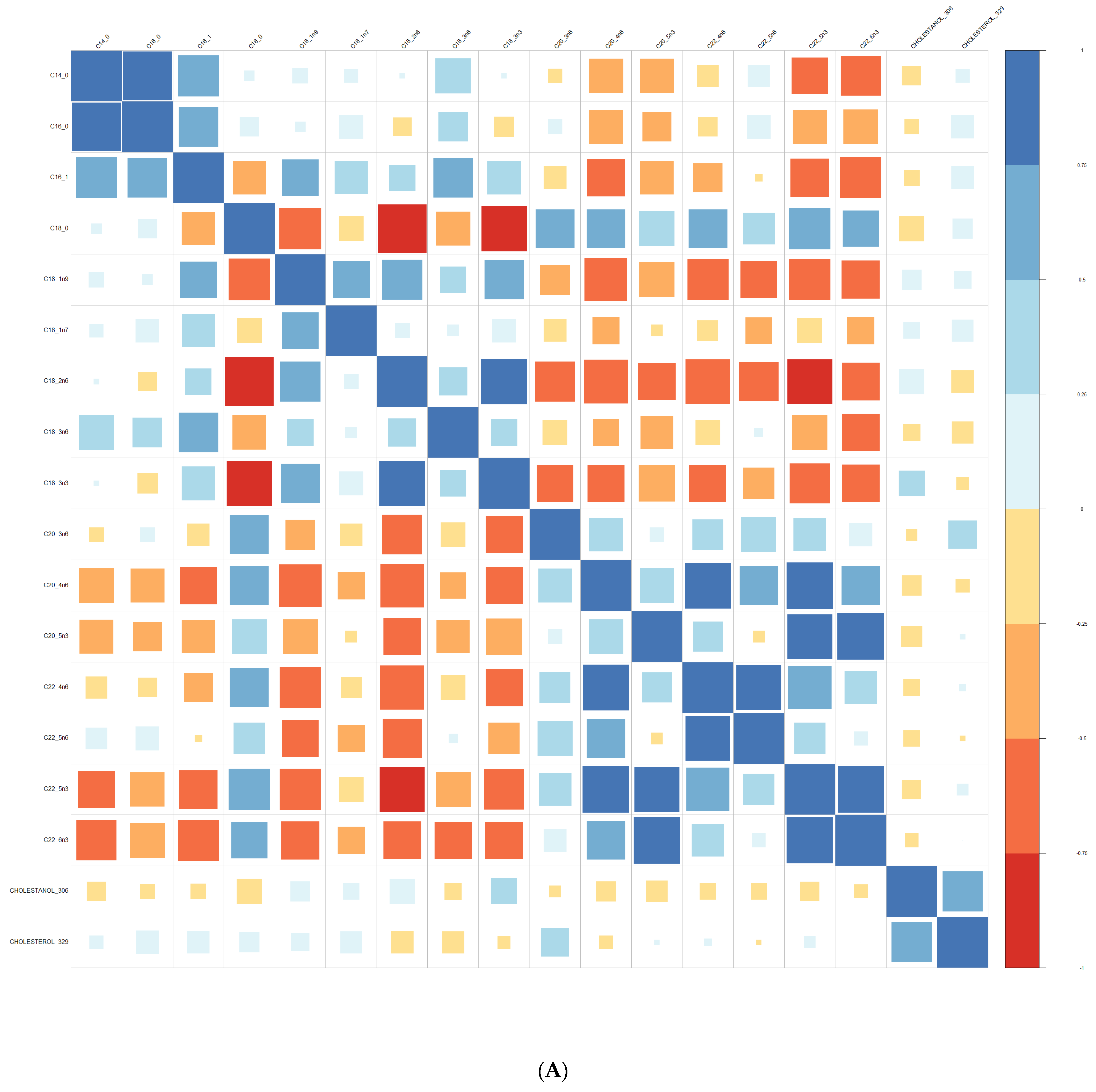
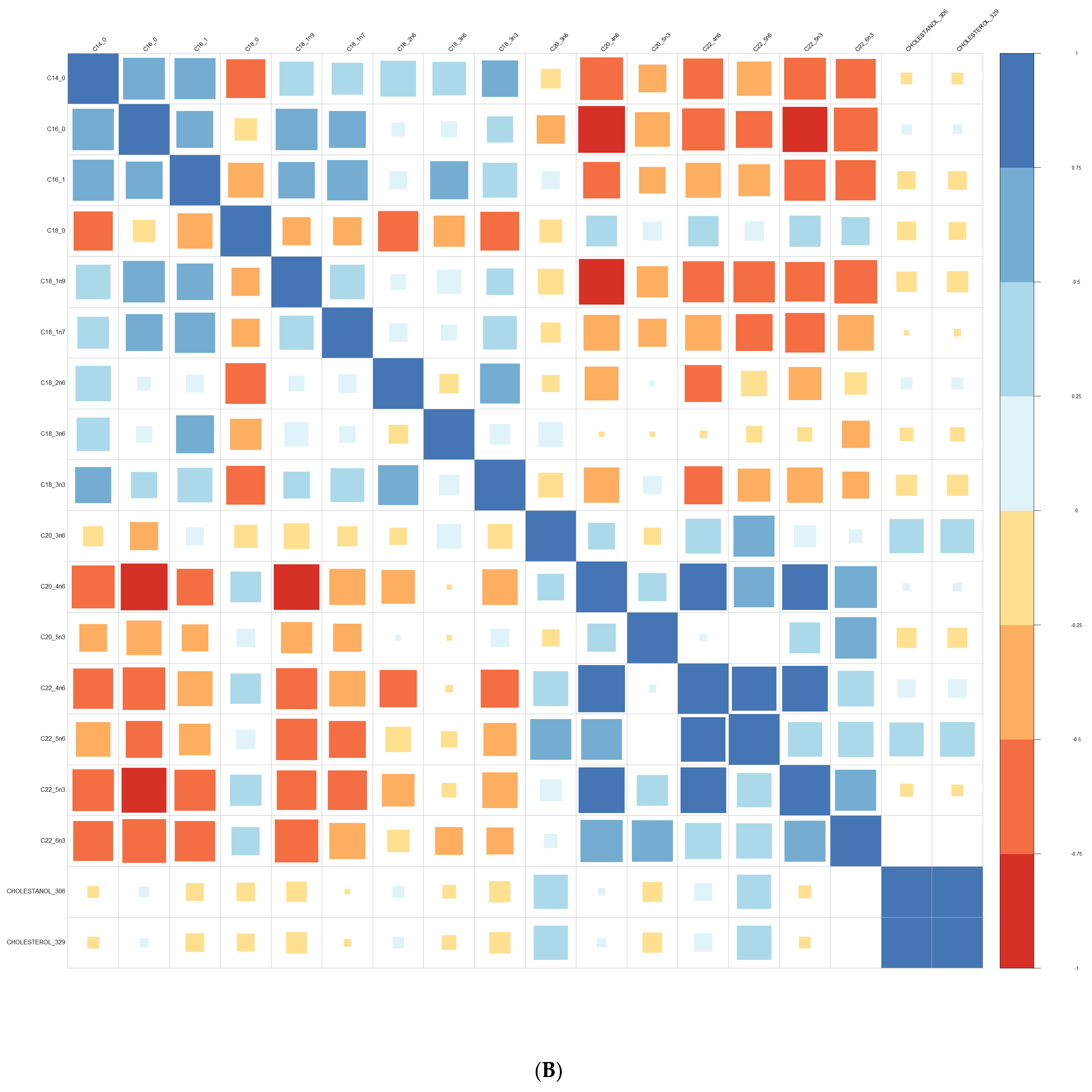
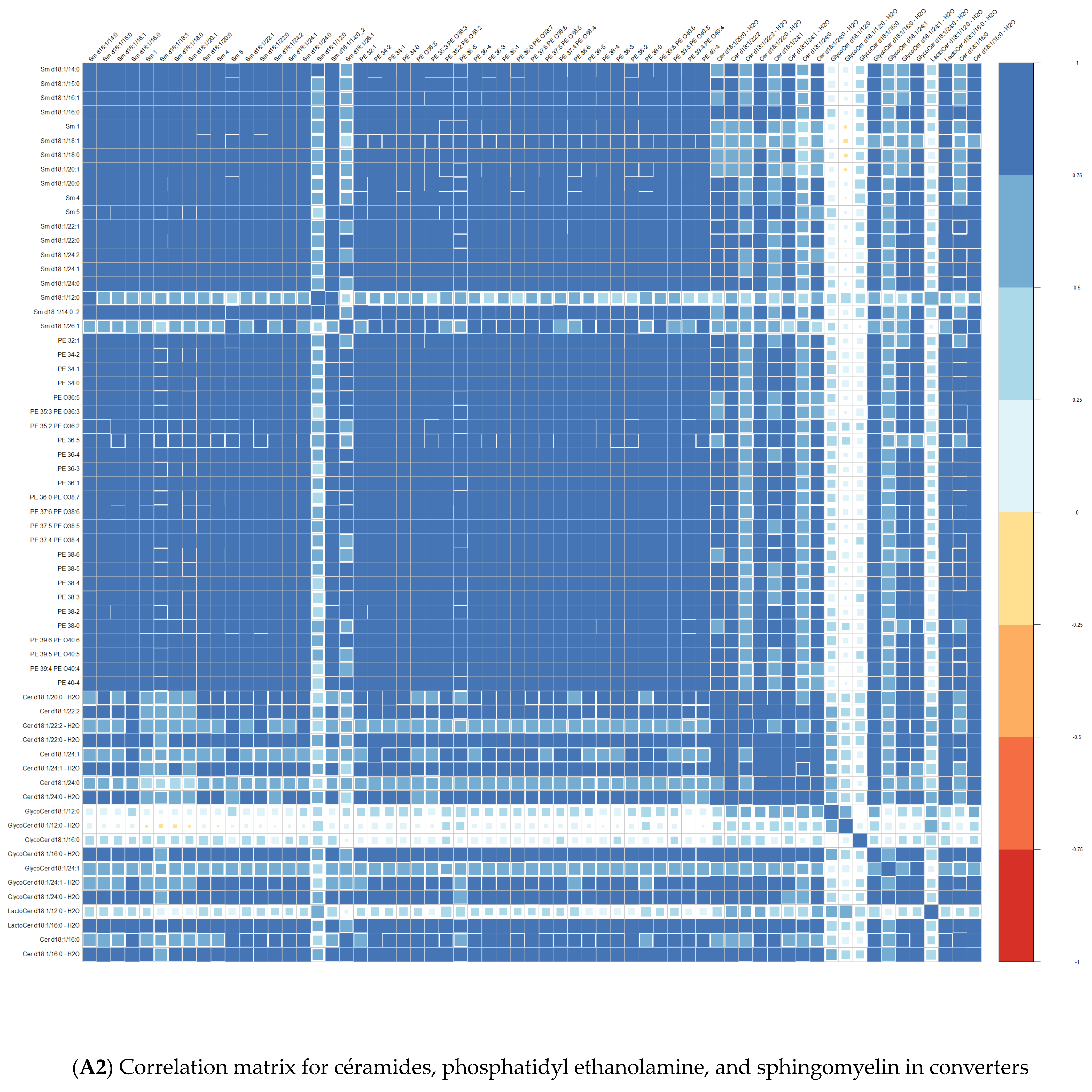
3.3. Lipid Composition over Time
4. Discussion
4.1. Membrane Lipids as Biomarkers of Conversion to Psychosis in UHR
4.2. Linolenic Acid (LA: C18:2n6) and Schizophrenia
4.3. Sterols in Psychiatry
4.4. Membrane Lipids for Personalised Medicine
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frajerman, A.; Scoriels, L.; Kebir, O.; Chaumette, B. Shared Biological Pathways between Antipsychotics and Omega-3 Fatty Acids: A Key Feature for Schizophrenia Preventive Treatment? Int. J. Mol. Sci. 2021, 22, 6881. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.A.; Lamichhane, S.; Dickens, A.; McGlinchey, A.; Ribeiro, H.C.; Sen, P.; Wei, F.; Hyötyläinen, T.; Orešič, M. Systems Biology Approaches to Study Lipidomes in Health and Disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158857. [Google Scholar] [CrossRef] [PubMed]
- Borroni, M.V.; Vallés, A.S.; Barrantes, F.J. The Lipid Habitats of Neurotransmitter Receptors in Brain. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, R.; Mircheva, K.; Vitkova, V.; Balashev, K.; Ivanova, T.; Tessier, C.; Koumanov, K.; Nuss, P.; Momchilova, A.; Staneva, G. Phospholipase A2-Induced Remodeling Processes on Liquid-Ordered/Liquid-Disordered Membranes Containing Docosahexaenoic or Oleic Acid: A Comparison Study. Langmuir 2016, 32, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Barrantes, F.J. Sphingolipid/Cholesterol Regulation of Neurotransmitter Receptor Conformation and Function. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2345–2361. [Google Scholar] [CrossRef]
- Arashiki, N.; Saito, M.; Koshino, I.; Kamata, K.; Hale, J.; Mohandas, N.; Manno, S.; Takakuwa, Y. An Unrecognised Function of Cholesterol: Regulating the Mechanism Controlling Membrane Phospholipid Asymmetry. Biochemistry 2016, 55, 3504–3513. [Google Scholar] [CrossRef]
- Arashiki, N.; Takakuwa, Y. Maintenance and Regulation of Asymmetric Phospholipid Distribution in Human Erythrocyte Membranes: Implications for Erythrocyte Functions. Curr. Opin. Hematol. 2017, 24, 167–172. [Google Scholar] [CrossRef]
- Roy, S.; Dasgupta, A.; Banerjee, U.; Chowdhury, P.; Mukhopadhyay, A.; Saha, G.; Singh, O. Role of Membrane Cholesterol and Lipid Peroxidation in Regulating the Na+/K+-ATPase Activity in Schizophrenia. Indian J. Psychiatry 2016, 58, 317–325. [Google Scholar] [CrossRef]
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.F.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.J.; Whiteford, H.A. Global Epidemiology and Burden of Schizophrenia: Findings from the Global Burden of Disease Study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef]
- Krebs, M.-O.; Kebir, O.; Jay, T.M. Exposure to Cannabinoids Can Lead to Persistent Cognitive and Psychiatric Disorders. Eur. J. Pain 2019, 23, 1225–1233. [Google Scholar] [CrossRef]
- McGorry, P. A Treatment-Relevant Classification of Psychotic Disorders. Aust. N. Z. J. Psychiatry 1995, 29, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Yung, A.R.; McGorry, P.D.; McFarlane, C.A.; Jackson, H.J.; Patton, G.C.; Rakkar, A. Monitoring and Care of Young People at Incipient Risk of Psychosis. Schizophr. Bull. 1996, 22, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Borgwardt, S.; Bechdolf, A.; Addington, J.; Riecher-Rössler, A.; Schultze-Lutter, F.; Keshavan, M.; Wood, S.; Ruhrmann, S.; Seidman, L.J.; et al. The Psychosis High-Risk State: A Comprehensive State-of-the-Art Review. JAMA Psychiatry 2013, 70, 107–120. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, G.S.; Radua, J.; Pereira, J.; Bonoldi, I.; Arienti, V.; Besana, F.; Soardo, L.; Cabras, A.; Fortea, L.; Catalan, A.; et al. Probability of Transition to Psychosis in Individuals at Clinical High Risk: An Updated Meta-Analysis. JAMA Psychiatry 2021, 78, 970–978. [Google Scholar] [CrossRef]
- Millan, M.J.; Andrieux, A.; Bartzokis, G.; Cadenhead, K.; Dazzan, P.; Fusar-Poli, P.; Gallinat, J.; Giedd, J.; Grayson, D.R.; Heinrichs, M.; et al. Altering the Course of Schizophrenia: Progress and Perspectives. Nat. Rev. Drug Discov. 2016, 15, 485–515. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III—The Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Snyder, M.A.; Gao, W.-J. NMDA Receptor Hypofunction for Schizophrenia Revisited: Perspectives from Epigenetic Mechanisms. Schizophr. Res. 2019, 217, 60–70. [Google Scholar] [CrossRef]
- Fakhoury, M. Role of the Endocannabinoid System in the Pathophysiology of Schizophrenia. Mol. Neurobiol. 2017, 54, 768–778. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Do, K.Q. Linking Early-Life NMDAR Hypofunction and Oxidative Stress in Schizophrenia Pathogenesis. Nat. Rev. Neurosci. 2016, 17, 125–134. [Google Scholar] [CrossRef]
- Müller, N. A Brief History of Immunological Research into Psychosis and Pathways for Immune Influence of the Brain. Curr. Top. Behav. Neurosci. 2019, 44, 1–8. [Google Scholar] [CrossRef]
- Frajerman, A.; Kebir, O.; Chaumette, B.; Tessier, C.; Lamazière, A.; Nuss, P.; Krebs, M.-O. Membrane lipids in schizophrenia and early phases of psychosis: Potential biomarkers and therapeutic targets? Encephale 2020, 46, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F.; Glen, A.I.M.; Vaddadi, K. The Membrane Hypothesis of Schizophrenia. Schizophr. Res. 1994, 13, 195–207. [Google Scholar] [CrossRef]
- Glen, A.I.; Glen, E.M.; Horrobin, D.F.; Vaddadi, K.S.; Spellman, M.; Morse-Fisher, N.; Ellis, K.; Skinner, F.S. A Red Cell Membrane Abnormality in a Subgroup of Schizophrenic Patients: Evidence for Two Diseases. Schizophr. Res. 1994, 12, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bentsen, H.; Solberg, D.K.; Refsum, H.; Gran, J.M.; Bøhmer, T.; Torjesen, P.A.; Halvorsen, O.; Lingjærde, O. Bimodal Distribution of Polyunsaturated Fatty Acids in Schizophrenia Suggests Two Endophenotypes of the Disorder. Biol. Psychiatry 2011, 70, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Tessier, C.; Sweers, K.; Frajerman, A.; Bergaoui, H.; Ferreri, F.; Delva, C.; Lapidus, N.; Lamaziere, A.; Roiser, J.P.; De Hert, M.; et al. Membrane Lipidomics in Schizophrenia Patients: A Correlational Study with Clinical and Cognitive Manifestations. Transl. Psychiatry 2016, 6, e906. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Mukherjee, S.; Correnti, E.E.; Kelkar, H.S.; Wakade, C.G.; Costa, R.M.; Scheffer, R. Plasma Membrane Phospholipid and Cholesterol Distribution of Skin Fibroblasts from Drug-Naive Patients at the Onset of Psychosis. Schizophr. Res. 1994, 13, 239–247. [Google Scholar] [CrossRef]
- Chaumette, B.; Kebir, O.; Mam-Lam-Fook, C.; Morvan, Y.; Bourgin, J.; Godsil, B.P.; Plaze, M.; Gaillard, R.; Jay, T.M.; Krebs, M.-O. Salivary Cortisol in Early Psychosis: New Findings and Meta-Analysis. Psychoneuroendocrinology 2016, 63, 262–270. [Google Scholar] [CrossRef]
- Yung, A.R.; Yuen, H.P.; McGorry, P.D.; Phillips, L.J.; Kelly, D.; Dell’Olio, M.; Francey, S.M.; Cosgrave, E.M.; Killackey, E.; Stanford, C.; et al. Mapping the Onset of Psychosis: The Comprehensive Assessment of At-Risk Mental States. Aust. N. Z. J. Psychiatry 2005, 39, 964–971. [Google Scholar] [CrossRef]
- Krebs, M.-O.; Magaud, E.; Willard, D.; Elkhazen, C.; Chauchot, F.; Gut, A.; Morvan, Y.; Bourdel, M.-C.; Kazes, M. Assessment of mental states at risk of psychotic transition: Validation of the French version of the CAARMS. Encephale 2014, 40, 447–456. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lamaziere, A.; Farabos, D.; Wolf, C.; Quinn, P.J. The Deficit of Lipid in Cultured Cells Contrasted with Clinical Lipidomics. Mol. Nutr. Food Res. 2013, 57, 1401–1409. [Google Scholar] [CrossRef]
- Lamaziere, A.; Richard, D.; Barbe, U.; Kefi, K.; Bausero, P.; Wolf, C.; Visioli, F. Differential Distribution of DHA-Phospholipids in Rat Brain after Feeding: A Lipidomic Approach. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Shillito, B.; Desurmont, C.; Barthélémy, D.; Farabos, D.; Després, G.; Ravaux, J.; Zbinden, M.; Lamazière, A. Lipidome Variations of Deep-Sea Vent Shrimps According to Acclimation Pressure: A Homeoviscous Response? Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 161, 103285. [Google Scholar] [CrossRef]
- Boussicault, L.; Alves, S.; Lamazière, A.; Planques, A.; Heck, N.; Moumné, L.; Despres, G.; Bolte, S.; Hu, A.; Pagès, C.; et al. CYP46A1, the Rate-Limiting Enzyme for Cholesterol Degradation, Is Neuroprotective in Huntington’s Disease. Brain 2016, 139, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Chevy, F.; Humbert, L.; Wolf, C. Sterol Profiling of Amniotic Fluid: A Routine Method for the Detection of Distal Cholesterol Synthesis Deficit. Prenat. Diagn. 2005, 25, 1000–1006. [Google Scholar] [CrossRef]
- Dickens, A.M.; Sen, P.; Kempton, M.J.; Barrantes-Vidal, N.; Iyegbe, C.; Nordentoft, M.; Pollak, T.; Riecher-Rössler, A.; Ruhrmann, S.; Sachs, G.; et al. Dysregulated Lipid Metabolism Precedes Onset of Psychosis. Biol. Psychiatry 2021, 89, 288–297. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Zhou, J.; Shi, H.; Su, X.; Shao, M.; Yang, Y.; Wang, X.; Zhao, J.; Guo, D.; et al. Potential Plasma Biomarker Panels Identification for the Diagnosis of First-Episode Schizophrenia and Monitoring Antipsychotic Monotherapy with the Use of Metabolomics Analyses. Psychiatry Res. 2023, 321, 115070. [Google Scholar] [CrossRef] [PubMed]
- Amminger, G.P.; Schäfer, M.R.; Klier, C.M.; Slavik, J.-M.; Holzer, I.; Holub, M.; Goldstone, S.; Whitford, T.J.; McGorry, P.D.; Berk, M. Decreased Nervonic Acid Levels in Erythrocyte Membranes Predict Psychosis in Help-Seeking Ultra-High-Risk Individuals. Mol. Psychiatry 2012, 17, 1150–1152. [Google Scholar] [CrossRef]
- Alqarni, A.; Mitchell, T.W.; McGorry, P.D.; Nelson, B.; Markulev, C.; Yuen, H.P.; Schäfer, M.R.; Berger, M.; Mossaheb, N.; Schlögelhofer, M.; et al. Comparison of Erythrocyte Omega-3 Index, Fatty Acids and Molecular Phospholipid Species in People at Ultra-High Risk of Developing Psychosis and Healthy People. Schizophr. Res. 2019, 226, 44–51. [Google Scholar] [CrossRef]
- Alqarni, A.; Mcintyre, K.J.; Brown, S.H.J.; Meyer, B.J.; Mitchell, T.W. A High-Throughput Method for the Analysis of Erythrocyte Fatty Acids and the Omega-3 Index. Lipids 2018, 53, 1005–1015. [Google Scholar] [CrossRef]
- Hathaway, D.; Pandav, K.; Patel, M.; Riva-Moscoso, A.; Singh, B.M.; Patel, A.; Min, Z.C.; Singh-Makkar, S.; Sana, M.K.; Sanchez-Dopazo, R.; et al. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect. Chemother. 2020, 52, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Fritsche, K. Linoleic Acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.Y. Linoleic Acid-Good or Bad for the Brain? NPJ Sci. Food 2020, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, A.; Wang, C.; Ramamurthy, J.; Zhang, E.; Guadagno, E.; Trakadis, Y. Metabolomics in Patients with Psychosis: A Systematic Review. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 580–588. [Google Scholar] [CrossRef]
- Jones, H.J.; Borges, M.C.; Carnegie, R.; Mongan, D.; Rogers, P.J.; Lewis, S.J.; Thompson, A.D.; Zammit, S. Associations between Plasma Fatty Acid Concentrations and Schizophrenia: A Two-Sample Mendelian Randomisation Study. Lancet Psychiatry 2021, 8, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Hoen, W.P.; Lijmer, J.G.; Duran, M.; Wanders, R.J.A.; van Beveren, N.J.M.; de Haan, L. Red Blood Cell Polyunsaturated Fatty Acids Measured in Red Blood Cells and Schizophrenia: A Meta-Analysis. Psychiatry Res. 2013, 207, 1–12. [Google Scholar] [CrossRef]
- van der Kemp, W.J.M.; Klomp, D.W.J.; Kahn, R.S.; Luijten, P.R.; Hulshoff Pol, H.E. A Meta-Analysis of the Polyunsaturated Fatty Acid Composition of Erythrocyte Membranes in Schizophrenia. Schizophr. Res. 2012, 141, 153–161. [Google Scholar] [CrossRef]
- Berger, M.E.; Smesny, S.; Kim, S.-W.; Davey, C.G.; Rice, S.; Sarnyai, Z.; Schlögelhofer, M.; Schäfer, M.R.; Berk, M.; McGorry, P.D.; et al. Omega-6 to Omega-3 Polyunsaturated Fatty Acid Ratio and Subsequent Mood Disorders in Young People with at-Risk Mental States: A 7-Year Longitudinal Study. Transl. Psychiatry 2017, 7, e1220. [Google Scholar] [CrossRef]
- Berger, M.; Nelson, B.; Markulev, C.; Yuen, H.P.; Schäfer, M.R.; Mossaheb, N.; Schlögelhofer, M.; Smesny, S.; Hickie, I.B.; Berger, G.E.; et al. Relationship Between Polyunsaturated Fatty Acids and Psychopathology in the NEURAPRO Clinical Trial. Front. Psychiatry 2019, 10, 393. [Google Scholar] [CrossRef]
- Pawełczyk, T.; Trafalska, E.; Kotlicka-Antczak, M.; Pawełczyk, A. The Association between Polyunsaturated Fatty Acid Consumption and the Transition to Psychosis in Ultra-High Risk Individuals. Prostaglandins Leukot. Essent. Fat. Acids 2016, 108, 30–37. [Google Scholar] [CrossRef]
- Kidnapillai, S.; Bortolasci, C.C.; Panizzutti, B.; Spolding, B.; Connor, T.; Bonifacio, K.; Sanigorski, A.; Dean, O.M.; Crowley, T.; Jamain, S.; et al. Drugs Used in the Treatment of Bipolar Disorder and Their Effects on Cholesterol Biosynthesis—A Possible Therapeutic Mechanism. World J. Biol. Psychiatry 2019, 20, 766–777. [Google Scholar] [CrossRef]
- Jacobs, M.L.; Faizi, H.A.; Peruzzi, J.A.; Vlahovska, P.M.; Kamat, N.P. EPA and DHA Differentially Modulate Membrane Elasticity in the Presence of Cholesterol. Biophys. J. 2021, 120, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid Rafts: Heterogeneity on the High Seas. Biochem. J. 2004, 378, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Hezghia, A.; Shaikh, S.R.; Cenido, J.F.; Stark, R.E.; Mann, J.J.; Sublette, M.E. Regulation of Monoamine Transporters and Receptors by Lipid Microdomains: Implications for Depression. Neuropsychopharmacology 2018, 43, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Zhen, J.; Reith, M.E.A. Importance of Cholesterol in Dopamine Transporter Function. J. Neurochem. 2012, 123, 700–715. [Google Scholar] [CrossRef]
- Das, D.; Peng, X.; Lam, A.-T.N.; Bader, J.S.; Avramopoulos, D. Transcriptome Analysis of Human Induced Excitatory Neurons Supports a Strong Effect of Clozapine on Cholesterol Biosynthesis. Schizophr. Res. 2021, 228, 324–326. [Google Scholar] [CrossRef]
- Berginer, V.M.; Foster, N.L.; Sadowsky, M.; Townsend, J.A.; Siegel, G.J.; Salen, G. Psychiatric Disorders in Patients with Cerebrotendinous Xanthomatosis. Am. J. Psychiatry 1988, 145, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Fraidakis, M.J. Psychiatric Manifestations in Cerebrotendinous Xanthomatosis. Transl. Psychiatry 2013, 3, e302. [Google Scholar] [CrossRef]
- Wong, J.C.; Walsh, K.; Hayden, D.; Eichler, F.S. Natural History of Neurological Abnormalities in Cerebrotendinous Xanthomatosis. J. Inherit. Metab. Dis. 2018, 41, 647–656. [Google Scholar] [CrossRef]
- Degos, B.; Nadjar, Y.; Amador, M.D.; Lamari, F.; Sedel, F.; Roze, E.; Couvert, P.; Mochel, F. Natural History of Cerebrotendinous Xanthomatosis: A Paediatric Disease Diagnosed in Adulthood. Orphanet J. Rare Dis. 2016, 11, 41. [Google Scholar] [CrossRef]
- Guidara, W.; Messedi, M.; Naifar, M.; Maalej, M.; Khrouf, W.; Grayaa, S.; Maalej, M.; Bonnefont-Rousselot, D.; Lamari, F.; Ayadi, F. Plasma Oxysterols in Drug-Free Patients with Schizophrenia. J. Steroid Biochem. Mol. Biol. 2022, 221, 106123. [Google Scholar] [CrossRef]
- What is Arteriosclerotic Brain Disorder? Definition of Arteriosclerotic Brain Disorder (Psychology Dictionary) 2018. Available online: https://psychologydictionary.org/arteriosclerotic-brain-disorder/ (accessed on 21 April 2023).
- Brown, R.B. Phospholipid Packing Defects and Oxysterols in Atherosclerosis: Dietary Prevention and the French Paradox. Biochimie 2019, 167, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, M.H.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.; Cardoos, A.; Walker, R.; Mischoulon, D. Inflammation as a Predictive Biomarker for Response to Omega-3 Fatty Acids in Major Depressive Disorder: A Proof-of-Concept Study. Mol. Psychiatry 2016, 21, 71–79. [Google Scholar] [CrossRef]
- Amminger, G.P.; Mechelli, A.; Rice, S.; Kim, S.-W.; Klier, C.M.; McNamara, R.K.; Berk, M.; McGorry, P.D.; Schäfer, M.R. Predictors of Treatment Response in Young People at Ultra-High Risk for Psychosis Who Received Long-Chain Omega-3 Fatty Acids. Transl. Psychiatry 2015, 5, e495. [Google Scholar] [CrossRef]
- Susai, S.R.; Healy, C.; Mongan, D.; Heurich, M.; Byrne, J.F.; Cannon, M.; Cagney, G.; Wynne, K.; Markulev, C.; Schäfer, M.R.; et al. Evidence That Complement and Coagulation Proteins Are Mediating the Clinical Response to Omega-3 Fatty Acids: A Mass Spectrometry-Based Investigation in Subjects at Clinical High-Risk for Psychosis. Transl. Psychiatry 2022, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, P.; Tang, M.; Liu, Y.; Guo, W.; Lang, B.; Wang, J.; Wu, H.; Tang, H.; Yu, Y.; et al. Reduced Erythrocyte Membrane Polyunsaturated Fatty Acid Levels Indicate Diminished Treatment Response in Patients with Multi- versus First-Episode Schizophrenia. NPJ Schizophr. 2022, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Adrien, V.; Bosc, N.; Fumat, H.; Tessier, C.; Ferreri, F.; Mouchabac, S.; Tareste, D.; Nuss, P. Higher Stress Response and Altered Quality of Life in Schizophrenia Patients with Low Membrane Levels of Docosahexaenoic Acid. Front. Psychiatry 2023, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, X.; Wang, D.; Jiang, J.; Li, M.; Qing, Y.; Yang, X.; Zhang, J.; Zhang, Y.; Wan, C. Association between Arachidonic Acid and the Risk of Schizophrenia: A Cross-National Study and Mendelian Randomization Analysis. Nutrients 2023, 15, 1195. [Google Scholar] [CrossRef] [PubMed]


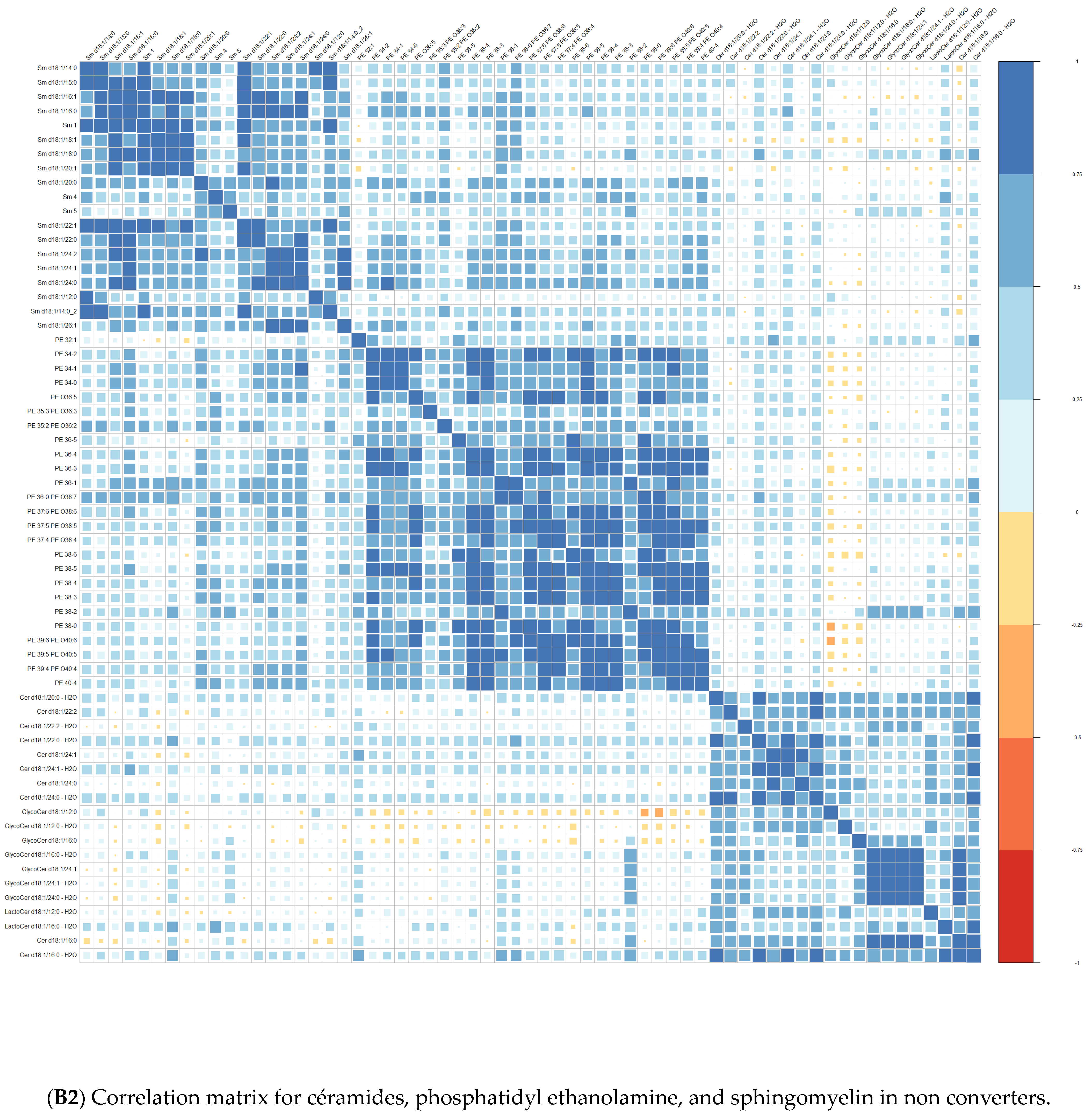


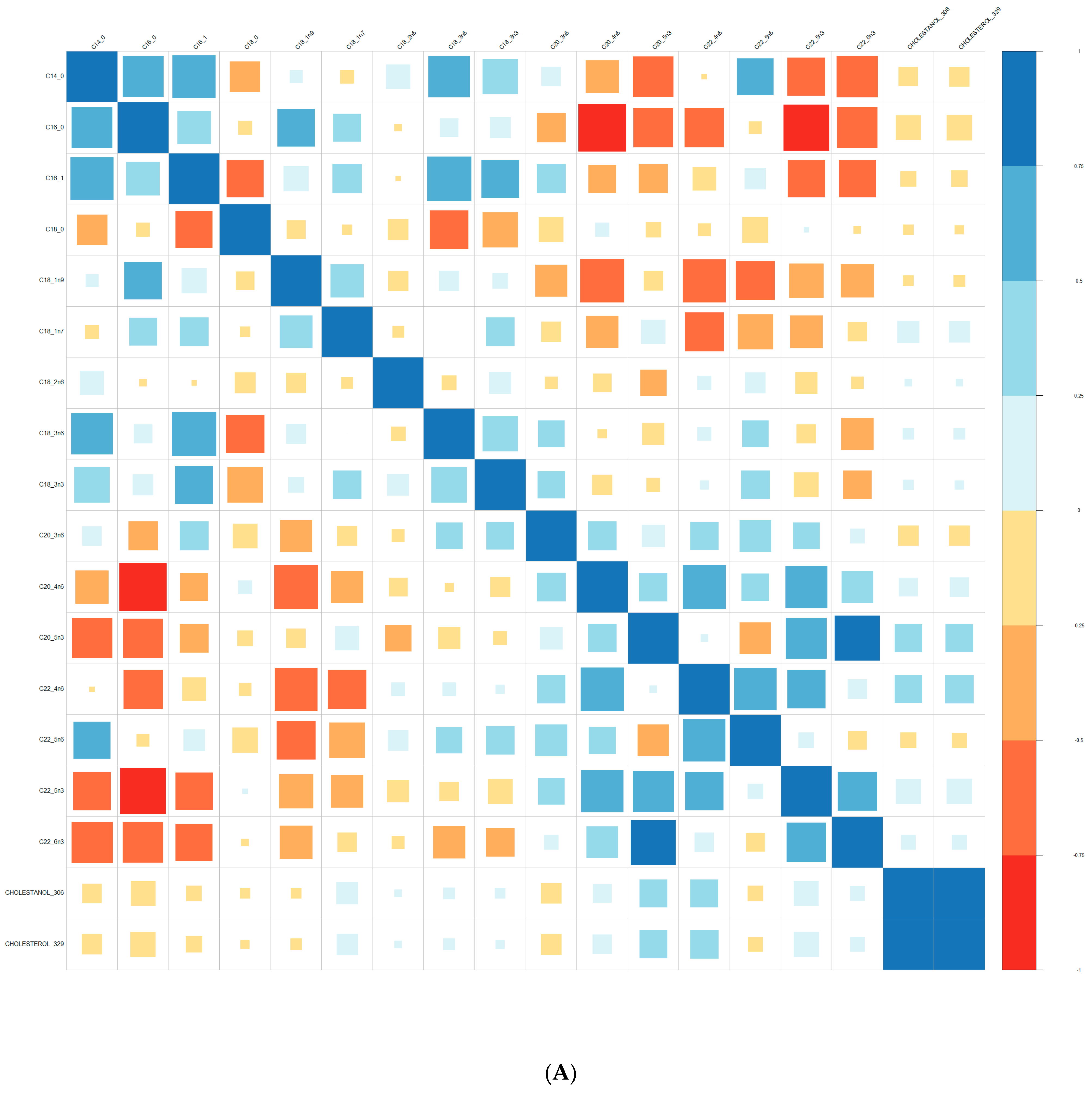
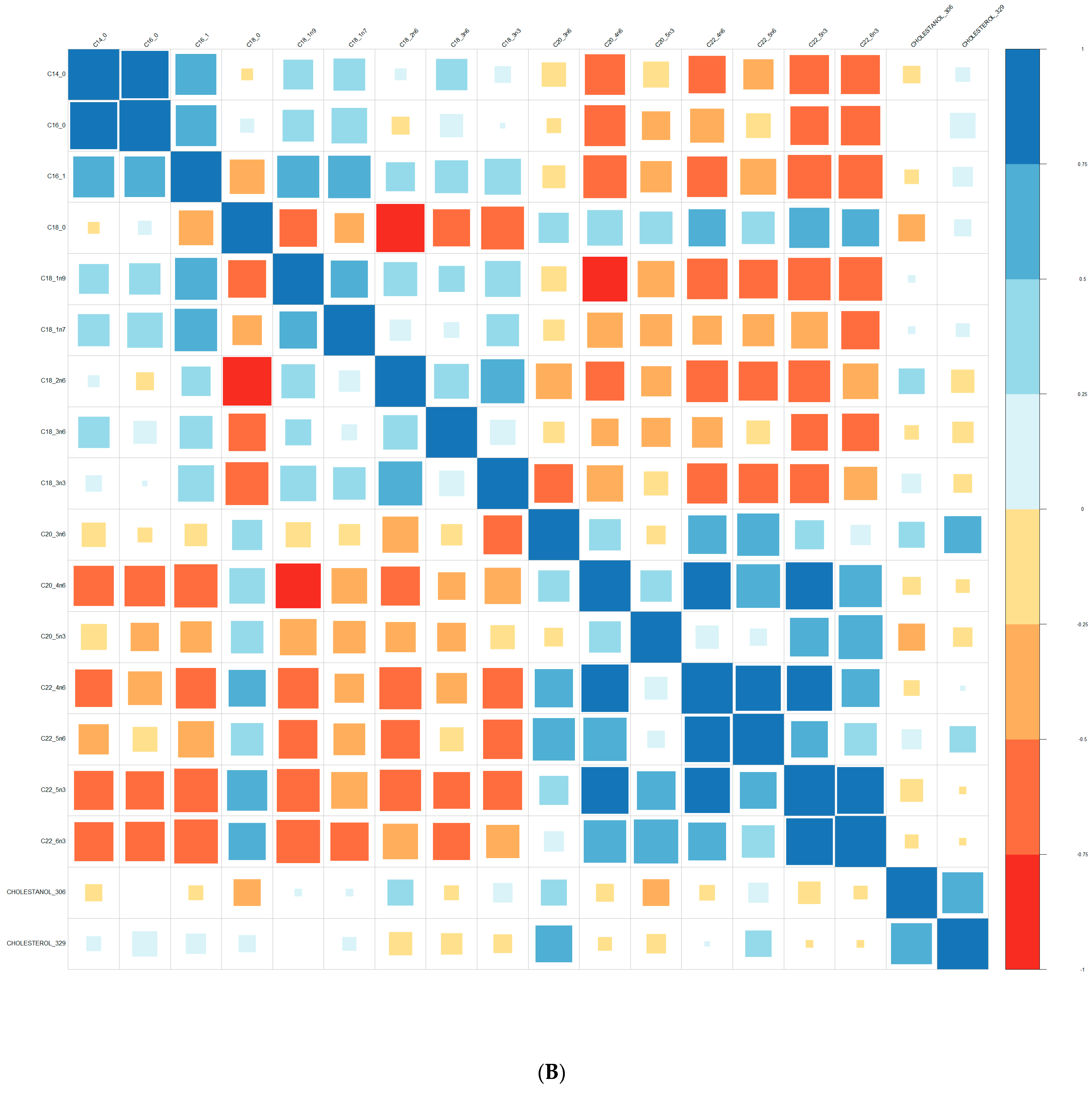
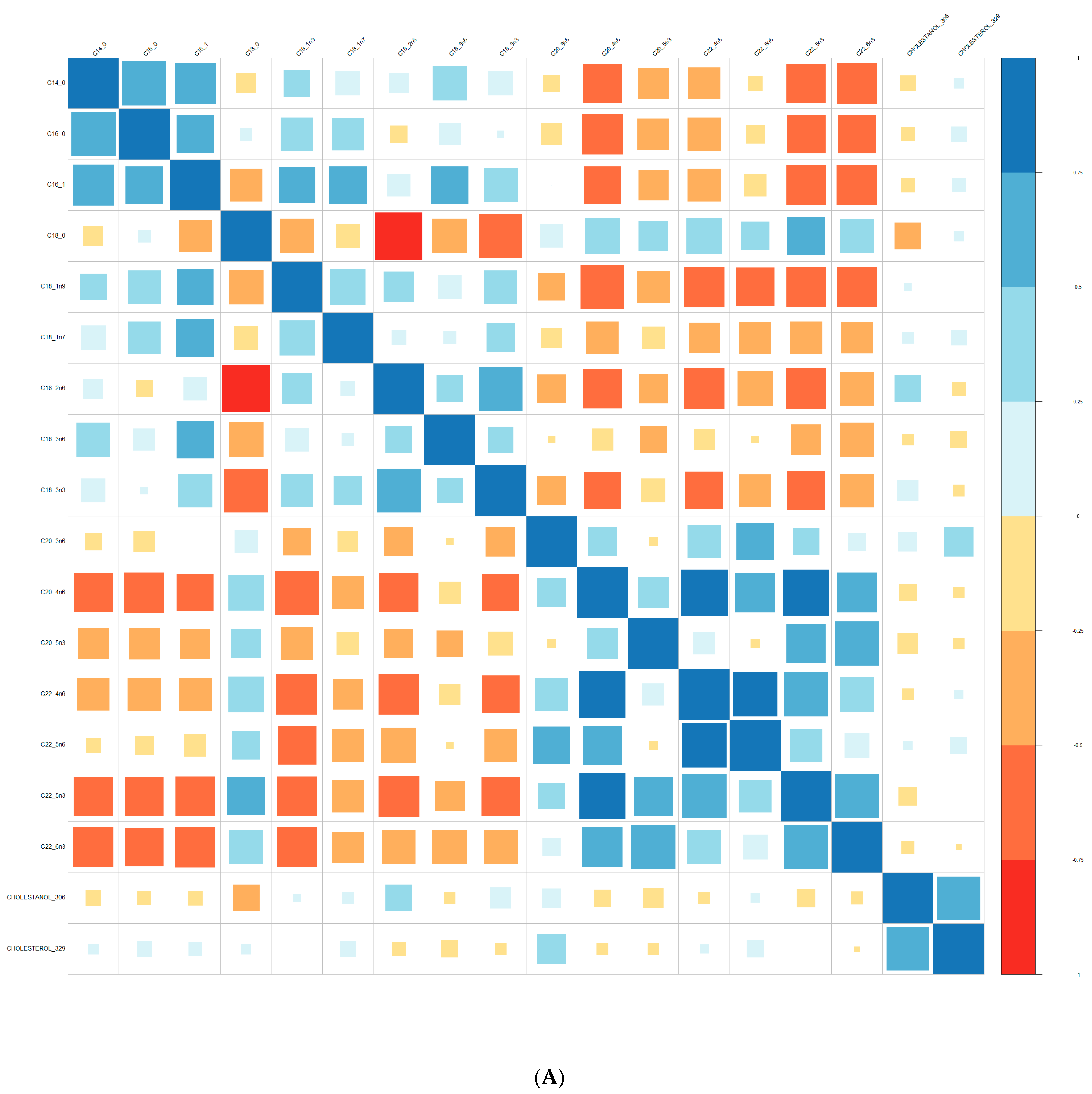
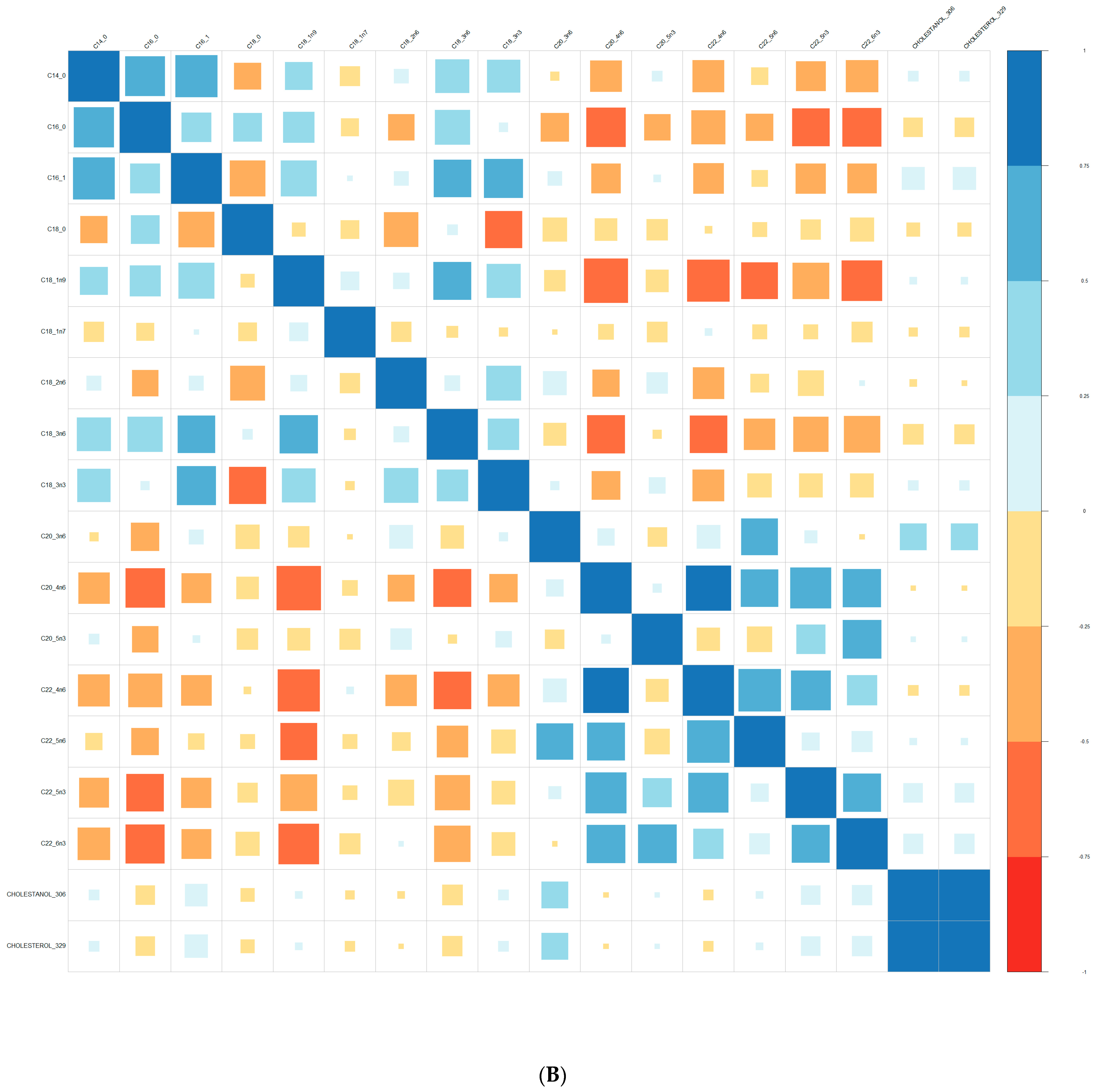

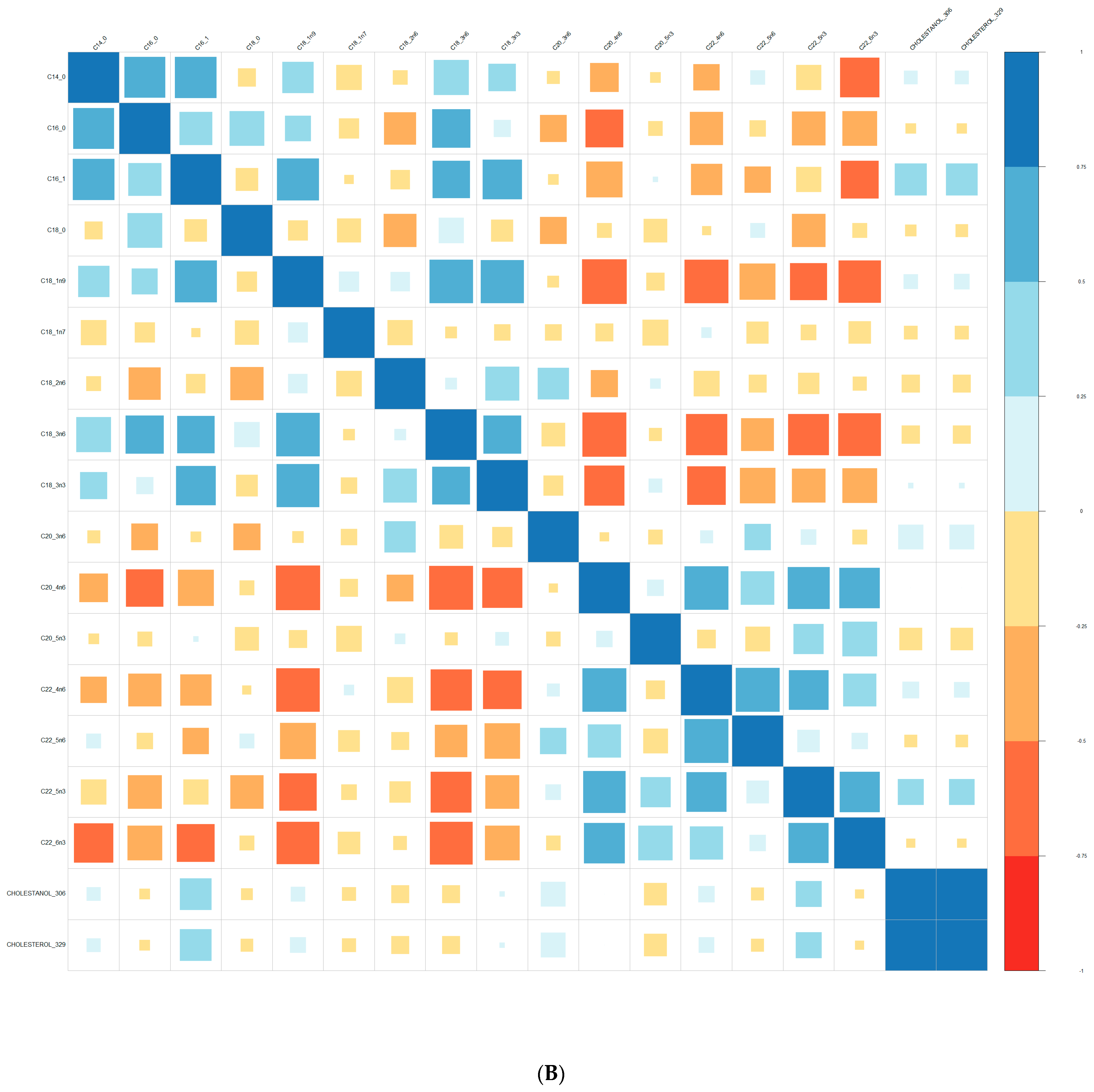
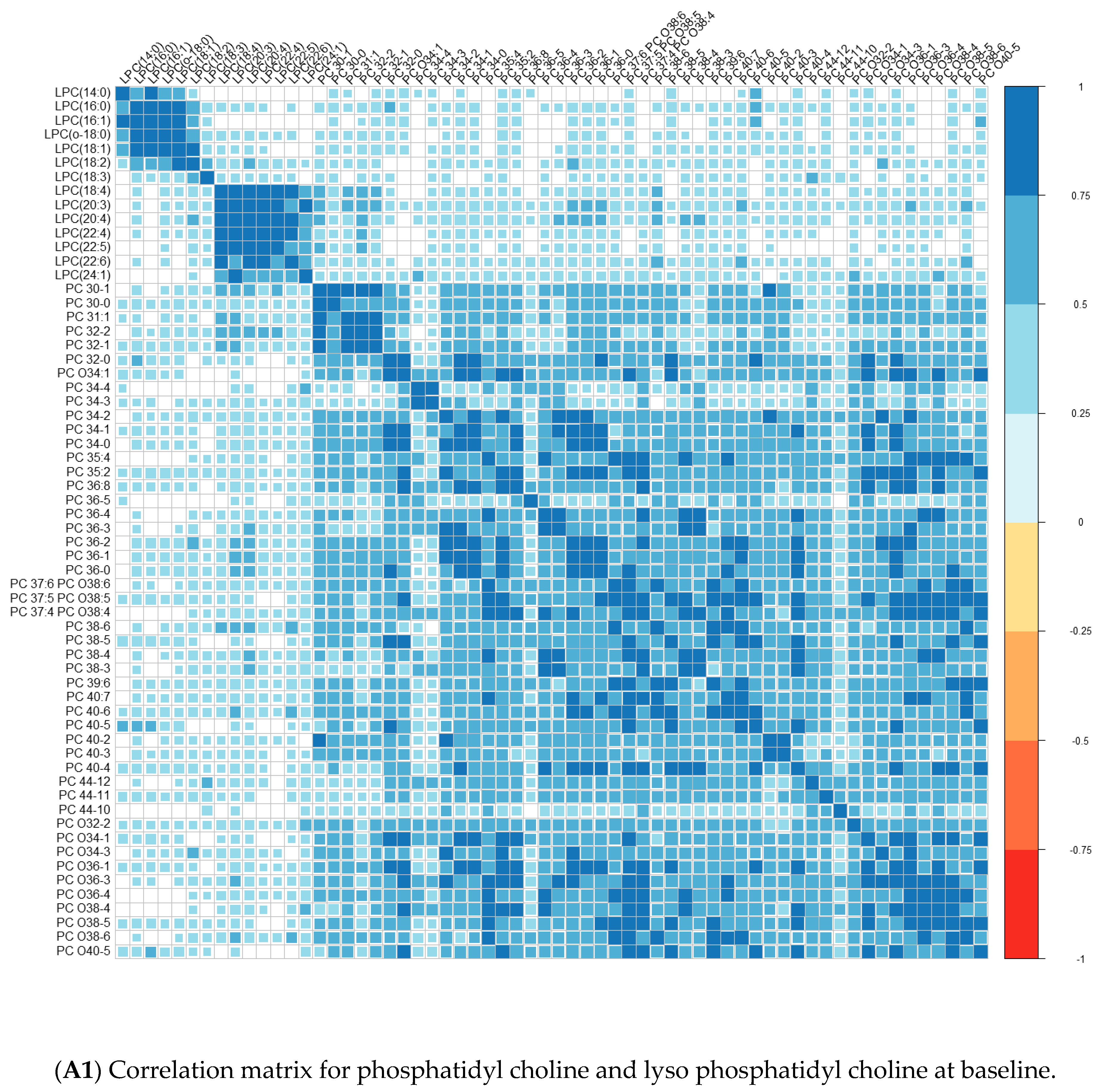
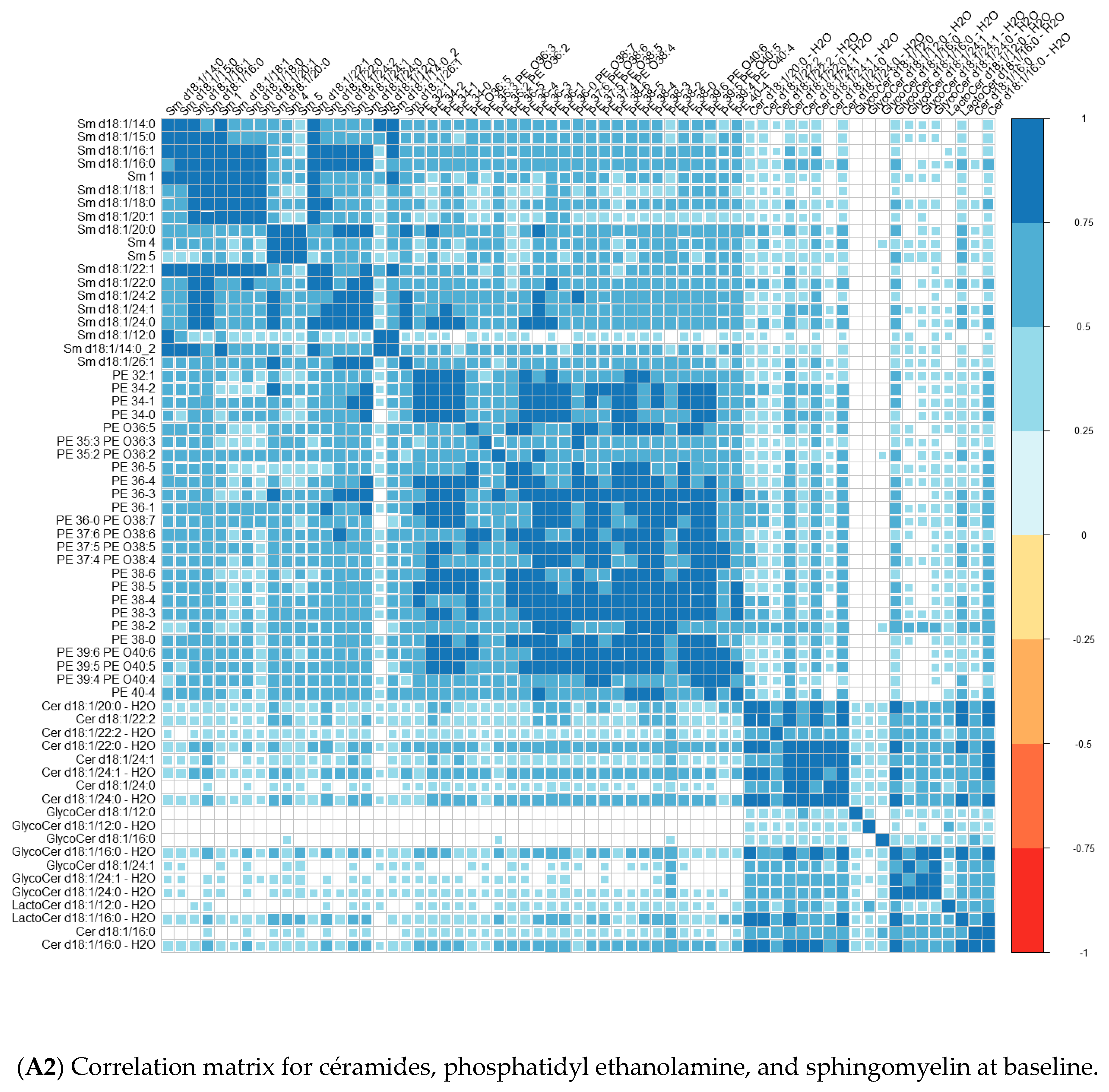
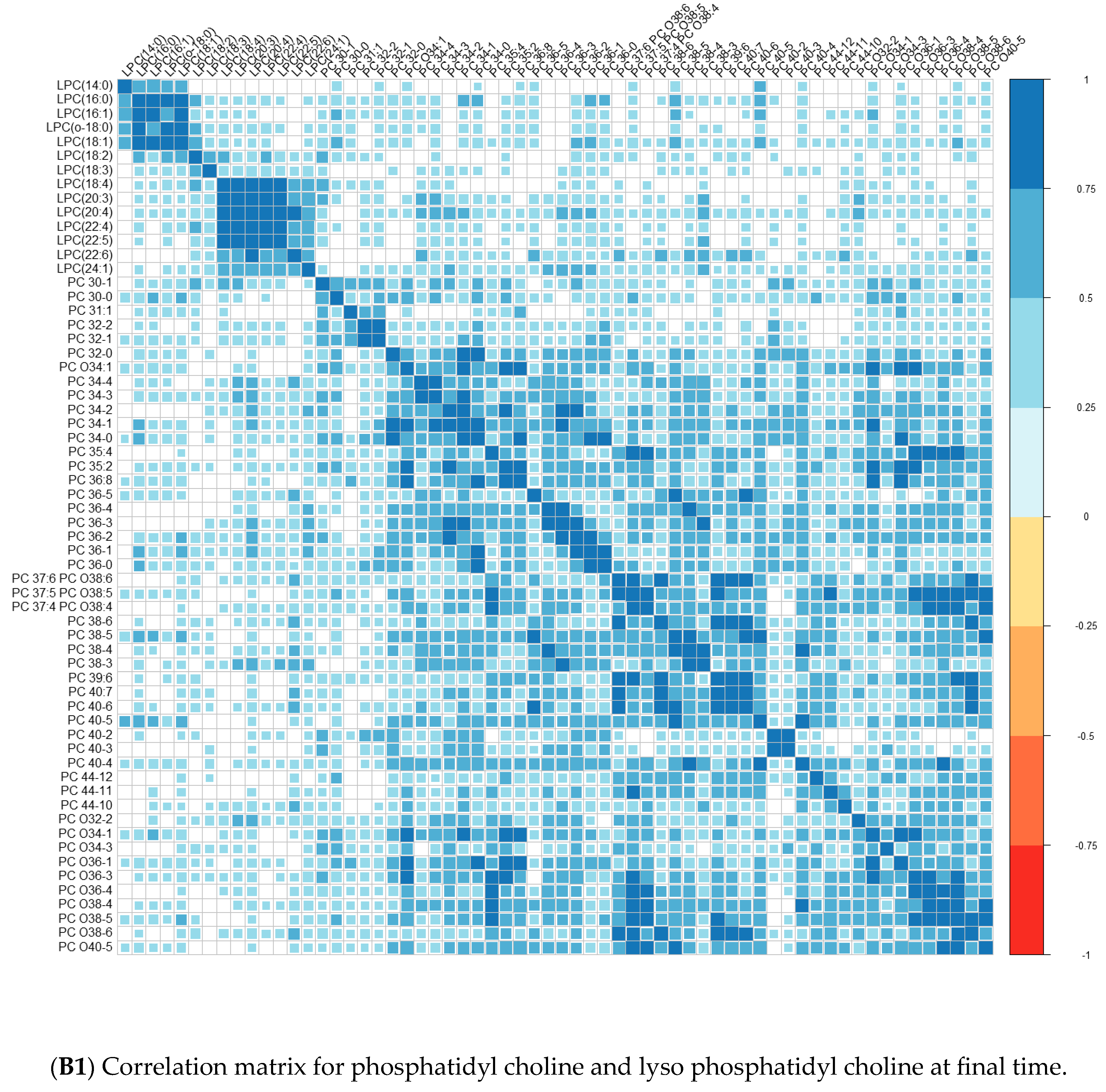
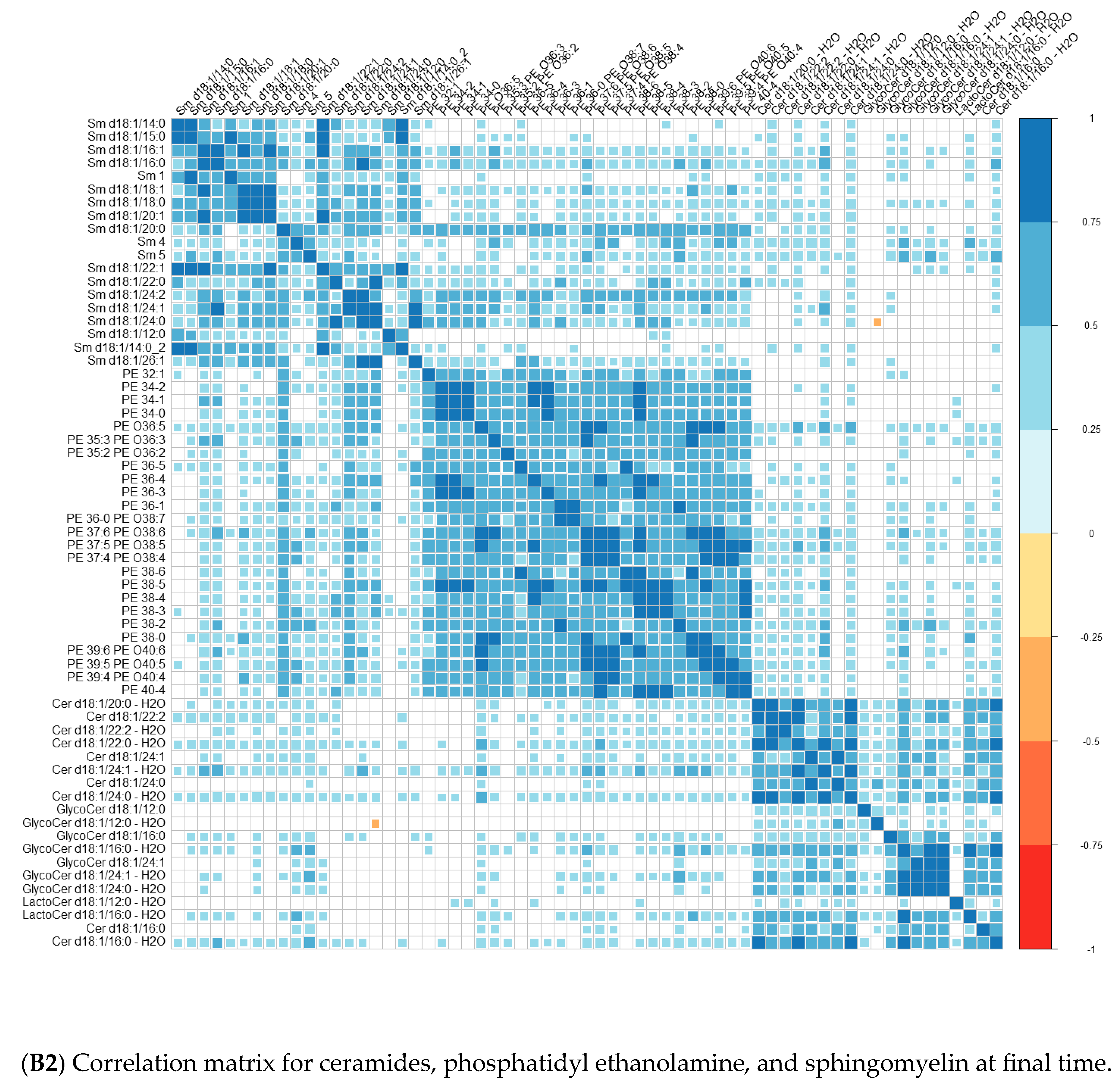
| Converters | Non-Converters | p-Value | |||
| N | Mean (SD) | N | Mean (SD) | ||
| Age (year) | 29 | 20.21 (2.43) | 32 | 21.5 (3.46) | 0.10 |
| BMI | 29 | 21.98 (3.49) | 32 | 21.9 (3.06) | 0.92 |
| CRP | 22 | 2.3 (3.85) | 28 | 2.21 (2.44) | 0.92 |
| GLY | 24 | 4.57 (0.93) | 30 | 4.62 (0.92) | 0.83 |
| HDL | 25 | 1.21 (0.46) | 27 | 1.32 (0.37) | 0.33 |
| TRIG | 25 | 1.02 (0.59) | 29 | 0.99 (0.53) | 0.88 |
| SOFAS | 28 | 48.04 (10.13) | 32 | 46.75 (9.25) | 0.61 |
| PANSSTOT | 28 | 67.86 (25.3) | 32 | 71.72 (17) | 0.49 |
| SANS | 24 | 23.83 (18.01) | 32 | 22.63 (16) | 0.79 |
| SAPS | 24 | 16.75 (14.54) | 32 | 13.28 (9.18) | 0.28 |
| MADRS | 24 | 20.5 (7.35) | 32 | 21.19 (9.31) | 0.77 |
| CPZ EQ | 24 | 23.88 (43.91) | 32 | 17.77 (46.8) | 0.62 |
| N | Percent | N | Percent | ||
| Men (%) | 17 | 58.60% | 18 | 56.30% | 0.91 |
| Cannabis use last month | 0.55 * | ||||
| 0 | 13 | 45% | 18 | 62% | |
| 1–2 | 2 | 7% | 1 | 3% | |
| 3–9 | 4 | 14% | 1 | 3% | |
| >10 | 3 | 10% | 5 | 17% | |
| NA | 7 | 24% | 7 | 15% | |
| Membrane Lipids (%) | Body Mass Index Rho (Spearman) | p | Plasmatic Cholesterol Rho (Spearman) | p | Triglycerides Rho (Spearman) | p |
|---|---|---|---|---|---|---|
| Omega-3 | −0.08 | 0.53 | 0.06 | 0.66 | −0.40 | 0.003 |
| Omega-6 | −0.07 | 0.57 | 0.15 | 0.30 | −0.25 | 0.07 |
| Omega-9 | 0.12 | 0.38 | −0.04 | 0.76 | 0.33 | 0.02 |
| Total PUFA | −0.07 | 0.62 | 0.09 | 0.51 | −0.37 | 0.005 |
| Cholestanol | −0.01 | 0.95 | −0.25 | 0.08 | 0.16 | 0.28 |
| Cholesterol | 0.08 | 0.57 | −0.12 | 0.42 | 0.10 | 0.47 |
| PC | 0.14 | 0.27 | −0.03 | 0.84 | 0.21 | 0.13 |
| PE | −0.24 | 0.06 | 0.12 | 0.38 | −0.02 | 0.91 |
| PS | −0.04 | 0.75 | 0.06 | 0.69 | 0.04 | 0.77 |
| SM | −0.07 | 0.57 | −0.21 | 0.12 | −0.12 | 0.40 |
| Low LA Level | High LA Level | Total | |
|---|---|---|---|
| Converters | 6 (26%) | 23 (61%) | 29 |
| Non-converters | 17 (74%) | 15 (39%) | 32 |
| Total | 23 | 38 | 61 |
| Low LA Level | Low LA Level | p-Value | p-Value Adjusted | |
|---|---|---|---|---|
| C14_0 | 0.275217 | 0.331053 | 0.2 | 0.35556 |
| C16_0 | 22.26739 | 22.01053 | 0.7 | 0.74667 |
| C16_1 | 0.733043 | 0.740263 | 0.6 | 0.74667 |
| C18_0 | 17.962174 | 16.466842 | 0.001 | 0.00320 |
| C18_1n9 | 16.847826 | 17.370526 | 0.4 | 0.58182 |
| C18_1n7 | 1.175217 | 1.160789 | 0.7 | 0.74667 |
| C18_3n6 | 0.086957 | 0.084474 | 0.7 | 0.74667 |
| C18_3n3 | 0.123043 | 0.179211 | 0.0005 | 0.00267 |
| C20_3n9 | 0.295652 | 0.283158 | 0.3 | 0.48000 |
| C20_3n6 | 1.616957 | 1.625263 | 0.8 | 0.80000 |
| C20_4n6 | 15.975652 | 13.988421 | 0.001 | 0.00320 |
| C20_5n3 | 0.678696 | 0.603158 | 0.07 | 0.14000 |
| C22_4n6 | 2.83 | 2.194737 | 0.0003 | 0.00267 |
| C22_5n6 | 0.516087 | 0.427368 | 0.05 | 0.11429 |
| C22_5n3 | 2.184783 | 1.737632 | 0.0004 | 0.00267 |
| C22_6n3 | 4.635652 | 3.984737 | 0.05 | 0.11429 |
| Inclusion | Final Time | p | |
|---|---|---|---|
| C16_0 | 22.107377 | 22.95463 | 0.002 |
| C18_3n6 | 0.08541 | 0.134074 | <0.001 |
| LPC(18:3) | 0.006299 | 0.006612 | 0.050 |
| PS 32:0 | 0.33473 | 0.33627 | 0.045 |
| PS 36:0 | 0.002418 | 0.007212 | <0.001 |
| Converters | Non-Converters | p | |
|---|---|---|---|
| C22_5n6 | 0.003209 | −0.006645 | 0.005 |
| LPC(18:3) | 0.000216 | −0.000042 | 0.04 |
| PC O34:1 | 0.00128 | −0.002841 | 0.03 |
| PC 38-3 | 0.006624 | −0.000174 | 0.04 |
| Cer d18:1/22:2—H2O | 0.008777 | −0.000666 | 0.02 |
| Cer d18:1/16:0—H2O | 0.018843 | −0.021831 | 0.05 |
| LactoCer d18:1/12:0—H2O | 0.00433 | −0.001459 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frajerman, A.; Chaumette, B.; Farabos, D.; Despres, G.; Simonard, C.; Lamazière, A.; Krebs, M.-O.; Kebir, O. Membrane Lipids in Ultra-High-Risk Patients: Potential Predictive Biomarkers of Conversion to Psychosis. Nutrients 2023, 15, 2215. https://doi.org/10.3390/nu15092215
Frajerman A, Chaumette B, Farabos D, Despres G, Simonard C, Lamazière A, Krebs M-O, Kebir O. Membrane Lipids in Ultra-High-Risk Patients: Potential Predictive Biomarkers of Conversion to Psychosis. Nutrients. 2023; 15(9):2215. https://doi.org/10.3390/nu15092215
Chicago/Turabian StyleFrajerman, Ariel, Boris Chaumette, Dominique Farabos, Gaétan Despres, Christelle Simonard, Antonin Lamazière, Marie-Odile Krebs, and Oussama Kebir. 2023. "Membrane Lipids in Ultra-High-Risk Patients: Potential Predictive Biomarkers of Conversion to Psychosis" Nutrients 15, no. 9: 2215. https://doi.org/10.3390/nu15092215
APA StyleFrajerman, A., Chaumette, B., Farabos, D., Despres, G., Simonard, C., Lamazière, A., Krebs, M.-O., & Kebir, O. (2023). Membrane Lipids in Ultra-High-Risk Patients: Potential Predictive Biomarkers of Conversion to Psychosis. Nutrients, 15(9), 2215. https://doi.org/10.3390/nu15092215






